Blue-emissions Modulated by Packing Forces in Alkaline-earth Metal Organic Frameworks Based on Thiophene-2,5-dicarboxylic: Structures and Theoretical Calculations①
CHEN Jia-Yue
Blue-emissions Modulated by Packing Forces in Alkaline-earth Metal Organic Frameworks Based on Thiophene-2,5-dicarboxylic: Structures and Theoretical Calculations①
CHEN Jia-Yue②
(350007)
Solvothermal reactions of Ca(NO3)2, Sr(NO3)2with thiophene-2,5-dicarboxylic in DMF afforded two new inorganic-organic hybrid frameworks, [M(TDC)(DMF)](M = Ca (1), Sr (2), TDC = thiophene-2,5-dicarboxylic, DMF = N,N΄-dimethylformamide), which have been characterized by single-crystal X-ray diffraction, powder X-ray diffraction, elemental analysis and IR spectra. Both compounds feature three-dimensional (3D) frameworks based on the versatile coordinated modes (3-2:2,3-2:1,2-2:1) of carboxylic groups in tdc ligands. C–H···S hydrogen bonds and C–H···interactions contribute to the stabilization of the structures. They exhibit weaker packing force compared with their literature isomers. Consequently, blue and blue/green luminescence of two compounds has been observed. Their luminescence mechanism can be ascribed to ligand-to-metal charge transfer (LMCT) compared with the ligand-centered luminescence in their isomers. Electronic structural calculations illustrate that under the condition of weaker packing forces, larger gaps can be achieved, which facilitate the LMCT. This work suggests that the introduction of S-heteroatom can result in more electrons rich in the metal centers, thus giving rise to metal-involved luminescence.
alkaline-earth metal complex, thiophene-2,5-dicarboxylic, photoluminescence, theoretical calculation;
1 INTRODUCTION
In the last decade, hybrid inorganic-organic frameworks have captured the interest of chemists not only due to their intriguing architectures and topologies, but also to their fascinating applications such as luminescence, semiconductors, storage materials and magnetic materials[1, 2]. However, in this field, most attention has been focused on the incorporation of transition metal ions and rare-earth ions as coordination centers[3, 4], whereas much less attention is paid to alkaline earth metal-based com- pounds. Recently, the inorganic-organic frame- works based on main group alkaline-earth metals have received more and more research attention due to their low polarizability, versatile coordination modes for the construction of high dimensional materials with robust structures[5]and interesting properties such as tunable fluorescent indicators, ion sensing, macrocyclic ion receptors[6, 7]. Among them, the divalent calcium and strontium cations (Ca2+and Sr2+) have higher abundances in earth and play important roles in new functional materials and biological processes, for example, oxygen-evolving complexes such as photosystem II and metallo- proteins[8-10].So far, numerous alkaline earth metal-based inorganic-organic hybrids constructed from aromatic carboxylate ligands have been reported[11-17]. But alkaline earth metal compounds based on S-hetero carboxylate ligands are much less. Because of the bigger radius of the S atom than the C, N and O atoms, its lone pair of electrons can be more easily delocalized within the heterocycle, so the ligand exhibits good charge-transfer ability[18].
Thiophene-2,5-dicarboxylic acid (H2TDC) is a typical S-hetero aromatic ligand in constructing high-dimensional coordinated networks with enhan- ced thermal stabilities and luminescent properties, which stem from diverse coordination modes of the carboxylate groups (including monodentate, biden- tate, tridentate, et al) and the more delocalized rigid heterocyclic ring[19-21]. Up to now, to our knowledge, H2tdc/alkaline-earth metal MOFs are still rare, and their photoluminescence was mainly violet and dominated by organic moiety[22, 23]. How to control their emission is still a big challenge. In this work, we report the solvothermal synthesis, structural characterization and blue/green emissions of two new 3-D s-block coordination networks using TDC as the linker. Our study suggests that the network topology and the presence of coordinated solvent molecules are important factors in controlling the luminescence properties of the synthesized ma- terials.
2 EXPERIMENTAL
2. 1 Materials and methods
All chemicals of regent grade were obtained from commercial sources and used without further puri- fication.Elemental analyses for C, H and N were performed on a Vario MICRO elemental analyzer. IR spectra were recorded on a Perkin-Elmer Spectrum- 2000 FTIR spectrophotometer (4000~400 cm-1). Fluorescence spectrum was carried out on a PW2424 spectrometer.
2. 2 Computational details
The band structure calculation was based on density function theory (DFT)[24], in which wave functions were explained in a plane wave basis set and the spin polarized version of the PW-91 GGA was employed for the exchange-correlation func- tional in the CASTEP code[25].The number of plane waves included in the basis was determined by a cutoff energyEof 550 eV.
2. 3 Synthesis
Synthesis of [Ca(TDC)(DMF)](1) 1 was pre- pared by solvothermal method. Thiophene-2,5-dicar- boxylic acid (H2tdc, 0.0344 g, 0.2 mmol) was dissolved in 5 mL DMF, and the suspension was stirred till complete dissolution. Then Ca(NO3)2(0.0164 g, 0.1 mmol) was added and kept stirring for 1 h. The mixture was removed into a 25 mL Teflon-lined autoclave, which was heated to 120 °C and held at this temperature for 72 h. Then the autoclave was cooled to room temperature in two days. Colorless block single crystals were obtained and washed with ether (0.0170 g, yield 71.3% based on Ca). Anal. Calcd. for C18H18Ca2N2O10S2(566.62): C, 38.16; H, 3.20; N, 4.94%. Found: C, 38.32; H, 3.38; N, 4.45%. IR(cm-1): 3089(m), 1562(w), 1369(w), 1118(w), 1030(s), 770(s), 684(s), 458(s).
Synthesis of [Sr(TDC)(DMF)](2) The synthesis process of 2 is the same with that of 1 except that Sr(NO3)2(0.0211 g, 0.1 mmol) was used as the starting material. Colorless block single crystals were obtained with the yield of 74.6% (0.0246 g, based on Sr). Compared with the synthesis condition of reported analogs[22, 23], the reaction temperature in this work is higher (120. 100 °C), which leads to structural difference. Anal. Calcd. for C9H9NO5SSr (330.85): C, 32.67; H, 2.74; N, 4.23%. Found: C, 32.82; H, 2.82; N, 4.31%. IR(cm-1): 3224(w), 3089(m), 1570(w), 1377(w), 1118(w), 1022(s), 770(s), 677(W), 458(s).
2. 4 X-ray crystallography
Block crystals of 1 and 2 were mounted on glass fibers. The intensity data were collected on a Rigaku Weissenberg IP diffractometer with a graphite- monochromated Moradiation (= 0.71073 Å) at 293(2) K by using an-2scan mode. The multi-scan absorption corrections were applied. The structurewas solved by direct methods with SHELXS-97program and refined by full-matrix least-squarestechniques on2with SHELXL-97 program[26].The non-hydrogenatoms were refined with anisotropic thermal displacement coefficients, andhydrogen atoms were determined with theoreticalcalculations and refined isotropically.Crystal data of 1: monoclinic, space group21/withM= 566.62,= 10.078(2),= 14.262(3),= 19.603(5) Å,= 116.54(2)°,= 2520.7(10) Å3,= 4,D=g/cm3,(000) =,(Mo) =mm–1, the final= 0.0410 and= 0.1178,= 0.940, (Δ/)max= 0.000, (Δ)max= 0.850and (Δ)min= –0.524 e/Å3. Crystal data of 2: monoclinic, space group21/withM= 330.85,= 6.0067(10),= 16.926(3),= 11.679(2) Å,= 91.394(3)°,= 1187.1(3) Å3,= 4,D=g/cm3,(000) =,(Mo) = 4.725 mm–1, the final= 0.0329 and= 0.1054,= 1.195, (Δ/)max= 0.000, (Δ)max=1.401 and (Δ)min= –0.997 e/Å3. Important bond lengths are listed in Table 1, and hydrogen bond details are given in Table 2.

Table 1. Selected Bond Lengths (Å) for Compounds 1 and 2

Table 2. Hydrogen Bridging Details of Compound 1
3 RESULTS AND DISCUSSION
3. 1 Structure description
1 crystallizes in monoclinic space group21/, which is an isomer of [Ca2(TDC-2H)2(DMF)2] (space group21/)[22]. There are two crystallogra- phically independent Ca centers in the lattice, which exhibit different coordination numbers and coor- dination environments. As illustrated in Fig. 1a, Ca(1) is coordinated by seven O atoms to give a distorted pentagonal bipyramidal geometry: four oxygen atoms (O(5), O(6), O(7), O(8)) from two carboxylate groups, two (O(3) and O(6)a, a: ––1, ––1, ––1) from two different carboxylate groups and one (O(10)) from one DMF molecule. O(3) and O(10) occupy the apical positions with a trans angle of 172.58(11)°. The Ca(1) center lies on the pentagonal plane with the deviation less than 0.01 Å. The Ca(1)–O bond distances range from 2.2941(17) to 2.5050(16) Å (Table 1), which are consistent with those in reported calcium carboxylate com- plexes[15, 27, 28]Comparably, Ca(2) is in a six-coor- dinated distorted octahedral environment with five oxygen atoms from five different carboxylate groups and one oxygen from the DMF molecule, where O(8) and O(9) occupy the axial positions and other four locate at the equator plane. The Ca(2)–O bond distances are in the normal range of 2.259(2)~2.4355(16) Å. The Ca(1)O7pentagonal bipyramid and Ca(2)O6octahedron are connected into a Ca2O10dimmer via edge-sharing mode with the Ca(1)–Ca(2) separation of 3.6702(9) Å, and two Ca2O10dimmers are linked into a Ca4O18tetramer via edge-sharing (defined by O(6)–O(6)a) with the Ca(1)–Ca(1)adistance of 3.6656(10) Å. These Ca–Ca distances are obviously shorter than those of reported binuclear complexes (Ca–Ca distances of 3.860(2) and 3.891(4) Å[29, 30]), which imply the existence of Ca···Ca interactions in 1.
Adjacent Ca4O18tetramers are extended into a 1-D chain along the-axis by two bridged carboxylate groups (Fig. 1a). Neighbouring 1-D chains are bri- dged by one of the crystallographically independent thiophene-2,5-dicarboxylic acid ligands to give a 2-D layer along-plane (Fig. 1b), in which two carboxylate groups of tdc ligand adopt the3-2:2(each oxygen atom coordinates to two metal atoms, and the carboxylic group coordinates to three metal atoms) and2-2:1(one oxygen atom connects two metal ions, the other connects one metal atom, and the carboxylic group coordinates to two metal atoms) coordination modes (Scheme 1, a). Finally, adjacent 2-D layers are extended to be a 3-D network via another crystallographically independent bridged tdc ligand (Fig. 1c), where this tdc presents the2-1:1(two oxygen atoms connect two metal ions, and the carboxylic group coordinates to two metal atoms) coordinated mode (Scheme 1, b).
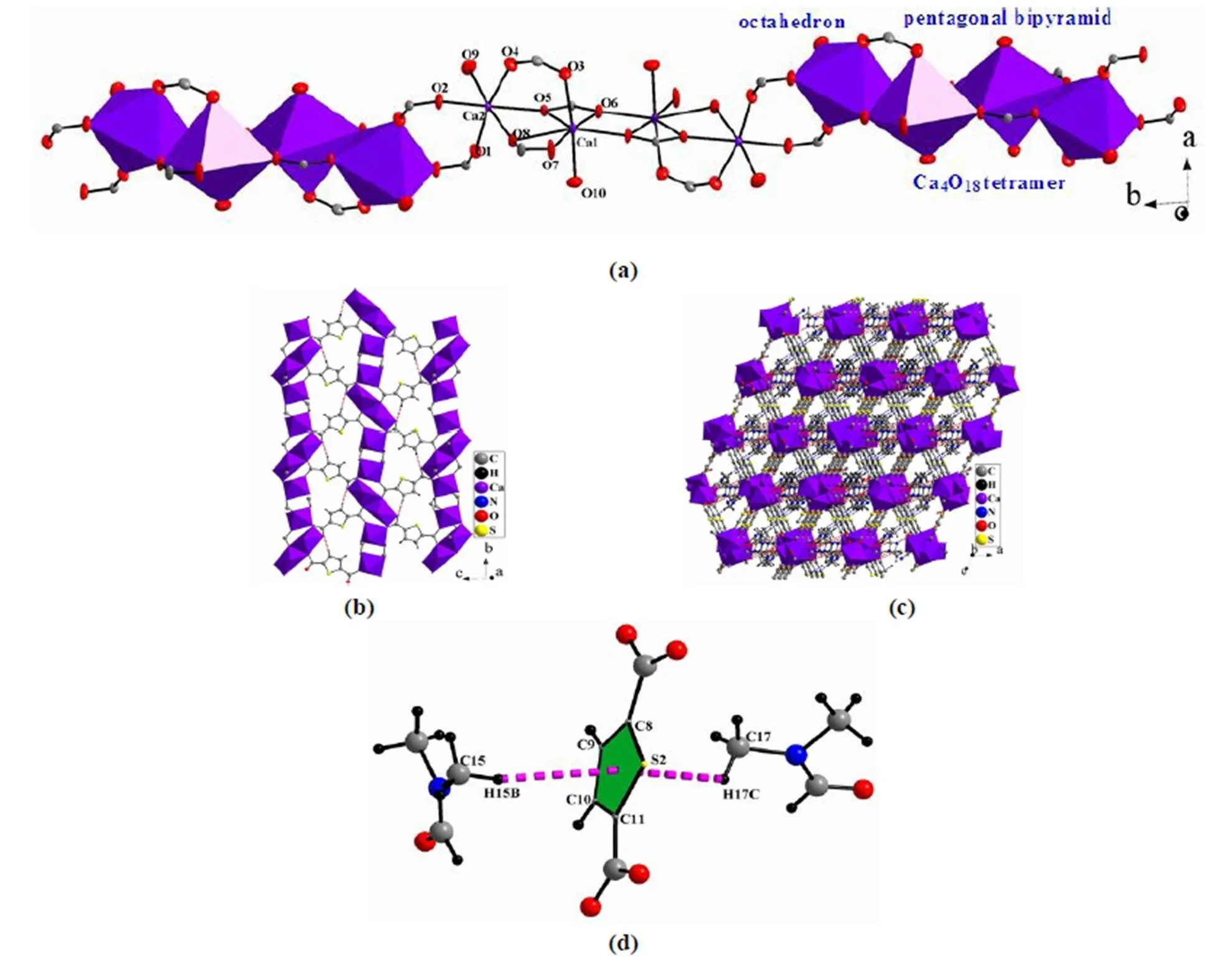
Fig. 1. (a) 1-D chain constructed from Ca4O18tetramers bridged by carboxylate groups; (b) 2-D layer along theplane based on 1-D chains; (c) 3-D network of 1 showing hydrogen bonds; (d) C–H···interactions between tdc and DMF in 1
Scheme 1. Coordinated modes of tdc ligand in this work
Specially, hydrogen bonds between tdc and DMF molecules can be observed (Table 2, blue imaginary lines for C–H···S hydrogen bonds and red imaginary lines for C–H···O hydrogen bonds in Fig. 1a and 1c). In addition, C–H···interactions can also be detected between tdc ligand and DMF (Fig. 1d). All these weak interactions contribute to the stabilization of structure. Comparably, in [Ca2(TDC-2H)2(DMF)2][22],one of Ca centers is in five-coordinated square bipyramidal geometry. Consequently, in 1, a new coordinated mode3-2:1is presented compared with those in [Ca2(TDC-2H)2(DMF)2]. The strengths of C–H···S hydrogen bonds of two isomers are generally the same, but one of the C–H···O hydrogen bonds in 1 (C(18)–H(18A)···O(4)) is weaker (H···A distance 2.60. 2.31 Å).
2 is also an isomer of Sr(TDA)(DMF)[22, 23], which crystallizes in monoclinic with space group21/. Its asymmetric unit contains one Sr atom, one tdc ligand and one DMF molecule. The Sr atom is eight-coordinated with distorted di-cap triangular prism geometry, whose O donors stem from six tdc ligands and one DMF molecule. The Sr–O bond distances range from 2.438(3) to 2.739(3) Å (Table 1). Two SrO8polyhedra are connected into a Sr2O12dimmer via a face-sharing mode with the Sr–Sr distance of 3.5144(8) Å. Furthermore, neighboring Sr2O12dimmers are linked into a 1-D chain along the-axisan edge-sharing mode with the Sr–Sr distance to be 3.9396(9) Å (Fig. 2a). These Sr–Sr lengths imply the presence of Sr···Sr interactions compared with the data in literatures[31]. But in [Sr(TDC-2H)(DMF)][22],these two Sr–Sr distances are 3.470(2) and 3.928(2) Å, suggesting a more relaxed packing in 2. 1-D chains were further bridged by tdc ligands along theanddirections to give a 3-D network (Fig. 2b), in which a cavity with the size of 9.788 × 16.966 Å2can be found ((Fig. 2b). DMF molecules locate in these cavities. As a result, two carboxylate groups of tdc ligand adopt the3-2:2and3-2:1modes (Scheme 1c). There are no hydrogen bonds and···stacking interactions in the lattice, but a clear C–H···interaction between tdc ligand and DMF can be found, which stabilizes the structure (Table 3, Fig. 2c). In [Sr(TDC- 2H)(DMF)][22],due to the disorder of DMF molecu- les, a lager cavity (9.838 × 17.058 Å2) is given. In all, under the higher synthesis temperature (120 ºC), compounds in this work exhibit a more relaxed packing compared with the reported isomers (for example, 1.851 g/cm3for 2 and 1.893 g/cm3for Sr(TDA)(DMF))[22].

Table 3. C–H···π Interaction Parameters for Compounds 1 and 2

Fig. 2. (a) 1-D chain constructed from Sr2O12dimmer bridged by carboxylate groups; (b) 3-D network of 2 based on bridged tdc ligands; (d) C–H···interactions between tdc and DMF in 2
3. 2 Fluorescence properties
The purities of bulk compounds 1 and 2 have been proved by powder X-ray diffraction (PXRD). The results show that theexperimental patterns arein good agreement with the simulatedones, indicating the good phase purities (Fig. 3).The minor difference in intensities between the simulated and experimental patterns may be due to the preferred orientation of the powder samples during the collec- tion of the experimental PXRD data.

(a)
(b)
Fig. 3. Powder X-ray diffraction (PXRD) patterns for compounds1 (a) and 2 (b)
The luminescence properties of 1 and 2 were studied in the solid state at room temperature. 1 exhibits blue/green emission with peak locating at 506 nm upon irradiation at 397 nm, and 2 produces blue emission at 478 nm when excited at 378 nm (Fig. 4). The emission of free H2tdc has been reported to be at 365 nm[22, 23, 32].According to the literatural alkaline earth metal complexes with crown ethers ligand, the photoluminescence of the crown ether ligands can be greatly enhanced by incorporation with alkaline earth metal ions[33]. Compared with the emission of free H2tdc, its Ca/Sr(II) complexes exhibited great red shifts with emission maximum at 506 and 478 nm. Therefore, the observed photoluminescence of complexes 1 and 2 is not the contribution of*-n/* of tdc ligand, but the component of ligand-to-metal charge transfer (LMCT) or metal-to-ligand charge transfer (MLCT). This situation is different from their isomers and other tdc-based complexes, whose emissions only stem from the*-n/* of organic ligands[11, 22, 23]. Why the emissions can change from tdc-centered to ligand-to-metal characters? A number of parameters, including crystal packing and the coordination environment of metal centers and linkers, can influence their luminescence[22, 23]. In 1, both different crystal packing and coordination environ- ment of metal centers compared to its isomer can be observed, but in 1, the coordination environments are the same, and only more relaxed crystal packing is given. Therefore, the weaker packing forces in this work might give rise to the presence of ligand- to-metal charge transfer (LMCT) and thus produce blue or blue/green fluorscence. In addition, the emit light of 1 exhibits red-shift by approximate 28 nm compared with 2, which may be due to the different binding modes of tdc ligands. The single coordina- ted mode of tdc in 2 may result in a more rigid structure of ligand than 1, which gives rise to more delocalized electrons between the ligand and metals.
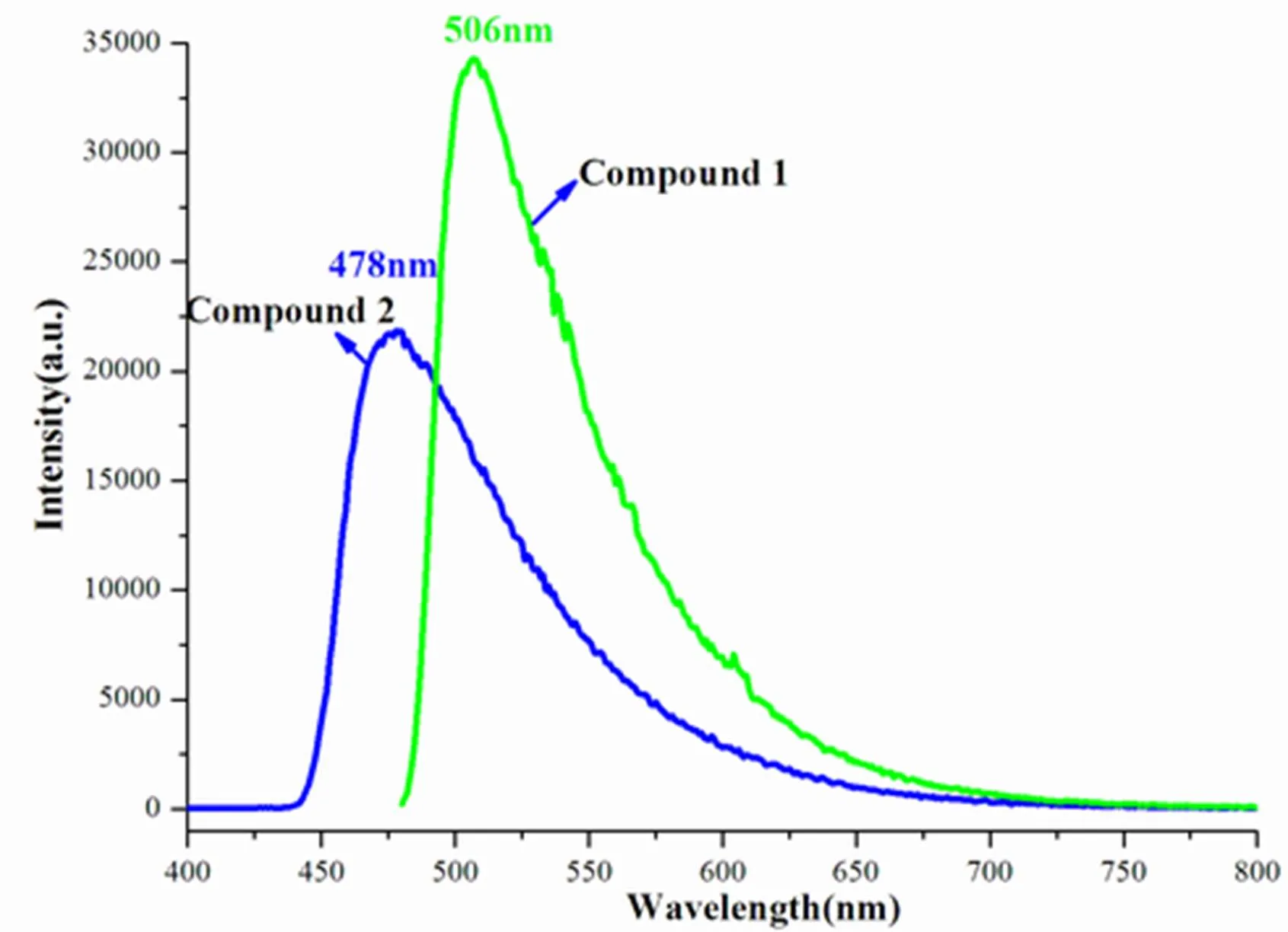
Fig. 4. Solid-state emission spectra of compounds 1 and 2
3. 3 Electronic structures
In order to look further insight into the blue photoluminescence of 1 and 2 compared with the literature compounds, DFT calculations illustrated by band structures along with high symmetry points of the first Brillouin zone and density of states (DOS) were executed using the CASTEP code in Materials Studio 8.0. The band structures of 1, 2 and their corresponding isomers, total/partial DOS ([Ca2(TDC-2H)2(DMF)2] and1 were set as examples)along certain symmetry directions are given in Figs. 5 and 6. The calculated band gaps based on GGA-PBE are 3.03 (for 1) and 3.35 eV (for 2), both of which are direct band gaps, but the gaps of their corres- ponding isomers are 1.32 and 0.54 eV. Judging from these data, under the weaker packing force in this work, much larger band gags can be found. The larger band gaps of 1 and 2 suggest that the higher energies are needed when the electrons transfer from ground states to excited states, which is consistent with its lower excited wave length (378 nm for 2. 397 nm for 1). As shown by the total and partial DOS diagrams (Fig. 6), in [Ca2(TDC-2H)2(DMF)2], the energy gaps of bonding- and anti-bondingorbitals in tdc are small, which facilitates the* transfer among tdc ligand. But in 1, the energy gaps are much larger. The top of VBs between −5 and Fermi energy originate from thebonding orbitials of tdc, and the bottom of CBs are the contribution of p-* antibonding orbitals of tdc ligands mixed with small amount of Ca-3/Sr-4states. These orbital components prove that the intense luminescence of two compounds stem from the ligand-to-metal charge transfer (LMCT). The engagement of metalorbitals in the frontier orbitals might be led by the introduction of S-atom of aromatic ring, which results in more delocalized electrons and conse- quently, more electrons rich in the metal centers. This situation can not be found in benzene-ring based aromatic polycarboxylic ligands[34].
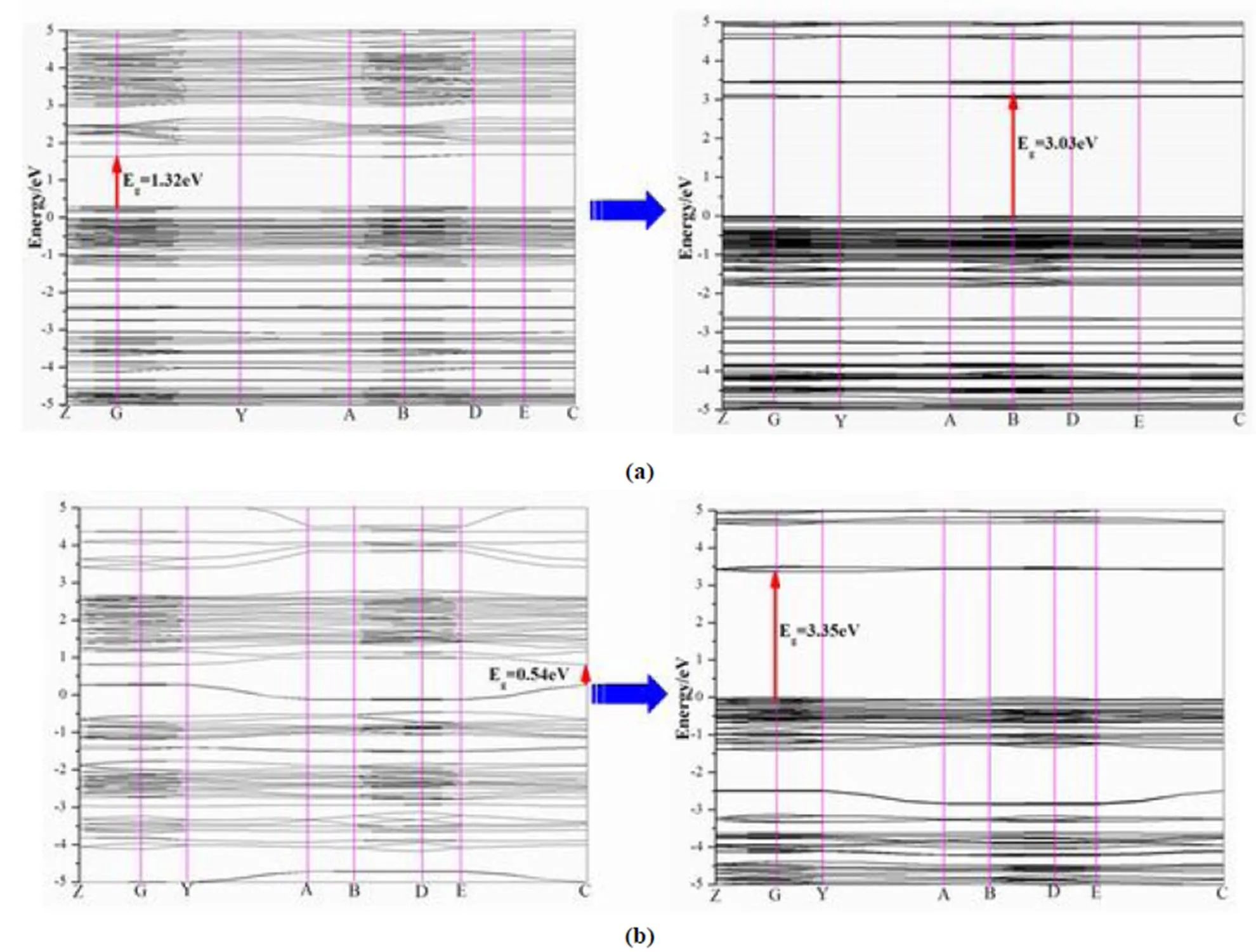
Fig. 5. Band structures of [Ca2(TDC-2H)2(DMF)2] and 1 (a); band structures of Sr(TDA)(DMF) and 2 (b)
Fig. 6. Total and partial density of states of [Ca2(TDC-2H)2(DMF)2] in literature (a) and 1 (b)
4 CONCLUSION
Two new three-dimensional frameworks, [M(TDC)(DMF)](M = Ca (1), Sr (2)) exhibit blue and blue/green emissions, in which weaker packing forces compared with the reported isomers can be found. Their luminescence mechanism can be ascribed to ligand-to-metal charge transfer (LMCT) compared with the ligand-centered luminescence in their isomers, which were verified by electronic structural calculations. Interestingly, the introduction of S-heteroatom can result in more electrons rich in the metal centers, thus giving rise to metal-involved luminescence.
(1) Zheng, S. T.; Bu, J. T.; Yi, Y. F.; Wu, T.; Zuo, F.; Feng, P. Y.; Bu, X. H. Pore space partition and charge separation in cage-within-cage indium-organic frameworks with high CO2uptake.2010, 132, 17062–17064.
(2) Luo, X. L.;Sun, L. B.;Zhao, J.; Li, D. S.; Wang, D. M.; Li, G. H.;Huo, Q. S.;Liu, Y. L.Three metal-organic frameworks based on binodal inorganic building units and hetero-O,N donor ligand: solvothermal syntheses, structures, and gas sorption properties.,, 4901–4907.
(3) Liang, J. B.; Ma, R. Z.; Ebina, Y.; Geng, F. X.; Takayoshi Sasaki, T. New family of lanthanide-based inorganic-organic hybrid frameworks: Ln2(OH)4[O3S(CH2)nSO3]·2H2O (Ln = La, Ce, Pr, Nd, Sm; n = 3, 4) and their derivatives2013, 52, 1755−1761.
(4) Maniam, P.;Stock, N.Investigation of porous Ni-based metal-organic frameworks containing paddle-wheel type inorganic building unitshigh-throughput methods.,, 5085–5097.
(5) Banerjee, D.; Parise, J. B. Recent advances in-block metal carboxylate networks.2011, 11, 4704–4720.
(6) Collot, M.; Loukou, C.; Yakovlev, A. V.; Wilms, C. D.; Li, D.; Evrard, A.; Zamaleeva, A.; Bourdieu, L.; Léger, J. F.; Ropert, N.; Eilers, J.; Oheim, M.; Feltz, A.; Mallet, J. M. Calcium rubies: a family of red-emitting functionalizable indicators suitable for two-photon Ca2+imaging2012, 134, 14923−14931.
(7) Bar-Shir, A.; Gilad, A. A.; Chan, K. W. Y.; Liu, G.; van Zijl, P. C. M.; Bulte, J. W. M.; McMahon, M. T. Metal ion sensing using ion chemical exchange saturation transfer19F magnetic resonance imaging.2013, 135, 12164−12167.
(8) Gatt, P.; Petrie, S.; Stranger, R.; Pace, R. J. 3D chemical image using TOF-SIMS revealing the biopolymer component spatial and lateral distributions in biomass.2012, 51, 12025−12028.
(9) Tsui, E. Y.; Tran, R.; Yano, J.; Agapie, T. Redox-inactive metals modulate the reduction potential in heterometallic manganese-oxido clusters.2013, 5, 293−299.
(10) Park, Y. J.; Cook, S. A.; Sickerman, N. S.; Sano, Y.; Ziller, J. W.; Borovik, A. S. Heterobimetallic complexes with MIII-(-OH)-MIIcores (MIII= Fe, Mn, Ga; MII= Ca, Sr, and Ba): structural, kinetic, and redox properties.2013, 4, 717−726.
(11) Zhang, X.; Huang, Y. Y.; Zhang, M. J.; Zhang, J.; Yao, Y. G. A series of Ca(II) or Ba(II) inorganic-organic hybrid frameworks based on aromatic polycarboxylate ligands with the inorganic M−O−M (M = Ca, Ba) connectivity from 1D to 3D.2012, 12, 3231−3238.
(12) Burgess, K. M. N.; Xu, Y.; Leclerc, M. C.; Bryce, D. L. Alkaline-earth metal carboxylates characterized by43Ca and87Sr solid-state NMR: impact of metal-amine bonding.2014, 53, 552−561.
(13) Du, S. F.; Ji, C. Q.; Xin, X. L.; Zhuang, M.; Yu, X. Y.; Lu, J. T.; Yukun Lu, Y. K.; Sun, D. F. Syntheses, structures and characteristics of four alkaline-earth metalorganic frameworks (MOFs) based on benzene-1,2,4,5-tetracarboxylicacid and its derivative ligand.2017, 1130, 565−572.
(14) Zou, R. Q.; Zhong, R. Q.; Han, S. B.; Xu, H. W.; Baurrell, A. K.; Henson, N.; Cape, J. L.; Hickmott, D. D.; Timofeeva,T. V.; Larson, E.; Zhao, Y. S. A porous metal-organic replica of-PbO2for capture of nerve agent surrogate.2010, 132, 17996–17999.
(15) Zhu, H. F.; Zhang, Z. H.; Sun, W. Y.; Okamura, T.; Veyama, N. Syntheses, structures, and properties of two-dimensional alkaline earth metal complexes with flexible tripodal tricarboxylate ligands.2005, 5, 177–182.
(16) Wiesbrock, F.; Schimdbaur, H. Crystal structures of rubidium and cesium anthranilates and salicylates.. 2003, 42, 7283–7289.
(17) Kam, K. C.; Young, K. L. M.; Cheetham, A. K. Chemical and structural diversity in chiral magnesium tartrates and their racemic and meso analogues.. 2007, 7, 1522–1532.
(18) Xu, K. X.Chemical Industry, Beijing 1986.
(19) Wang, G.; Huang, C. C.; Huang, X. H.; Liu, D. S. Three-dimensional lanthanide thiophenedicarboxylate framework with an unprecedented (4,5)-connected topology.2008, 8, 795–798.
(20) Yesilel, O. Z.; Ilker, I.; Buyukgungor, O. Three copper(II) complexes of thiophene-2,5-dicarboxylic acid with dissimilar ligands: synthesis, IR and UV-Vis spectra, thermal properties and structural characterizations2009, 28, 3010–3016.
(21) Li, H. H.; Zeng, X. H.; Wu, H. Y.; Jie, X.; Zheng, S. T.; Chen, Z. R. Incorporating guest molecules into honeycomb structures constructed from uranium(VI)-polycarboxylates: structural diversities and photocatalytic activities for the degradation of organic dye,2015, 15, 10–13.
(22) Chen, X. Y.; Plonka, A. M.;Banerjee, D.; Parise, J. B. Synthesis, structures and photoluminescence properties of a series of alkaline earth metal-based coordination networks synthesized using thiophene-based linkers.2013, 13, 326−332.
(23) Chen, Q.; Guo, P. C.; Zhao, S. P.; Liu, J. L.; Ren, X. M. A rhombus channel metal-organic framework comprised of Sr2+and thiophene-2,5-dicarboxylic acid exhibiting novel dielectric bistability.. 2013, 15, 1264–1270.
(24) Perew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple.1996, 77, 3865–3868.
(25) Segall, M.; Probert, M.; Pickard, C.; Hasnip, P.; Clark, S.; Refson, K.; Payne, M.2006.
(26) Sheldrick, G. M.,University of Göttingen, Germany 1997.
(27) Onoda, A.; Yamada, Y.; Doi, M.; Okamura, T.; Ueyama, N. Dinuclear calcium complex with weakly N–H···O hydrogen-bonded sulfonate ligands.2001, 40, 516–521.
(28) Pan, L.; Frydel, T.; Sander, M. B.; Huang, X.; Li, J. The effect of pH on the dimensionality of coordination polymers.2001, 40, 1271–1283.
(29) Ueyama, N.; Takeda, J.; Yamada, Y.; Onoda, A.; Okamura, T.; Nakanura, A. Dinuclear calcium complexes with intramolecularly N–H···O hydrogen-bonded dicarboxylate ligands.1999, 38, 475–478.
(30) Bahl, A. M.; Krishnaswamy, S.; Massand, N. G.; Burkey, D. J.; Hanusa, T. P. Heavy alkaline-earth polyether carboxylates. the crystal structure of {Ca[OOC(CH2)O(CH2)2]2O(H2O)2}2..1997, 36, 5413–5415.
(31) Dan, M.; Cheetham, A. K.; Rao, C. N. R. Diverse structures and dimensionalities in hybrid frameworks of strontium and lanthanum with isomeric dihydroxybenzoates.2006, 45, 8227–8238.
(32) Zhou, L.; Wang, C. G.; Zheng, X. F.; Tian, Z. F.; Wen, L. L.; Qua, H.; Li, D. F. New metal-organic frameworks based on 2,5-thiophenedicarboxylate and pyridine- or imidazole-based spacers: syntheses, topological structures, and properties.2013, 42, 16375–16386.
(33) Prodi, L.; Bolletta, F.; Zaccheroni, N.; Watt, C. I. F.; Mooney, N. J. A new family of luminescent sensors for alkaline earth metal ions.1998, 4, 1090–1094.
(34) Liang, P. C.; Liu, H. K.; Yeh, C. T.; Lin, C. H.; Zima, V. Supramolecular assembly of calcium metal-organic frameworks with structural transformations.2011, 11, 699–708.
8 May 2018;
25 August 2018 (CCDC 1839708 and 1839709)
① This work was supported by the Science and Technology Funding Project of Fujian Provincial Department of Transportation (No. 201337)
. E-mail: fjfzcjy@126.com
10.14102/j.cnki.0254-5861.2011-2064
- 结构化学的其它文章
- Two Cd(II) Coordination Polymers Based on a Flexible Tricarboxylate Ligand: Syntheses, Structures, and Photoluminescence and Catalytic Properties①
- A Selective Luminescent Acetone Sensing Coordination Polymer Constructed from Zinc(II) Ions and Imidazolyl-bearing Ligands①
- Syntheses, Crystal Structures and DNA-binding Properties of Zn(II), Ni(II) and Co(II) Compounds Containing Thiazole Derivatives①
- A New Luminescent Cd(II) Coordination Polymer Constructed with 2-(Carboxymethoxy)benzoic Acid①
- Synthesis, Single-crystal Structure and Fluorescence Property of a New Boron Compound Based on 2-(2΄-Hydroxyphenyl)-1H-benzimidazole①
- Theoretical Investigations on the Structural and Electronic Properties of WO3Polymorphs①

