Syntheses, Crystal Structures and DNA-binding Properties of Zn(II), Ni(II) and Co(II) Compounds Containing Thiazole Derivatives①
QI Shuang LI Yu-Han YANG Yu HE Yu-Ting CAI Jian LIN Shi-Wei YUE Shu-Mei
Syntheses, Crystal Structures and DNA-binding Properties of Zn(II), Ni(II) and Co(II) Compounds Containing Thiazole Derivatives①
QI Shuang LI Yu-Han YANG Yu HE Yu-Ting CAI Jian LIN Shi-Wei YUE Shu-Mei②
(130032)
Zn(II), Ni(II) and Co(II) compounds (1~3) based on 2-(2-pyridyl)benzothiazole (bpt) and terephthalic acid (PTA) were synthesized. The crystal structures of [Zn(bpt)(PTA)2] (1), [Ni(bpt)(PTA)2] (2), and [Co(bpt)(PTA)2] (3) have been determined by single-crystal X-ray diffraction analysis, which shows that all the three complexes belong to monoclinic system with space group21/. Time-dependent density functional theory (TD-DFT) calculation is performed on a reference structure of compound 3. The excited electrons mainly localized at the* of ligand 2-(2-pyridyl)benzothiazole, which will be convenient for them to bind with the DNA reacting sites. Fluorescence spectroscopy, ultraviolet (UV) spectroscopy andviscosity were used to characterize the interaction of the compounds withCalf thymus DNA (CT-DNA).The results indicate that compounds 1~3 bind to CT-DNA and have a strong interaction with DNA. The compounds can probably bind to CT-DNA via a non-intercalative mode as concluded by studying the viscosity of a DNA solution in the presence of the compounds. This combination can effectively break DNA, which speculates that these three compounds may interact with the cancer cell DNA in this binding mode, thereby damaging the cancer cells.
calf thymus DNA, 2-(2-pyridyl)benzothiazole, single-crystal structures, spectroscopy;
1 INTRODUCTION
Heterocyclic compounds, especially nitrogen-con-taining heterocyclic metal compounds and their derivatives, are widely used as intermediates in various fields, such as pharmaceuticals, dyes, and other fine chemical products[1-6]. Benzothiazole and benzimidazole metal derivatives have displayed good photoluminescence, magnetic properties and biolo-gical activity[7-13]. Metal compounds are widely used in various fields[14-16]. The interactions between transition metal compounds and DNA have been a subject of passionate research. Many compounds coordinated with metal ions have been studied and used in the treatment of cancer[17-21]. More and more researches on the interaction between metal compounds and DNA are reported. For example, Barone reported the synthesis of nickel(II), copper(II) and zinc(II) compounds of the same ligand, which showed similar coordinate geometry and binding patterns for DNA[22]. Darabi prepared mononuclear cobalt(II) compounds and monitored the interaction of CT-DNA and bovine serum albumin (BSA) with the compound using different analytical methods[23]. Karastogianni synthesized and characterized a mononuclear compound [Mn(thienyl-2-carboxy-late)2(H3tea)], H3tea triethanolamine. DNA binding properties and antioxidant activity have also been investigated[24, 25].
The mechanism of interaction between compounds and DNA is still not very clear, and there are still some controversies. Therefore, understanding the mechanism of interaction between these compounds and DNA is essential in order to reveal its anticancer mechanism and to synthesize novel anticancer drugs[26-28]. At present, the non-covalent interactions between drug molecules and DNA reported in the literature include electrostatic binding, groove binding and intercalation interactions[29, 30]. If we can find compounds that can target DNA destruction, there will be further development in anticancer drugs and cancer treatment.
Our research group has been working on the synthesis and properties of imidazole and thiazole metal compounds recently. T.T. Hou synthesized a Cd(II) metal compound with 2-(2-pyridyl)benzimi-dazole as its ligand[31]. X.R. Yue synthesized a Cd(II) metal compound with 2-(2-pyridyl)benzothiazole as ligand[32]. We wish to further understand the ways in which thiazole metal compounds interact with DNA, so that we can analyze the prospects of this approach in the development of anticancer drugs.
We discuss here the syntheses of three metal compounds based on 2-(2-pyridyl) benzothiazole and the interaction of these compounds with DNA. We hope it could offer further help to anti-cancer drug research and development.
2 EXPERIMENTAL
2. 1 Materials and methods
All chemicals were of analytical grade and used without any further purification. CT-DNA was purchased from Sigma and stored at 2~8 °C. The Tris buffer (hydroxymethyl) purchased from J&K with purity of analytical grade was used for preparing all solutions for CT-DNA binding studies. The TU-1901 dual-beam recording spectrophotome-ter was used to measure the UV visible spectrum of composite in the range of 200~500 nm. RF-5301PC fluorescence spectrophotometer was available for fluorescence spectrometer.
2. 2 Preparation of 2-(2-pyridyl) benzothiazole (bpt)
20 mL of polyphosphoric acid was added to a 250 mL round-bottomed flask. 2-Picolinic acid (3.1 g, 25 mmol) and 2-aminothiophenol (3.1 g, 25 mmol) were added into this flask respectively when the tempera- ture was raised to 110 °C. Then the mixture was heated to 180 °C for 3 h and poured into 300 mL of ice-water while it was still hot, followed by constant stirring and adjusting to pH = 7 with NaOH solution. The ligand can be extracted by methyl alcohol after filtration and drying in 56% yield. Anal. Calcd. (%) for C11H8N2S: C, 66.00; H, 4.00; N, 1.4. Found (%): C, 65.97; H, 3.98; N, 1.41.
2. 3 Preparation of compounds 1~3
ZnCl2·2H2O (1.75 g, 10 mmol), bpt (2.12 g, 10 mmol) and PTA (1.66 g, 10 mmol) were dissolved in the mixed solvents of methanol (3 mL), DMF (1 mL), and water (1 mL) in a beaker of 25 mL. After being heated and stirred on magnetic stirrer for 1 hour, the mixed solution was then transferred to a stainless-steel reaction kettle with polytetrafluoroethylene lining, which was then placed into an electro-thermostatic blast drying oven at 80oC for complete reaction for 5 days. After natural cooling for 2 days, light yellow crystals were obtained.Light yellow crystals were isolated by filtration, washed with methanol and driedin49% yield based on Zn. Anal Calcd. (%) for C20H12N2O4ZnS (1): C, 54.32; H, 2.72; N, 6.34. Found (%): C, 54.30; H, 2.71; N, 6.35.
Compound 2 was synthesized in a similar proce-dure to 1, by changing ZnCl2·2H2O (1.75 g, 10 mmol) to NiCl2·6H2O (2.37 g, 10 mmol). After naturally cooling for 2 days, light green crystals were obtained. Light green crystals were isolated by filtration, washed with methanol and dried in 52% yield based on Ni. Anal. Calcd. (%) forC20H12N2NiO4S (2): C, 55.16; H, 2.75; N, 6.43. Found (%): C, 55.15; H, 2.73; N, 6.42.
Compound 3 was synthesized in a similar proce-dure to 1 by substituting ZnCl2·2H2O (1.75 g, 10 mmol) with CoCl2·6H2O (2.37 g, 10 mmol). After naturally cooling for 2 days, red-brown crystals were obtained by filtration, washed with methanol and dried (51% yield based on Co). Anal. Calcd. (%) forC20H12N2O4CoS (3): C, 55.13; H, 2.75; N, 6.43.Found (%): C, 55.12; H, 2.73; N, 6.41.
2. 4 X-ray crystallography
Suitable single crystals of 1~3 were put in fine-focus sealed tube for X-ray data collection performed on a Bruker SMART APEX CCD area-detector dif-fractometer equipped with graphite-monochromated Moradiation (= 0.71073 Å) at 293(2) K by using anscan mode. The data of all complexes were refined by full-matrix least-squares on2and the structures were solved using SHELXS-97 and refined with SHELXL-97[33]. A summary for crystal data and structure refinement of 1~3 is given in Table 1. Selected bond lengths and bond angles of these three compoundsare listed in Tables 2~4.
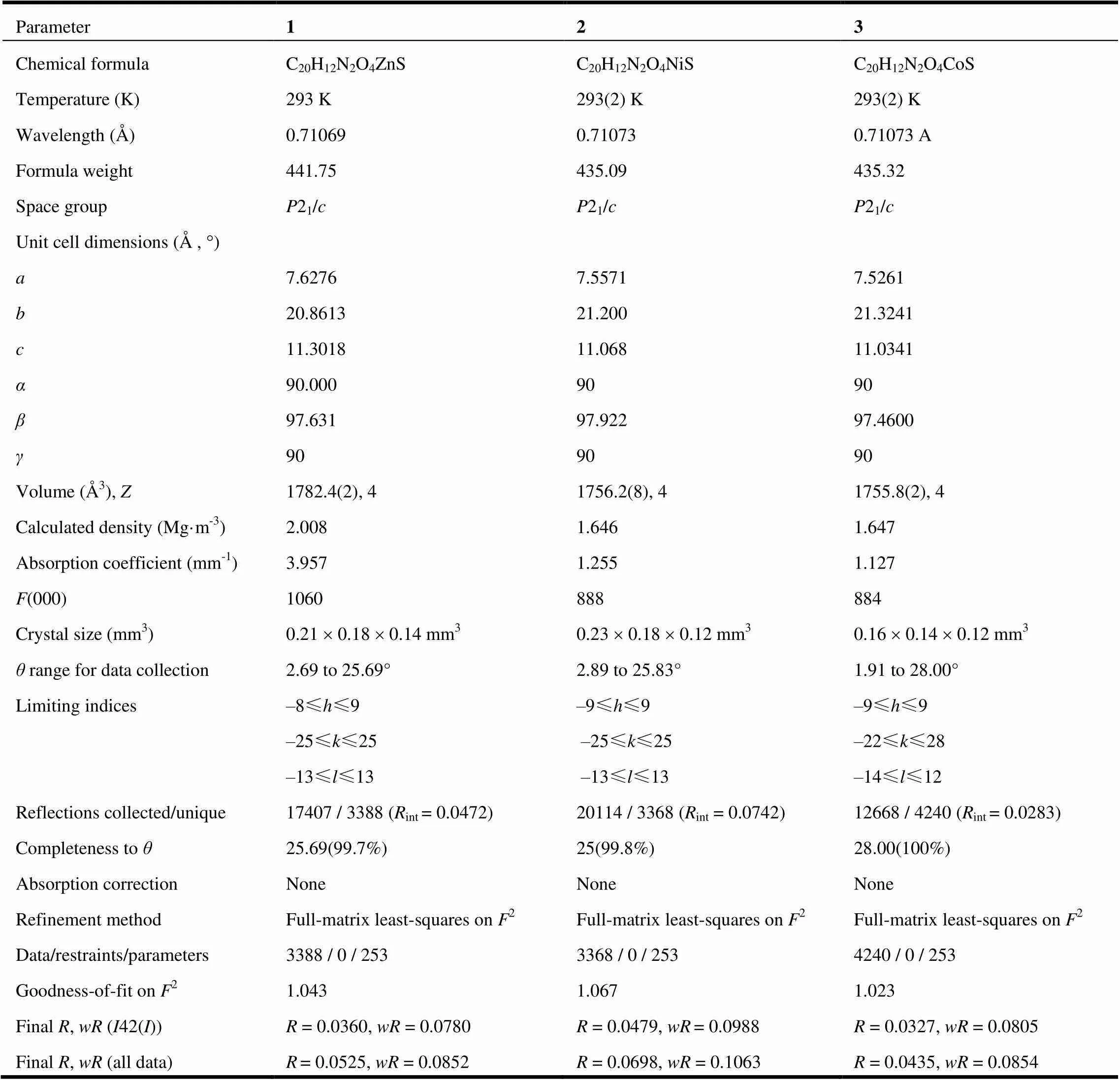
Table 1. Crystal Data and Structure Refinement for 1~3
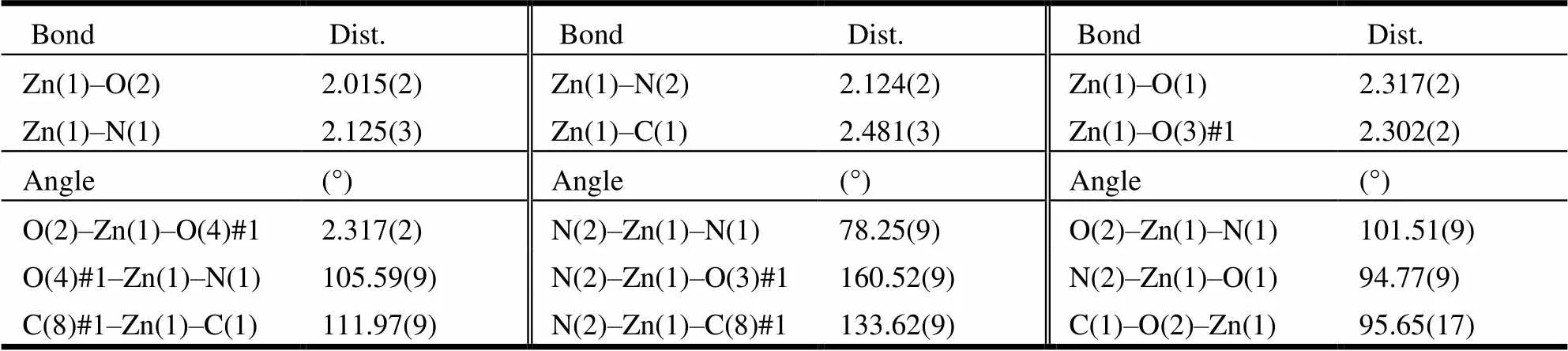
Table 2. Selected Bond Lengths (Å) and Bond Angles (°) of Compound 1
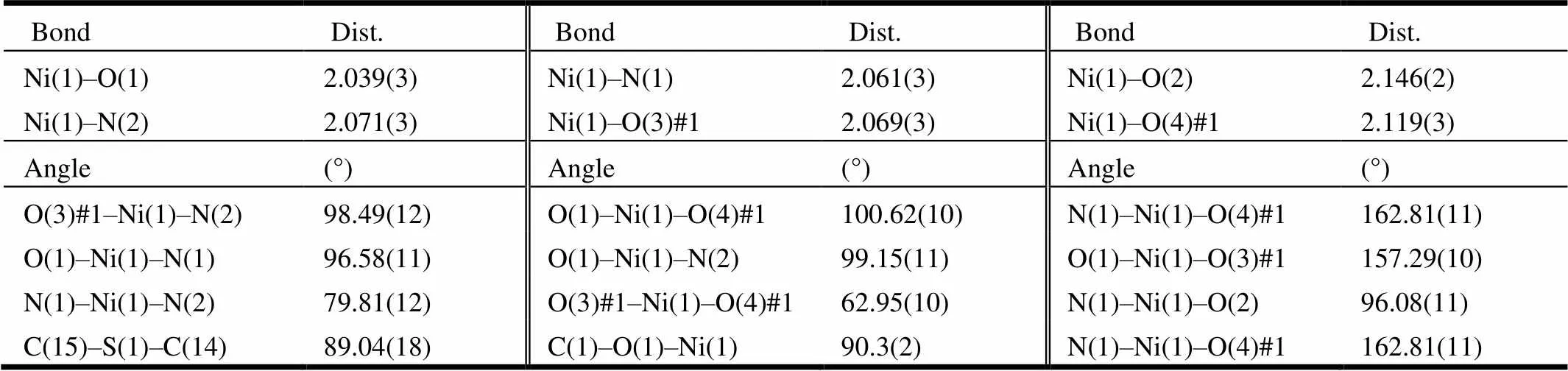
Table 3. Selected Bond Lengths (Å) and Bond Angles (°) of Compound 2

Table 4. Selected Bond Lengths (Å) and Bond Angles (°) of Compound 3
2. 5 TD-DFT
The geometrical structure of ground state was optimized in gas phase by the density functional theory (DFT)[34]with the Becke’s LYP (B3LYP) exchange-correlation functional calculus, using a single crystal structure as initial structure[35]. During the simulations, the basis sets of 6-31g(d)[36, 37]were applied for C, H, O, N and Si, LANL2Dz[38]was applied for Re and Br. All calculation was finished with PCGAMESS[36].
2. 6 DNA binding studies
Experiments on the interaction between com-pounds and DNA, including absorption spectral traces, emission spectroscopy and viscosity measure-ments, conform to the standard methods and prac-tices previously used in our laboratory[39]. By varying the concentration of CT-DNA added, the con-centration of metal compound was fixed and the interaction between the compound and DNA was analyzed by using fluorescence and UV spectroscopy. Viscosity measurements were carried on a Ubbelohde viscometer in a thermostated water-bath maintained at 20 °C by varying the concentration of added metal compounds. Sample concentration ratio is shown in Table 5.
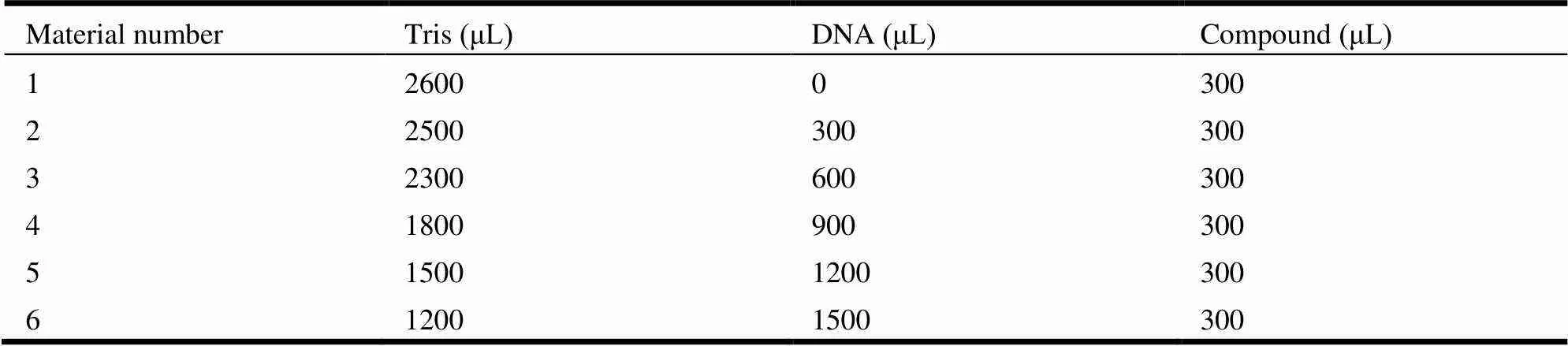
Table 5. Concentration Ratio of Mixed Solution
3 RESULTS AND DISCUSSION
3. 1 Crystal structure description
The crystal structures of compounds 1~3 are shown in Figs. 1~3.
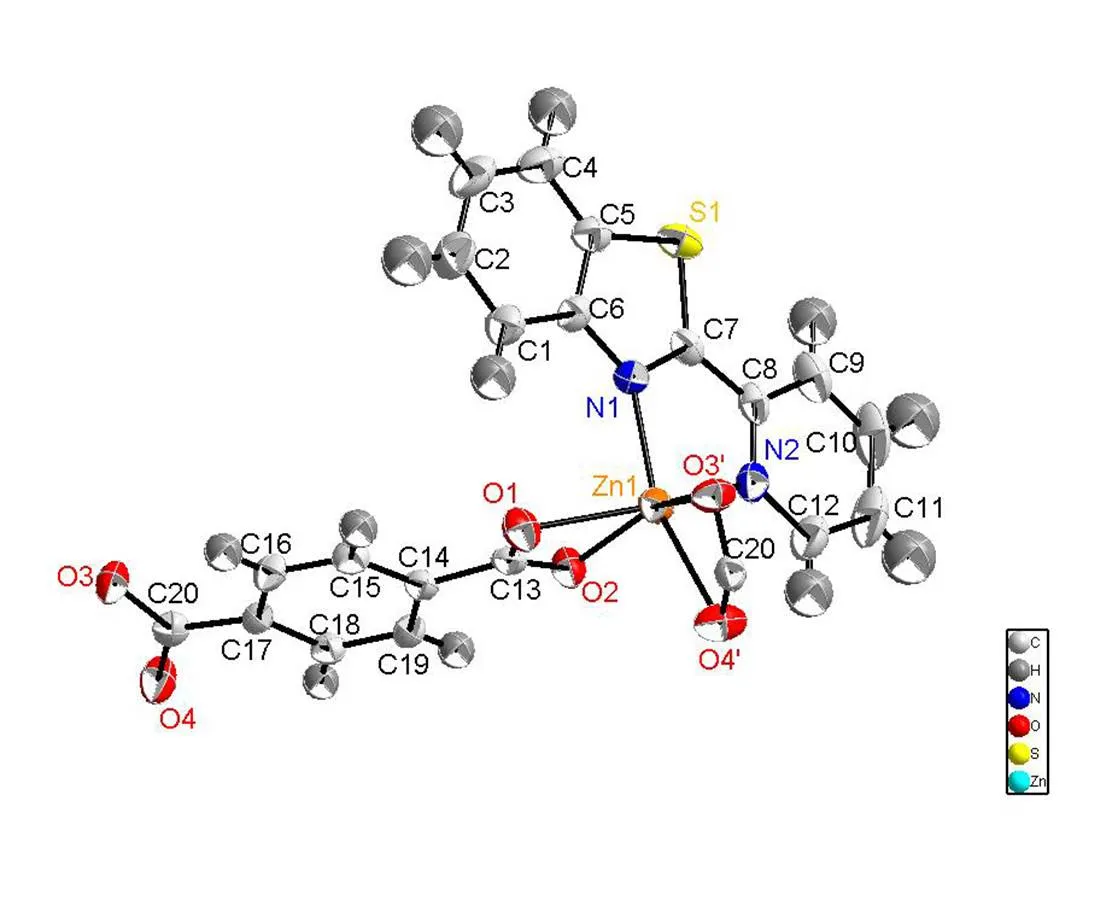
Fig. 1. Molecular structure of compound 1 showing 50% displacement ellipsoids
Fig. 2. Molecular structure of compound 2 showing 50% displacement ellipsoids
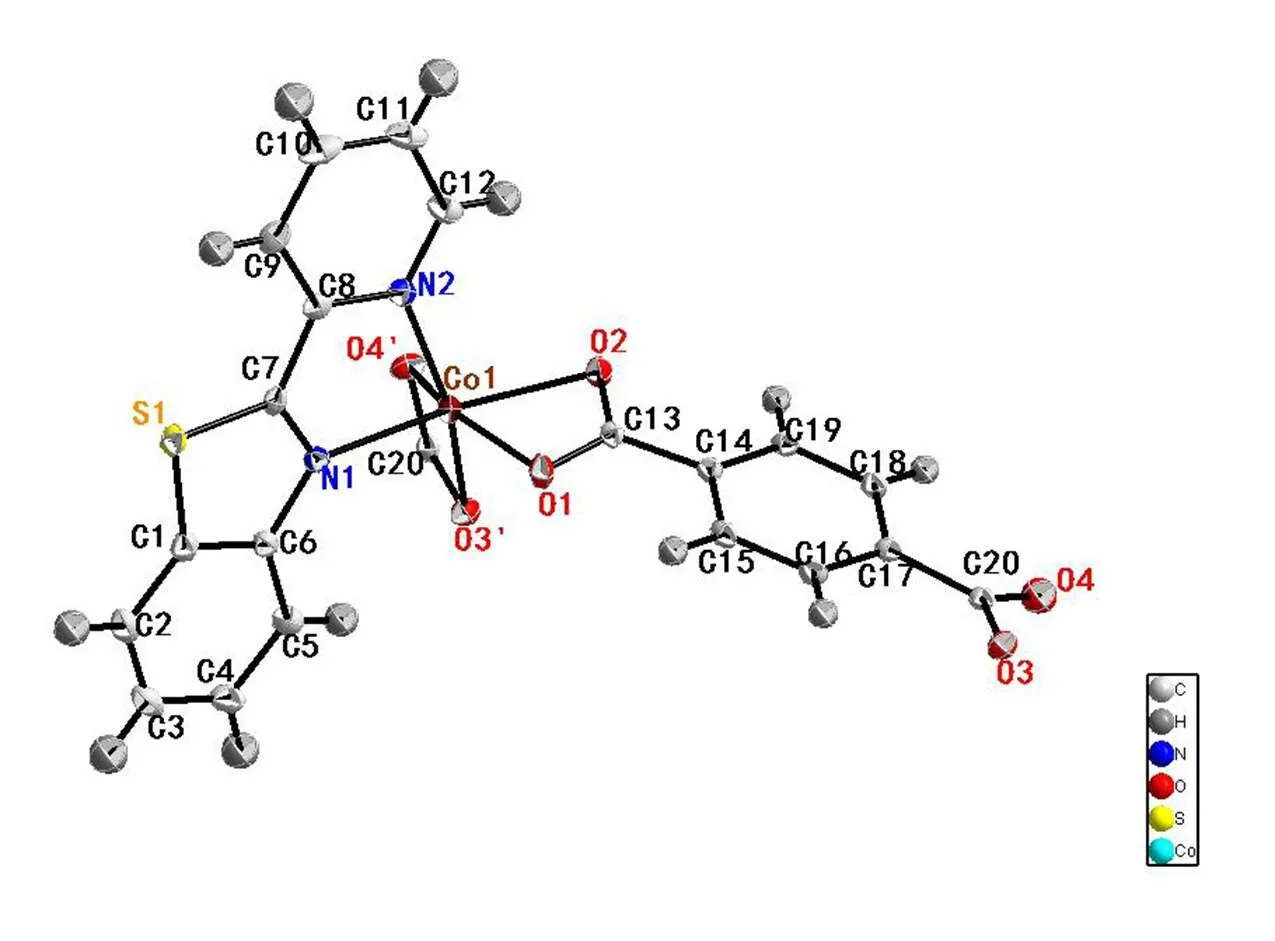
Fig. 3. Molecular structure of compound 3 showing 50% displacement ellipsoids
Single-crystal X-ray structural analysis shows that compounds 1~3 crystallize in the monoclinic system with space group21/. For compound 1, each Zn(II) ion is six-coordinated by two nitrogen atoms from 2-(2-pyridyl)benzothiazole ligand and four oxygen atoms from two terephthalic acids. The geometry around Zn(II) center forms the twisted octahedral structure. The Zn–O bond lengths are within the range of 2.015(2) to 2.302(2) Å, with an average value of 2.166 Å. The Zn(1)–N(2) (thiazole, 2.124(2) Å) bond length is similar to Zn(1)–N(1) (pyridyl, 2.125(3) Å), indicating that the nitrogen atom of pyridyl shows similar coordination ability to thiazole[26]. The bond lengths are within the ranges of 60.11(8)°~160.52(9)°, deviating from 90°, 94.77(9)°~105.59(9)°, deviating from 180°. Compounds 2 and 3 are also twisted octahedra with 6 coordination structure. For compound 2, the asym-metric unit consists of one Ni(II) cation, two nitrogen atoms from 2-(2-pyridyl)benzothiazole ligand and four oxygen atoms from two terephthalic acids form the distorted octahedral structure. Ni–O bond lengths are within the range of 2.039(3)~2.146(3) Å, with an average value of 2.093 Å. The Ni–N bond length is (2.071(3) Å), which compares to that of analogous compound[26]. The bond lengths are within the range of 62.95(10)°~100.62(10)°, deviating from 90°, 157.29(10)°~162.81(11)°, deviating from 180°. For compound 3, two nitrogen atoms from 2-(2-pyridyl)benzothiazole ligands and four oxygen atoms from terephthalic acids with central atom cobalt form the structure with six coordinates. The O–Co bond distances range from 2.0452(14)~2.2147(14) Å, with an average value of 2.1298 Å. The bond lengths are within the range of 61.96(5)°~101.40(6)°, deviating from 90°, 154.61(6)° to 162.74(6)° devia-ting from 180°.
The packing diagrams of compounds 1~3 are shown in Figs. 4~6. The intermolecular weak hydrogen bonds are observed among the oxygen atoms of terephthalic acid and the sulfur atom of 2-(2-pyridyl)benzothiazole ligand. The intermolecular interactions in compounds 1~3 lead to two-dimen-sional networks in the crystals.
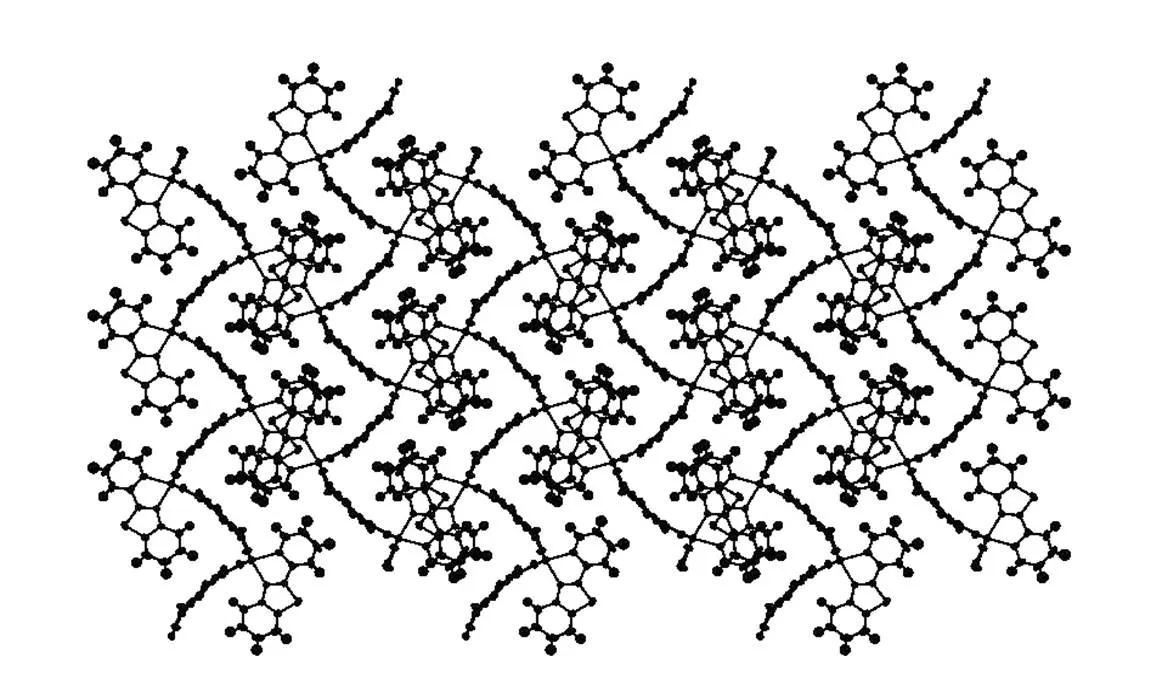
Fig. 4. Packing diagram of compound 1 in a unit cell viewed along theaxis
Fig. 5. Packing diagram of compound 2 in a unit cell viewed along theaxis
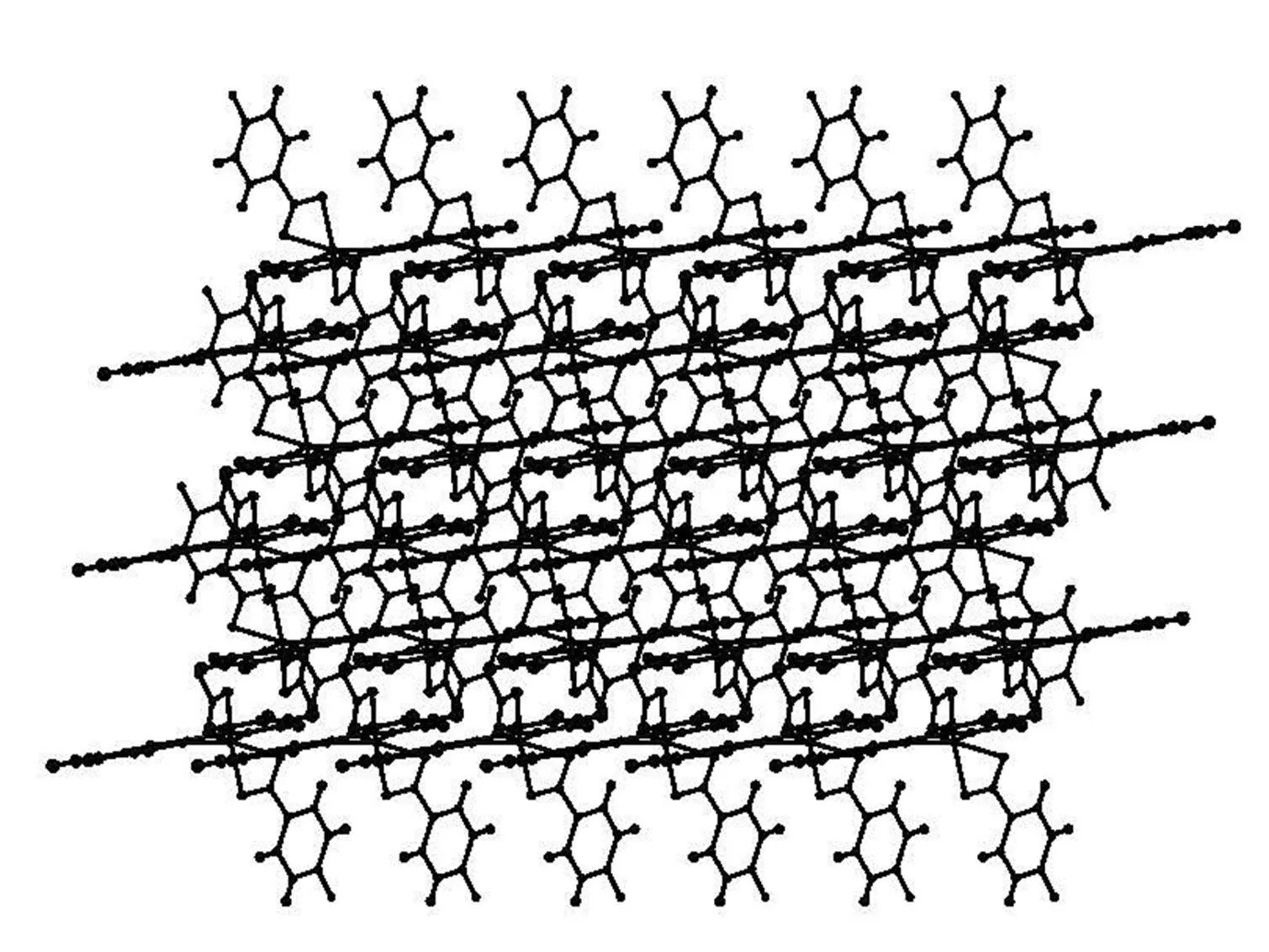
Fig. 6. Packing diagram of compound 3 in a unit cell viewed along the along theaxis
3. 2 Thermal stability and powder X-ray diffraction
Compounds 1~3 were subjected to ascertain the stability of supramolecular architecture by thermo gravimetric analysis (TGA), as shown in Fig. 7. For compound 1, no obvious weight loss was found before the decomposition of the framework occurring at about 415 °C. The TGA analysis showed that the framework has collapsed step by step around 415 and 575 °C. Apparently, compound 1 showed a weight loss of 67.8% in the temperature range of 415~580 °C. For compound 2, the TGA analysis showed that the framework has collapsed step by step around 427 and 496 °C. Apparently, compound 2 showed a weight loss of 79.2%. For compound 3, the TGA analysis showed that the framework has collapsed step by step around 431 and 487 °C. The weight loss of compound 3 was 72.6%. Powder XRD experiment was performed on compounds 1~3 to confirm the phase purity of bulk sample, and the experimental patterns of the as-synthesized sample are well consistent with the simulated ones, indicating the phase purity of the sample (Figs. 8~10).
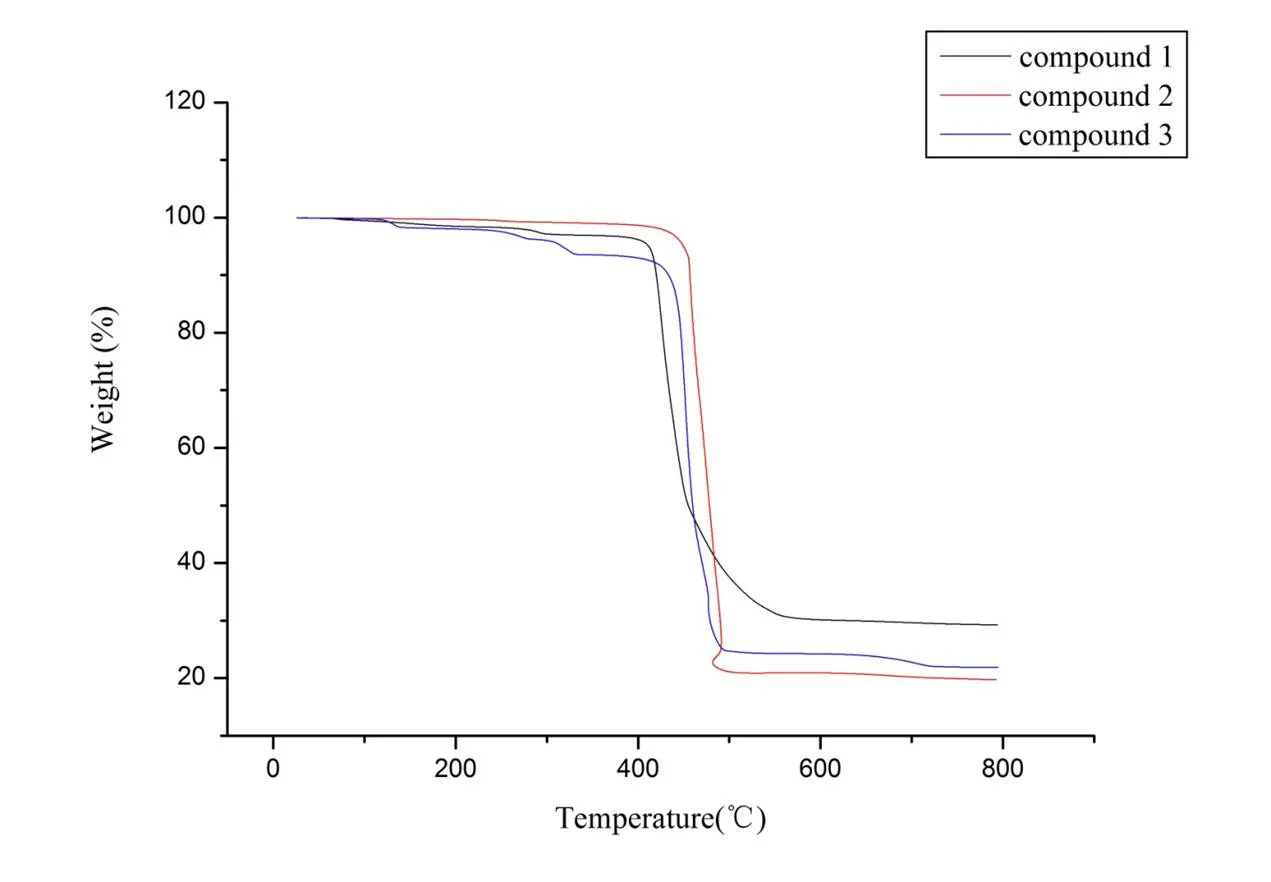
Fig. 7. TG curves of compounds 1~3
Fig. 8. Simulated and experimental XRPD patterns of compound 1
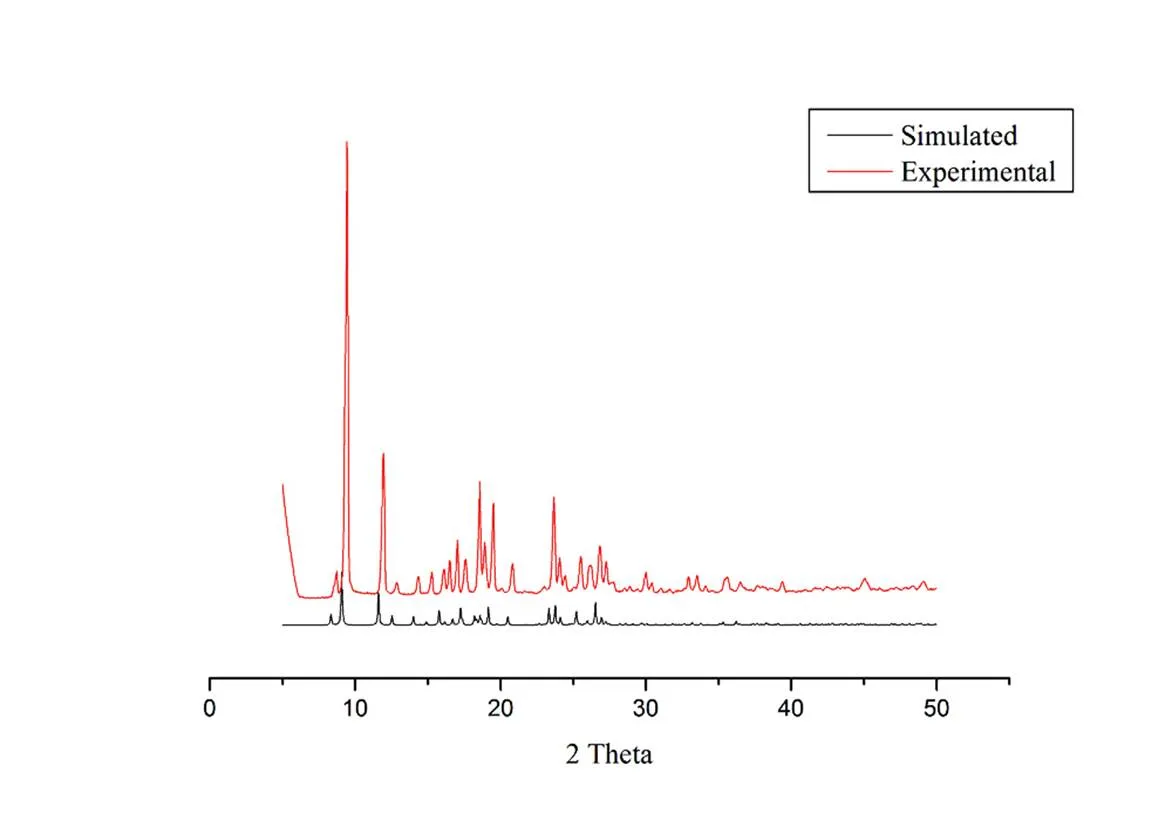
Fig. 9. Simulated and experimental XRPD patterns of compound 2
Fig. 10. Simulated and experimental XRPD patterns of compound 3
3. 3 TD-DFT
For a better understanding on the electronic structures of these compounds, time-dependent den-sity functional theory (TD-DFT) calculation is performed on a reference structure of compound 3. Corresponding frontier molecular orbitals (HOMO and LUMO) are plotted and shown as Figs. 11 and 12. It is observed that the HOMO of this compound is mainly composed of metal ion and ligand 2-(2-pyridyl)benzothiazole, which means that this HOMO has a dominant metal character. Upon excitation, the electron cloud of this compound is migrated to LUMO which is essentially theof ligand 2-(2-pyridyl)benzothiazole. In this case, the onset electro- nic transition of this compound should have an obvious metal-to-ligand-charge-transfer (MLCT) character. Considering that the excited electrons are mainly localized at the* of ligand 2-(2-pyridyl)benzo-thiazole, it will be convenient for them to bind with DNA reacting sites and thus facilitate their reaction with DNA.

Fig. 11.Compound 3 HOMO
Fig. 12.Compound 3 LUMO
3. 4 Absorption spectral studies of DNA binding
Electron spectroscopy is one of the most useful tools for detecting the combination of metal com-pounds and DNA[40, 41]. If the interaction between compounds and DNA is covalent, the wavelength is red-shifted. As the ligand and DNA bases in the DNA-inserted compound can undergo-electron stacking, the-space orbital is coupled to the-electron orbital of the base. The coupledorbital is partially filled with electrons, which reduces the probability of transition and produces a color reduction effect. At the same time, the energy level decreases after coupling, which causes the→* transition energy to decrease, resulting in a red shift. If the interaction between molecules and DNA are electrostatic or groove, the UV-visible absorption spectrum peak will show a small red shift, but its color reduction effect is not obvious. Figs. 13~15 show the absorption spectrum of compounds 1~3 in the presence of different concentrations of DNA. In the UV region, the compounds represent a strong absorption band at 202 nm, and the maximum value may be inversely proportional to the conversion rate of the ligand-*. When the amount of CT-DNA gradually increases, the hyperchromic phenomenon occurs in the absorption spectrum of the compounds, and the degree of color increases with the increase of DNA concentration. The spectral changes of compounds in the presence of DNA are mostly likely to be the minor groove binding or electrostatic binding mode for the compounds to DNA[42, 43], possibly due to the damage of DNA double helix structure.
3. 5 Fluorescent studies of DNA binding
In order to further reveal the interaction between the compounds and CT-DNA, emission experiments were performed. In the compounds solution of constant concentration, the emission spectra of compounds 1~3 are shown in Figs. 16~18 as the CT-DNA concentration increases. The compounds exhibit strong fluorescence at 395 nm when excited at 285 nm at room temperature. Upon addition of increasing amounts in CT-DNA to the fixed amount of compounds, the fluorescence emission intensity was enhanced without any change in the position of emission bands, indicating the strong interaction of compounds with DNA. This increase in emission intensity means the change in the environment of the metal compound and the different degree to which the compound enters the hydrophobic environment inside the DNA. It avoids the quenching effect of solvent water molecules and limits the mobility of the compounds at the binding site, thus resulting in reduced relaxation vibration modes and higher emission intensity[44].

Fig. 13. Absorption spectra of compound 1 in Tris-HCl buffer (pH 7.0) in the absence and presence of increasing amount of DNA at room temperature. Inset: arrow shows change in intensity with increasing the concentration of DNA
Fig. 14. Absorption spectra of compound 2 in tris-HCl buffer (pH 7.0) in the absence and presence of increasing the amount of DNA at room temperature. Inset: arrow shows change in intensity with increasing the concentration of DNA
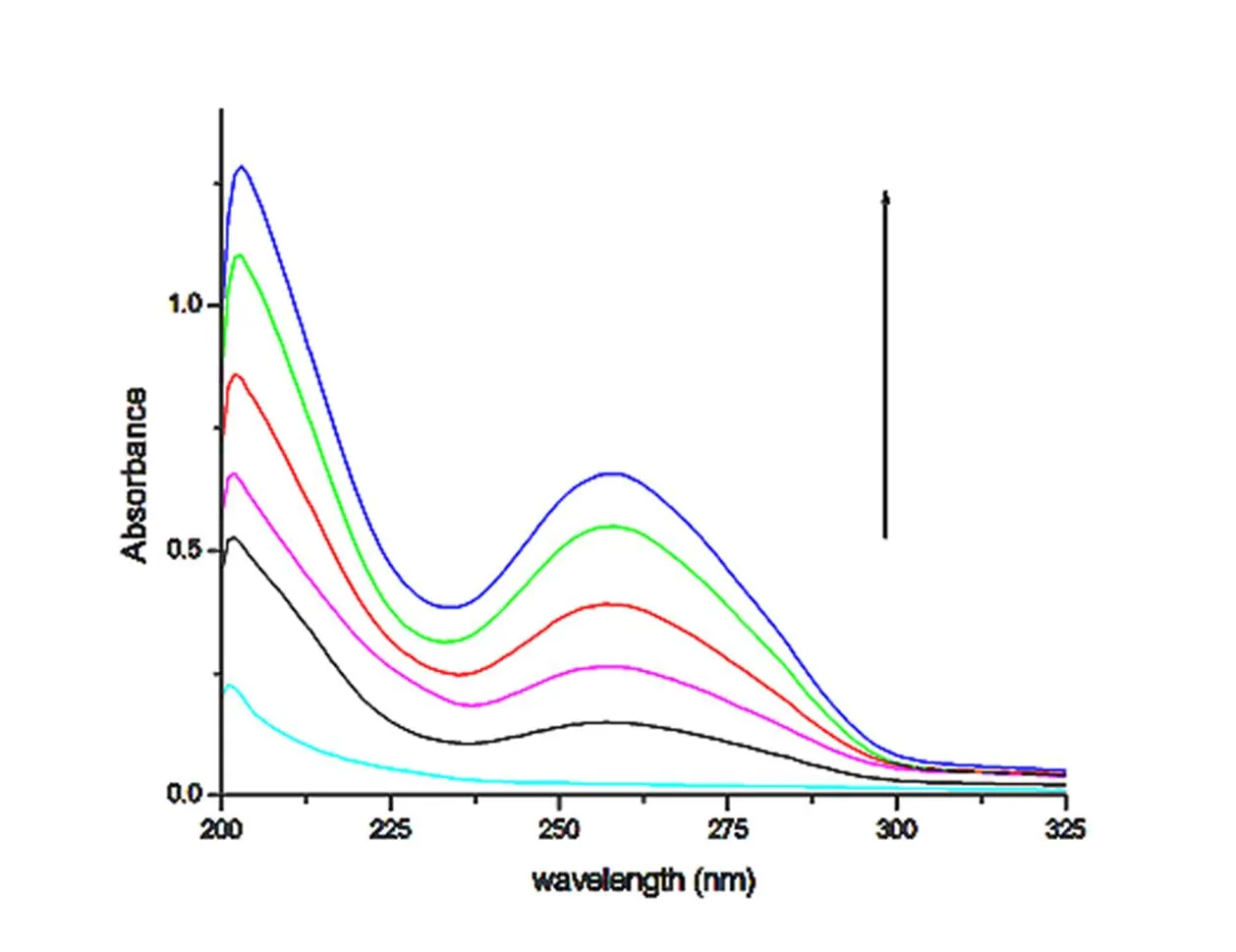
Fig. 15. Absorption spectra of compound 3 in tris-HCl buffer (pH 7.0) in the absence and presence of increasing the amount of DNA at room temperature. Inset: arrow shows change in intensity with increasing the concentration of DNA
Fig. 16. Emission spectra of compound 1 in tris-HCl buffer (pH 7.0) in the presence and absence of CT-DNA at room temperature. Arrow shows change in intensity with increasing the concentration of DNA
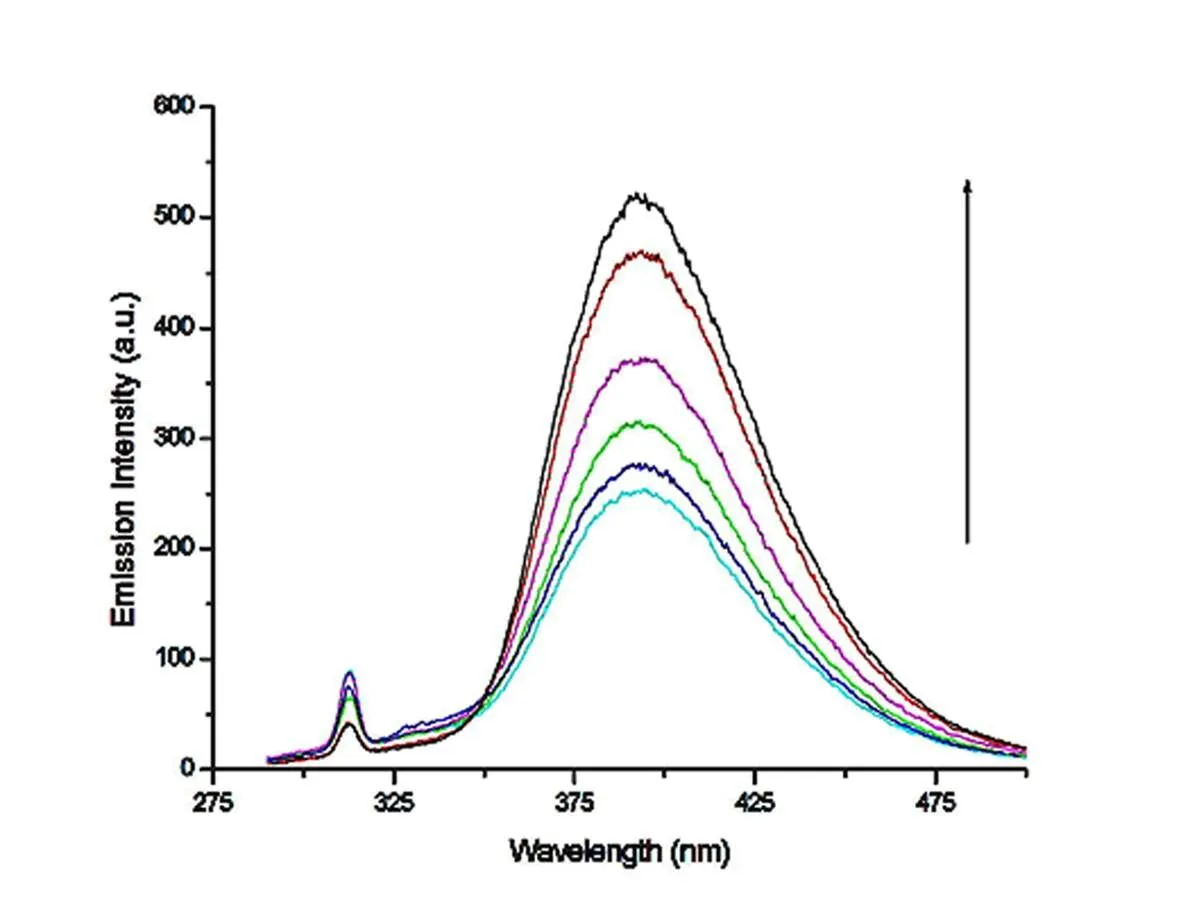
Fig. 17. Emission spectra of compound 2 in tris-HCl buffer (pH 7.0) in the presence and absence of CT-DNA at room temperature. Arrow shows change in intensity with increasing the concentration of DNA
Fig. 18. Emission spectra of compound 3 in tris-HCl buffer (pH 7.0) in the presence and absence of CT-DNA at room temperature. Arrow shows change in intensity with increasing the concentration of DNA
3. 6 Viscosity studies of DNA binding
To further reveal the binding mode between the three compounds to DNA, viscosity measurements on CT-DNA by varying the concentration of the added metal compounds were carried out. A classical intercalative mode causes an increase in the DNA viscosity becausethe lengthening of DNA helixas base pairs is separated in order to accommodate the binding ligand which increases the overall length of DNA[45]. However, non classical mode of interac-tions such as groove binding and electrostatic could bend (or kink) the DNA helix, reduces its length and causes the reduction or no change in the DNA viscosity with different concentrations[44]. The three curves in Fig. 19 are the viscosity curves for the interaction of compounds 1~3 with DNA. It can be seen from the figure that the viscosity curves for the interaction of compounds 1~3 with DNA fluctuate on a curve approaching a straight line. Therefore, the action mode of these three compounds with DNA is an electrostatic or groove one, which is consistent with the results obtained as a result of spectroscopy.

Fig. 19. Relative viscosity of CT-DNA upon addition of increasing the amounts ofcompounds 1~3 (r = 0.0~1.0).is the viscosity of DNA in the presence of compound, andηis the viscosity of DNA alone
4 CONCLUSION
Three new compounds 1~3 were synthesized and characterized respectively. Single-crystal X-ray diffraction shows that all the three compounds belong to monoclinic system with space group21/. All three compounds are twisted octahedra with 6 coordination structures. The intermolecular interac-tions in compounds 1~3 lead to two-dimensional networks in the crystals. The results of TD-DFT reveal that the excited electrons mainly localized at the* of ligand 2-(2-pyridyl)benzothiazole. It will be convenient for them to bind with the DNA reacting sites, and thus facilitate their reaction with DNA. The CT-DNA binding of compounds was investigated by absorption, fluorescence spectroscopy and viscosity measurements.With the increase of the amount of CT-DNA, there is a color enhancement effect in the ultraviolet spectrum, and the emission intensity of the fluorescence spectrum increases. The result indicates that the three compounds have similar properties, and they can probably bind to CT-DNA via an electrostatic or groove mode. This has potential research value for the study of the role of anticancer drugs and DNA. We hope to provide valuable information for the study of anticancer drugs.
(1) Yue, S. M.; Hou, T. T. Synthesis, crystal structure, photoluminescence and theoretical studies of a series of copper(I) compounds based on imidazole derivatives.2013, 15, 15–20.
(2) Guo, J. R.; Yan,J. W.; Chen,S. B.; G, L. Q. A simple structural modification to thiazole orange to improve the selective detection of G-quadruplexes.2016, 26, 76–85.
(3) Lewis, A.; Donald, M.; Scharbach, S. J.; Hamaway, S. J. The chemical biology of Cu(II) compounds with imidazole or thiazole containing ligands: synthesis, crystal structures and comparative biological activity.. 2016,157, 52–61.
(4) Zhou, W.; Zhang, J.; Sun, Z. G.; Zhu, Y. Y.; Jiao, C. J.; Shi, S. P. Synthesis, crystal structures, and luminescent properties of tin(II) and lead(II) carboxyphosphonates with 3D framework structures.. 2014, 47, 37–41.
(5) Yeap, G. Y.; Heng, B. T.; Faradiana, N.; Zulkifly, R. Synthesis, molecular structures and phase transition studies on benzothiazole-cored Schiff bases with their Cu(II) and Pd(II) complexes: crystal structure of (E)-6-methoxy-2-(4-octyloxy-2-hydroxybenzylideneamino)benzothiazole.2012, 1012, 1–11.
(6) Chen, X. W.; Jia, G. K.; Wang, Z. C. Synthesis, crystal structure and antitumor activities of (14S)-2,14-diphenyl-6,6a,11,12-tetrahydro-5H,10H,14H-[1,8] naphthyridino [1,2-c] pyrido [3,2,1-ij] quinazoline-3-carbonitrile... 2018, 37, 1411-1416.
(7) Lianka, N.; Motoki, S.; Nakai, M. DNA binding behaviors and nuclease activities of novel mixed-ligand ruthenium(II) compounds.. 2014, 46, 145–148.
(8) Soares, M. I. L.; Brito, A. F.; Laranjo, M. Chiral 6-hydroxymethyl-1H,3H-pyrrolo[1,2-c]thiazoles: novel antitumor DNA monoalkylating agents.m. 2010, 45, 4676–4681.
(9) Lu, Q.; Zhou, Z. X.; Mei, Y.; Wei, W.; Liu, S. Q. Detection of DNA damage by thiazole orange fluorescence probe assisted with exonuclease III.. 2013, 116, 958–963.
(10) Breydo, L.; Zang, H.; Gates, K. S. Synthesis and noncovalent DNA-binding properties of thiazole derivatives related to leinamycin.. 2004, 45, 5711–5716.
(11) Hoppenau, C. E.; Trana, V. T.; Kuscha, H.; Stromeyera, S. A. B.; Brausa, G. H. Verticillium dahliae VdTHI4, involved in thiazole biosynthesis, stress response and DNA repair functions, is required for vascular disease induction in tomato.. 2014, 108 14–22.
(12) Wang, J. J.; Sun, H. Y.; Li, C. B. A benzimidazole-pyridine-2,3-dicarboxylic acid bridged zinc(II) coordination complex-crystal structure, quantum chemistry and luminescence.. 2018, 37, 1502–1508.
(13) Nagaraj, K.; Arunachalam, S. Binding of a double-chain surfactant-cobalt(III) compound to CT DNA: effect of-cyclodextrin in the medium.. 2013, 62, 273–280.
(14) Feng, Y. Q.;Wang, L.; Xing, Z. Z.; Huang, Q. Z.; Ma, P. T. A new Cu(II) coordination polymer constructed from two kinds of ligands and rare [Ta2OF10]2−anion: synthesis, crystal structure and fluorescent properties.. 2018, 93, 15–19.
(15) Wu, H. L.; Yuan, J. K.; Zhang, Y. H.; Shi, F. R. Interactions of CT-DNA with hypocrellin A and its Al3+-hypocrellin A compound.2013, 404, 13–22.
(16) Kang, B. H.; Li, N. B.; Gao, Z. F.; Li, N. Thiazole orange as a fluorescent probe: label-free and selective detection of silver ions based on the structural change of i-motif DNA at neutral l pH.2016, 156, 141–146.
(17) Alvarez, N.; Veiga, N. Synthesis, structural characterization and DNA interaction of new copper-terpyridine compounds.2014, 68, 295–302.
(18) Ma, F.; Ge, X. F.; Huang, H. Y.; Yang, C. Interactions of CT-DNA with hypocrellin A and its Al3+–hypocrellin A compound.2013, 109, 158–163.
(19) Nagaraj, K.; Arunachalam, S. Binding of a double-chain surfactant-cobalt(III) compound to CT DNA: effect of-cyclodextrin in the medium.. 2013, 62, 273–280.
(20) Gajera, S. B.; Mehta, J. V.; Patel, M. N. DNA interaction, cytotoxicity, antibacterial and antituberculosis activity of oxovanadium(IV) compounds derived from fluoroquinolones and 4-hydroxy-5-((4-hydroxyphenyl)diazenyl)thiazole-2(3H)-thione.2015, 5, 21710–21720.
(21) Gjorgjieva, M.; Tomašič, T.; Barančokova, M.; Katsamakas, S.; Ilaš, J.; Tammela, P.; Mašič, L. P. Discovery of benzothiazole Scaffold-based DNA Gyrase B inhibitors.2016, 59, 8941–8954
(22) Barone, G.; Terenzi, A.; Lauria, A.; Almerico, A. M. DNA-binding of nickel(II), copper(II) and zinc(II) compounds: structure-affinity relationships.2013, 257, 2848–2862.
(23) Darabi, F.; Hadadzadeh, H.; Ebrahimi, M.; Khayamian, T.; Rudbari, H. A. The piroxicam compound of cobalt(II): synthesis in two different ionic liquids, structure, DNA- and BSA interaction and molecular modeling.2014, 409, 379–389.
(24) Karastogianni, S.; Dendrinou-Samara, C.; Ioannou, E.; Raptopoulou, C. P.; Girousi, S. Synthesis, characterization, DNA binding properties and antioxidant activity of a manganese(II) compound with NO6chromophore.2013, 118, 48–58.
(25) Movahedi, E.; Rezvani, A. R. Multispectroscopic DNA-binding studies and antimicrobial evaluation of new mixed-ligand silver(I) complex and nanocomplex: a comparative study.2018, 1160, 117–128.
(26) Solanki, A.; Kumar, S. B.; Doshi, A. A. Mononuclear copper(II) compounds with a tetradentate pyrazole based ligand: syntheses, structures, DNA binding study and antimicrobial activity.2013, 63, 147–155.
(27) Murthy, N. N.; Manikandamathavan, V. M. M.; Punitha, V. Novel mononuclear Cu(II) terpyridine compounds: impact of fused ring thiophene and thiazole head groups towards DNA/BSA interaction, cleavage and antiproliferative activity on HepG2 and triple negative CAL-51 cell line.. 2017, 135, 434–446.
(28) Lin, C. Q.; Yang, J. X.; Zhang, X.; Qin, Y. Y.; Yao, Y. G. Organic carboxylate ligand tuned topological variations in three Zn(II) coordination polymers: syntheses, crystal structures and photoluminescent properties.. 2018, 37, 1470–1478.
(29) Hu, W.; Deng, S. W.; Huang, J. Y.; Lu, Y. M.; Le, X. Y. Intercalative interaction of asymmetric copper(II) compound with DNA: experimental, molecular docking, molecular dynamics and TDDFT studies.2013, 127, 90–98.
(30) Nishizawa, S.; Sato, T.; Sato, Y. Triplex-forming peptide nucleic acid probe having thiazole orange as a base surrogate for fluorescence sensing of double-stranded RNA.2016, 138, 9397–9400.
(31) Yue, S. M.; Hou, T. T. Syntheses, crystal structures, and properties of nickel and cadmium compounds containing imidazole derivatives.. 2012, 65, 3895–3902.
(32) Yue, S. M.; Yue, X. R.; Chen, Y. N. Synthesis, crystal structures and DNA-binding properties of Cd(II), Cu(II) and Ni(II) compounds with 2-(2-pyridyl)benzothiazole.2015, 200, 1–6.
(33) Sheldrick, G. M.. University of Gottingen, Germany 1997 .
(34) Dhara, A. K.; Ghosh, S. K. Density-functional theory for time-dependent systems.1987, 35, 442–444.
(35) Mayo, S. L.; Olafso, B. D.; Goddard, W. A. A generic force field for molecular simulations.1990, 94, 8897–8909.
(36) Igor, A.; Payam, M.; Jérôme, C.; Luisa, C. D. Iridium(III) compounds with orthometalated quinoxaline ligands: subtle tuning of emission to the saturated red color.. 2007, 129, 8247–8258.
(37) Lundin, N. J.; Walsh, P. J.; Howell, S. L.; McGarvey, J. J.; Blackman, A. G.; Gordon, K. C. Synthesis and characterization of a multicomponent rhenium(I) compound for application as an OLED dopant.2005, 44, 3551–3560.
(38) Si, Z.; Li, X.; Li, X.; Xu, G.; Pan, C. Novel Re(I) dendrimers: synthesis, characterization and theoretical studies.2009, 38, 10592–10600.
(39) Schmidt, M. W.; Baldridge, K. K.; Boatz, J. A.; Elbert, S. T.; Gordon, M. S.; Montgomery, J. A. General atomic and molecular electronic structure system.1993, 14, 1347–1363.
(40) Machura, B.; Wolff, M.; Benoist, E.; Coulais, Y. Tricarbonyl rhenium(I) compound of benzothiazole – synthesis, spectroscopic characterization, X-ray crystal structure and DFT calculations.2013, 724, 82–87.
(41) He, X. J.; Zeng, L. L.; Yang, G.; Xie, L. J.; Sun, X. N.; Tan, L. F. DNA binding, photocleavage and topoisomerase inhibitory activity of polypyridyl ruthenium(II) compounds containing the same ancillary ligand and different main ligands.2013, 408, 9–17.
(42) Arjmand, F.; Jamsheera, A.; Mohapatra, D. K. Synthesis, characterization and in vitro DNA binding and cleavage studies of Cu(II)/Zn(II) dipeptide compounds.2013, 121, 75–85.
(43) Siddiqi, Z. A.; Sharma, P. K.; Shahid, M.; Khalid, M. Synthesis, structural characterization, DNA binding studies and antitumor properties of tin(II)-oxydiacetate compounds containing-diimine as auxiliary ligand.2013, 125, 171–178.
(44) Palanimuthu, D.; Samuelson, A. G. Dinuclear zinc bis(thiosemicarbazone) compounds: synthesis, in vitro anticancer activity, cellular uptake and DNA interaction study.2013, 408, 152–161.
(45) Raman, N.; Sakthivel, A.; Selvaganapathy, M. Effect of DNA interaction involving antioxidative 4-aminoantipyrine incorporating mixed ligand compounds having alpha-amino acid as co-ligand.2014, 1060, 63–74.
6 July 2018;
21 September 2018 (CCDC 1834106 (1), 1834105 (2) and 1834107 (3))
① Supported by the Foundation for Jilin of China of 2014 Human Resources Development, the Natural Science Project Foundation of Education Department of Jilin Province (No. 2015358) and the Natural Science Foundation of Changchun Normal University (No. 201203)
. Yue Shu-Mei, female, doctor, professor, majoring in Functional compound. E-mail: 162161620520613@qq.com
10.14102/j.cnki.0254-5861.2011-2127
- 结构化学的其它文章
- Two Cd(II) Coordination Polymers Based on a Flexible Tricarboxylate Ligand: Syntheses, Structures, and Photoluminescence and Catalytic Properties①
- A Selective Luminescent Acetone Sensing Coordination Polymer Constructed from Zinc(II) Ions and Imidazolyl-bearing Ligands①
- Blue-emissions Modulated by Packing Forces in Alkaline-earth Metal Organic Frameworks Based on Thiophene-2,5-dicarboxylic: Structures and Theoretical Calculations①
- A New Luminescent Cd(II) Coordination Polymer Constructed with 2-(Carboxymethoxy)benzoic Acid①
- Synthesis, Single-crystal Structure and Fluorescence Property of a New Boron Compound Based on 2-(2΄-Hydroxyphenyl)-1H-benzimidazole①
- Theoretical Investigations on the Structural and Electronic Properties of WO3Polymorphs①

