Anti-hemolytic, antibacterial and anti-cancer activities of methanolic extracts from leaves and stems of Polygonum odoratum
Nittaya Chansiw, Narisara Paradee, Kamonnaree Chotinantakul, Somdet Srichairattanakool
1School of Medicine, Mae Fah Luang University, Chiang Rai, 57100, Thailand
2Department of Biochemistry, Faculty of Medicine, Chiang Mai University, Chiang Mai, 50200, Thailand
Keywords:Polygonum odoratum Anti-hemolysis Antibacterial agents Anti-cancer agents
ABSTRACT Objective: To investigate anti-hemolytic, antibacterial and anti-cancer activities of leaf and stem extracts from Polygonum odoratum. Methods: Leaves and stems of Polygonum odoratum were extracted using methanol and their anti-hemolytic activity was assessed using 2, 2′-Azobis(2-methylpropionamidine) dihydrochloride which is known to generate free radical damage on cell membranes of red blood cells. This damage, represented by hemolysis, was measured using spectrophotometry. Antibacterial activity was tested by using a broth microdilution method to find minimal inhibitory concentrations against eight bacterial strains. Anti-cancer activity of the extracts was evaluated against a human promyelocytic leukemic cell line (HL-60) by using MTT assay for cell viability and flow cytometry for apoptosis induction and cell cycle analysis. Results: Both leaf and stem extracts have anti-hemolytic activity. The results showed a significantly increased percentage of inhibition in a concentration-dependent manner. Interestingly, the leaf extract showed anti-hemolytic activity to a greater extent than the stem extract. Antibacterial activity of the extracts, as indicated by their minimal inhibitory concentration, using 12.5, 50, 25, 25 µg/mL, was measured against Staphylococcus epidermidis,Enterococcus faecium, Enterococcus faecalis and Staphylococcus aureus. The leaf extracts also exhibited anti-cancer activity, demonstrated by significantly decreased cell viability of human promyelocytic cells (HL-60), with an IC50 of (350.00±1.85) µg/mL for 48 h and (38.00±0.92)µg/mL for 72 h. Additionally, HL-60 became apoptotic and accumulated in G1-phase after 48 hours of treatment. Conclusions: The extracts of Polygonum odoratum exhibit potential antihemolytic activity. They also have antibacterial activity by inhibiting growth of Gram-positive bacteria. The leaf extract shows anti-cancer activity against HL-60 to a greater extent than the stem extract, causing decreased viability, increased G1-phase accumulation and apoptosis induction.
1. Introduction
A number of plants are not only consumed as vegetables but are also used for medicinal purposes. Medicinal plants have been widely used in traditional medicine for the treatment of many health problems. Recently, there has been increasing interest in medicinal plants, because of their potency and few adverse side effects.
Many plants are a source of antioxidants, such as polyphenols and flavonoids, which can protect the body against free radical damage leading to cardiovascular disease, cancer, and increased effects of ageing[1]. Additionally, polyphenols possess various biological activities such as anti-cancer[2,3], antimicrobial[4,5], antiinflammatory and immunomodulation[6,7].
Polygonum odoratum (P. odoratum) is generally known as Phakpaew or Phakphai in Thailand. It is in the family Polygonaceae.Its leaves are green and slender. The stems are red, straight and about 30-35 cm tall. This plant has a strong coriander aroma and a hot spicy taste. It is usually used for adding flavor and aroma to food[8].It is also used to treat flatulence and to help to relieve constipation in Thai traditional medicine. The bioactive compounds in ethanolic leaf extract of this plant have been previously reported, it contains high levels of polyphenols that have been identified in high-performance liquid chromatography, such as gallic acid, apigenin, ferulic acid,quercetin, ellagic acid and p-coumaric acid[9]. Several compounds like caryophyllene, alpha-caryophyllene, drimenolanddecanal,(Z)-3hexenal, (Z)-3-hexenol, decanal, undecanal, dodecanal and 3-sulfanylhexanal and 3-sulfanyl-hexanol have also been isolated from this plant[8]. In spite of numerous bioactive compounds, there are few reports that demonstrate its biological activities. A recent study, published by our team, reported that methanolic extracts of this plant contained high concentrations of phenolic compounds and flavonoids that might explain these antioxidant and antiinflammatory activities[10]. However, other related biological activities have not been reported. This study aims to investigate antihemolytic, antibacterial and anti-cancer activities of methanolic extracts from the leaves and stems of P. odoratum.
2. Materials and methods
2.1. Chemicals and reagents
RPMI 1640 and fetal bovine serum were purchased from Gibco(NY, USA). Penicillin-Streptomycin, 3-(4, 5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) and 2, 2′-Azobis(2-methylpropionamidine) dihydrochloride (AAPH) were purchased from Sigma-Aldrich Inc. (St Louis, MO, USA). Phosphate buffered saline pH 7.0 (PBS), dimethylsulfoxide (DMSO) and methanol were of analytical grade.
2.2. Plants collection and extraction
P. odoratum was collected from a local market in Chiang Rai Province, Thailand during July-August 2016. The identity of the plant was confirmed by Dr. Jantrararuk Tovaranonte, School of Science, Mae Fah Luang University. The voucher specimen was deposited in the herbarium of the School of Medicine, Mae Fah Luang University (Herbarium number: MD2018080001-1).Leaves and stems of P. odoratum were separated and left at room temperature for 7-10 d and then crushed to a fine powder. Fifty gram portions of dried powder were extracted, using 750 mL of 95%methanol at 25 ℃ for 72 h with 150 rpm rotation. Next, the extracts were filtered 3 times and concentrated using a rotary evaporator to obtain leaf and stem extracts.
2.3. Anti-hemolytic assay
Hemolysis is an indicator of free radical damage affecting the membrane of red blood cells (RBC) which might be counteracted by antioxidants. In the assay, AAPH was used to generate free radicals, which could attack the RBC membrane and eventually cause hemolysis[11]. Whole blood specimens from physiologically normal volunteers were collected and centrifuged at 5 000 rpm for 10 min to separate packed red cell samples. The 5% hematocrit of RBC suspension was prepared in PBS solution, pH 7.4. The cell suspension was pre-incubated with various concentrations of leaf and stem extracts at 37 ℃ for 1 h. Ascorbic acid (AA) was used as the positive reference. Then, the treated cells were incubated with 50 mM AAPH solution at 37 ℃ for 3 h and the degree of hemolysis was analyzed by measuring optical density (OD) at 540 nm. The reaction without the extract was used as a control sample. The percentage of anti-hemolysis was calculated from the following equation:%Inhibition = 100×(1-ODsample)/ODcontrol
2.4. Antibacterial activity
2.4.1. Microorganisms and inoculum preparation
Eight bacterial strains were used in the test including four strains of Gram-positive bacteria: Staphylococcus epidermidis (S. epidermidis)(ATCC 12228), Streptococcus aureus (S. aureus) (ATCC 25923),Enterococcus faecalis (E. faecalis) (ATCC 19212), Enterococcus faecium (E. faecium) (TISTR 2058) and four strains of Gramnegative bacteria: Escherichia coli (ATCC 25922), Salmonella typhi(DMST 22842), Pseudomonas aeruginosa (DMST 4739) and Shigella flexneri (DMST 4423). All bacterial strains were picking numerous colonies and grown in Muller Hinton broth for 4-6 h at 37 ℃ with rotation 150 rpm. Then, the bacterial concentration was adjusted with the saline solution to match that of a 0.50 McFarland standard solution which gives a final concentration of 106CFU/mL.
2.4.2. Minimal inhibitory concentration (MIC)
MICs of P. odoratum extracts were assayed using a broth microdilution method[12]. The leaf and stem extracts were dissolved in 5% DMSO to obtain 100 mg/mL stock solution. Stock solution was diluted with Muller Hinton broth to achieve 0.195, 0.391, 0.781,1.563, 3.125, 6.25, 12.5, 25, 50, and 100 µg/mL. Afterward, 190 µL volumes of the extract were placed into 96-well plates and 10 µL of each inoculum was added into each well. The culture was incubated for 16-24 h at 37 ℃. Growth inhibition or microbial growth was determined by measuring the OD of the culture in the micro wells with the different concentrations of extracts at 625 nm using a microplate reader. A control was maintained, using 0.05% DMSO in culture medium and bacterial cells. Ampicillin and gentamicin were used as reference substances. MIC was defined as the lowest concentration of the extracts to inhibit the growth of microorganisms.
2.5. Anti-cancer activity
2.5.1. Cell culture
Human promyelocytic leukemic cell line (HL-60) was cultured in RPMI 1640, supplemented with 10% (v/v) fetal bovine serum, 100 U/mL penicillin and 100 µg/mL streptomycin, and maintained at 37 ℃ in a 5%CO2incubator.
2.5.2. Cell viability
Cell viability was investigated by using MTT assay[13]. HL-60 (1×104cells/well) were seeded into 96-well plates and treated with various concentrations of the extracts for 48 or 72 h. Following that,MTT solution (5 mg/mL in PBS), was added to the treated cells and incubated for another 4 h at 37 ℃. Then, the medium was removed and DMSO solution was added to dissolve the formazan crystals.Finally, the absorbance was measured at a wavelength of 540/630 nm. The untreated cells were used as a control group (100% of viable cells) for calculating the percentage of viable cells after the treatment.
2.5.3. Apoptotic induction analysis
The effect of the extracts on apoptotic induction was detected with fluorescent probes. Anexin Ⅴ-FITC and propidium iodide (PI)staining (Millipore, Canada) were used for measuring apoptosis cells[14]. Briefly, HL-60 (5×105cells/well) were seeded in a 24-well plate and then treated with various concentrations of the extracts (50,100 and 200 µg/mL) for 48 h. Next, the treated cells were washed with cold PBS and stained with fluorescent probes. Finally, the stained cells were analyzed, using a flow cytometer (FACSCanto Ⅱ,B.D Bioscience, USA).
2.5.4. Cell cycle analysis
Cell cycle distribution was investigated with PI staining(InvitrogenTM, Life Technologies, USA). Briefly, HL-60 (5×105cells/well) were seeded in a 24-well plate and then treated with various concentrations of the extracts for 48 h. Next, the treated cells were washed with cold PBS and fixed with ice cold 70% ethanol for 1 h. After that, the treated cells were washed and incubated with RNase (0.5 mg/mL) at 37 ℃ for 30 min. The treated cells were then stained with PI (50 µg/mL) in the dark at room temperature for 30 min, washed and finally analyzed, using a flow cytometer(FACSCanto Ⅱ, B.D Bioscience, USA).
2.6. Statistical analysis
The results were expressed as mean±SD of three independent measurements. Statistical analysis was determined by using Oneway Analysis of Variance (ANOVA), and a post-hoc test was also applied. Results were indicated as significant at P < 0.05.
3. Results
3.1. Anti-hemolytic activity of P. odoratum extracts
Anti-hemolytic activity was assessed by measuring the degree of hemolysis when AAPH generated free radical damage. The leaf and stem extracts of P. odoratum exhibited strong antioxidant activity,thereby protecting red blood cells from hemolysis. As shown in Figure 1, the results showed significantly increased percentage inhibition of hemolysis in a concentration-dependent manner. The leaf extract possessed stronger anti-hemolytic activity than the stem and its activity appeared to be close to that of ascorbic acid, a reference antioxidant.
3.2. Antibacterial activity of P. odoratum extracts
As shown in Table 1, both leaf and stem extracts were effective against Gram-positive bacteria including S. epidermidis, E. faecium,E. faecalis and S. aureus with MIC of 12.5, 50, 25, 25 µg/mL respectively. Among the eight bacteria tested, S. epidermidis was the most susceptible to the plant extracts, followed by E. faecalis and S.aureus and then E. faecium. However, the extracts were not effective against Gram-negative bacteria.
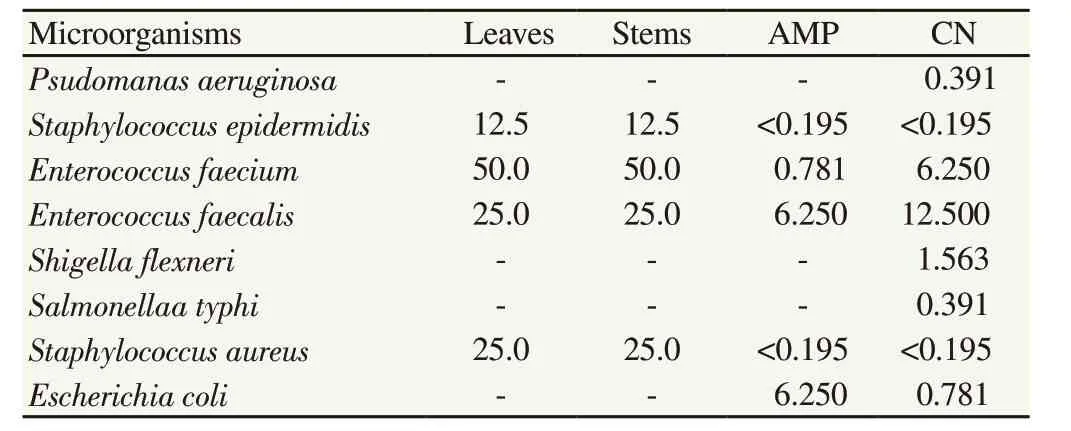
Table 1 Minimal inhibitory concentration of leaf and stem extracts of P. odoratum(µg/mL).
3.3. Anti-cancer activity of P. odoratum extracts
3.3.1. Toxic effect on cell viability of HL-60
As shown in Figure 2A, cell viability of HL-60 was significantly decreased in a concentration-dependent manner after 48 and 72 hours of exposure with leaf extracts. However, low concentrations of stem extract did not have an effect until levels of 500 µg/mL, as shown in Figure 2B. Leaf extract surprisingly demonstrated greater inhibitory effect with IC50of (350.00±1.85) µg/mL for 48 h and IC50of (38.00±0.92) µg/mL for 72 h.

Figure 2. Viability of HL-60 cells treated by leaf (A) and stem (B) extracts of P. odoratum at various concentrations for 48 and 72 h.Data obtained from three independent experiments are expressed as mean±SD. *P<0.05 when compared with the untreated cells.
3.3.2. Apoptosis induction on HL-60
Results from Figure 3 showed the percentage of apoptotic cells when treated with extracts for 48 h. Leaf and stem extracts exhibited significantly increased apoptotic cell production in a concentrationdependent manner. Interestingly, the leaf extract induced cell apoptosis to a greater extent than stem extract. The percentage of apoptotic cell approximately reached 7% at the highest concentration of leaf extract.

Figure 3. Percentage of cells showing apoptosis, treated by leaf and stem extracts of P. odoratum at various concentrations for 48 h.Data obtained from three independent experiments are expressed as mean±SD.*P<0.05 when compared with the untreated cells; #P<0.05 when compared with the stem extract.
3.3.3. Effect on cell cycle of HL-60
As shown in Figure 4, the distribution of cells in S-phase was diminished which means DNA synthesis in HL-60 was decreased after treatment with the extracts for 48 h. Thus, the percentage of cells in the G1-phase accumulated and led to an increase in a concentration-dependent manner. The leaf extract also reduced the cells in S-phase to a greater extent than the stem extract.
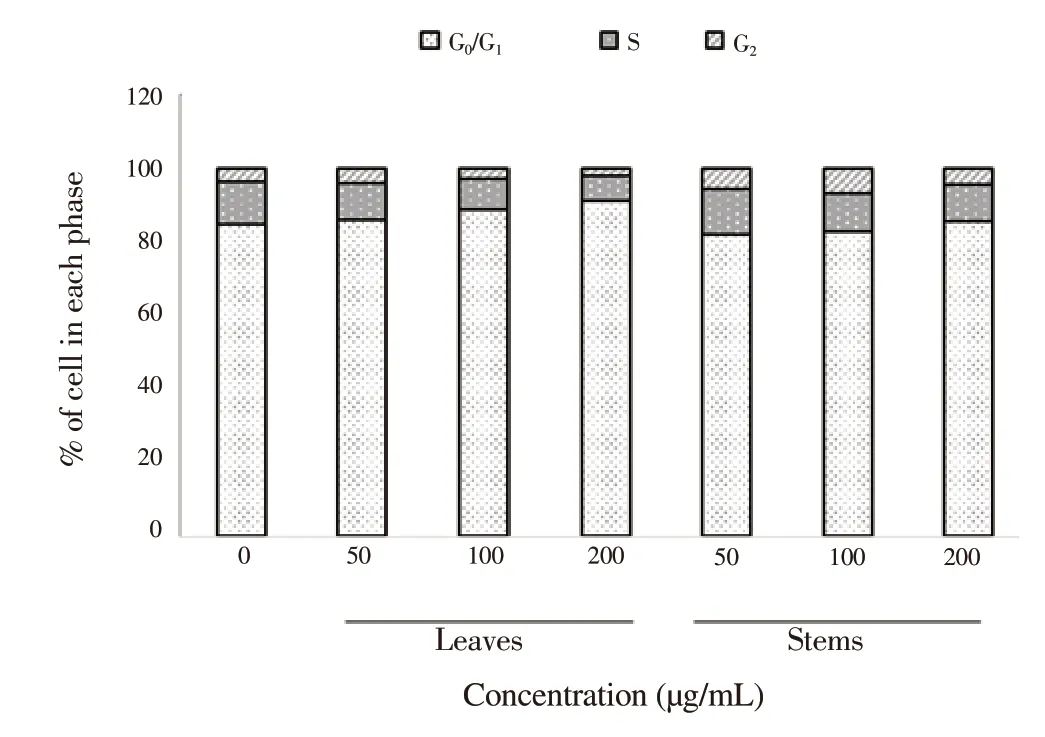
Figure 4. Cell cycle analysis as shown by percentages of cells in each phase,treated by leaf and stem extracts of P. odoratum at various concentrations for 48 h.Data were obtained from three independent experiments.
4. Discussion
The presented data demonstrated that methanolic extracts of P.odoratum exhibited strong antioxidant activity, thereby protecting RBC from hemolysis, as shown especially by the leaf extract. Our recent study confirmed that methanolic leaf and stem extracts of this plant contained high concentrations of phenolic compounds and flavonoids. Results from GC-MS analysis also showed a predominance of E-15-Heptadecenal and 3, 7, 11, 15-tetramethyl-2-hexadecen-1-ol in the extracts that might be the cause of the antioxidant activity and anti-inflammatory activities of these extracts[10]. However, identification of other bioactive compounds should be investigated further. Several studies have reported that ethanolic leaf extracts of this plant have good antioxidant activity.The extract contains high levels of polyphenols that are known to scavenge free radicals[9,15]. Polyphenols, such as phenolic acid and flavonoids, are responsible for the radical scavenging capacity of plants. Moreover, the phenolic compounds identified and quantified through HPLC, consist mainly of gallic acid, quercetin, ellagic acid, ferulic acid, apigenin and p-coumaric acid. Flavonoids also have other pharmaceutical activities including anti-allergic[16,17],anti-inflammatory[18-20], antimicrobial[21,22] and anti-cancer properties[23-25].
In this study, both leaf and stem extracts of P. odoratum also possessed high potential antibacterial effects against four of the eight tested bacterial strains by using broth microdilution method,indicating that both extracts strongly exhibited antibacterial activity with same MIC against four Gram-positive bacteria (S. epidermidis,E. faecium, E. faecalis and S. aureus). Interestingly, Gram-positive bacteria are more sensitive to the extracts than Gram-negative bacteria. This may be because the cell walls of Gram-positive bacteria are more sensitive to many of antimicrobial agents and natural products[26-29]. Moreover, Gram-negative bacteria have a lipopolysaccharide layer and periplasmic space that explains this relatively greater resistance[30-32]. Many reports have noted that ethanoic extracts of this plant have potential natural antimicrobial activity against Escherichia coli, S. aureus, Bacillus subtilis and Salmonella spp[33]. The hexane extract was effective against S.aureus, S. epidermidis, Streptococcus pneumonia and Streptococcus pyogenes[34]. The most susceptible bacteria for dichloromethane extraction were S. aureus, S. epidermidis, Streptococcus pyogenes and Salmonella typhi[34]. However, none of any reports demonstrate any anti-fungal or anti-viral activities[35].
We also demonstrated that leaf and stem extracts of P. odoratum also have anti-cancer activity against human promyelocytic leukemia cell line (HL-60). The leaf extract was more effective than the stem extract, causing a decrease of viability, increased apoptosis and arresting the cells in G1-phase. It has been reported that P.odoratum extract inhibits proliferation and induces apoptosis of human breast cancer cells (MCF-7 and MDA-MB-231)[15,36]. This is probably related to its strong antioxidant activity. However, the molecular mechanism involved in the extracts’ effects requires further elucidation.
In conclusion, P. odoratum exhibits potential anti-hemolytic,antibacterial and anti-cancer activities. Therefore the use of P.odoratum leaves may be expected to have beneficial health effects.
Conflict of interest statement
The authors confirm that this article content has no conflicts of interest.
Acknowledgements
The authors acknowledge the financial support of Mae Fah Luang University (No.59111040017) and thank the Department of Biochemistry, Faculty of Medicine, Chiang Mai University and School of Medicine, Mae Fah Luang University for providing facilities.
Funding
This work was financially supported by Mae Fah Luang University(No.59111040017).
 Asian Pacific Journal of Tropical Biomedicine2018年12期
Asian Pacific Journal of Tropical Biomedicine2018年12期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- A survey of biochemical and acute phase proteins changes in sheep experimentally infected with Anaplasma ovis
- Gac fruit extracts ameliorate proliferation and modulate angiogenic markers of human retinal pigment epithelial cells under high glucose conditions
- Anti-cancer and anti-inflammatory activities of aronia (Aronia melanocarpa) leaves
- Antidiabetic effects of Tetracarpidium conophorum seed on biomarkers of diabetesinduced nephropathy in rats
- Anti-cancer effects of hydro-alcoholic extract of pericarp of pistachio fruits
- Anti-insulin resistant effect of ferulic acid on high fat diet-induced obese mice
