Gac fruit extracts ameliorate proliferation and modulate angiogenic markers of human retinal pigment epithelial cells under high glucose conditions
Ali Abdulqader, Faisal Ali,2, Amin Ismail,3✉, Norhaizan Mohd Esa,3,4
1Department of Nutrition and Dietetics, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia
2Biochemistry & Molecular Biology Department, University Hospital, Faculty of Medicine and Health Sciences, Sana’a University, Yemen
3Research Center of Excellent, Non-Communicable Diseases (NNCD) Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, 43400,Serdang, Selangor, Malaysia
4Laboratory of Molecular Biomedicine, Institute of Bioscience, Universiti Putra Malaysia, 43400, Serdang, Malaysia
Keywords:Gac (Momordica cochinchinensis Spreng)High glucose Angiogenesis Human retinal pigment epithelial cells Proliferative diabetic retinopathy
ABSTRACT Objective: To investigate the impact of the extracts of Gac fruit parts (peel, pulp, seed, and aril) on the cell viability and angiogenesis markers of human retinal pigment epithelial (ARPE-19) cells under high glucose conditions. Methods: The effect of the extracts of Gac fruit peel,pulp, seed and aril on the ARPE-19 cells was determined using MTT viability assay, Trypan blue dye and morphological changes were observed using light microscopy. Enzyme-linked immunosorbent-based assay was performed to evaluate the effect of Gac fruit parts on the reactive oxygen species (ROS), vascular endothelial growth factor (VEGF) and pigmented epithelium-derived factor (PEDF) secretions. Results: High glucose (HG) at 30 mmol/L increased ARPE-19 cell viability and ROS and VEGF secretions. While, the exposure of ARPE-19 cells in high glucose condition to Gac fruit extracts led to inhibition of cell viability, induced morphological changes, decreased ROS and VEGF secretions, and increased PEDF level. Gac pulp, seed, and aril at 1 000 μg/mL showed significant inhibition activities [(7.5 ± 5.1)%, (2.7± 0.5)%, (3.2 ± 1.1)%, respectively] against HG-induced ARPE-19 cell viability. The findings also demonstrated that Gac aril at 250 μg/mL significantly decreased ROS and VEGF levels[(40.6 ± 3.3) pg/mL, (107.4 ± 48.3) pg/mL, respectively] compared to ROS [(71.7 ± 2.9) pg/mL] and VEGF [(606.9 ± 81.1) pg/mL] in HG untreated cells. Moreover, 250 μg/mL of Gac peel dramatically increased PEDF level [(18.2 ± 0.3) ng/mL] compared to that in HG untreated cells [(0.48 ± 0.39) ng/mL]. Conclusions: This study indicates that the extracts of Gac peel,pulp, seed and aril reduced cell viability, minimized ROS generations and showed angiogenic activities. Therefore, our findings open new insights into the potentiality of Gac fruit against HG-related diabetic retinopathy disease.
1. Introduction
Diabetic retinopathy (DR) is a microvascular destructive disease and is one of the common consequences of diabetes[1].Hyperglycaemia is a primary factor in the initiation and progression of vascular complications related to eye diseases in diabetes such as DR[2,3]. However, the mechanisms by which hyperglycaemia leads towards vascular dysfunction still require further investigation.Notwithstanding, it has been established that chronic exposure to hyperglycaemia by the microvascular in the retina is associated with several pathological changes. Of these changes, the over-production of reactive oxygen species (ROS) and the loss of homeostasis in the angiogenesis process have been identified as the major contributing factors[4-6]. Chronic exposure of retinal cells to hyperglycaemia gives chances to increase ROS by activation of different enzymatic pathways[7,8]. ROS is a toxic-free radical with impaired electron,such as superoxide radical, hydroxyl radical and hydrogen peroxide radical. Over-production of ROS may lead to increased oxidative stress and this could be the main pathogenesis of DR[9]. Increased ROS production could be either from large excesses of free radicals and/or a failure of the antioxidant systems such as vitamin E, A and C, carotenoids, polyphenols, glutathione or other radical degrading mechanisms to destroy the number of free radicals being formed[9].
Clinically, DR is categorised into two main classes, namely; nonproliferative DR (NPDR) and proliferative DR (PDR)[10]. PDR is the final stage of DR characterised by the uncontrolled growth of abnormal blood vessels that leak and bleed into the retina, inevitably leading to blindness[11]. The process of growing new blood vessels from pre-existing vessels is known as angiogenesis which is a very accurate process, tightly controlled by inhibitor and stimulator angiogenic markers[12,13]. The vascular endothelial growth factor(VEGF) is one of the most studied markers that acts as a stimulator for the angiogenesis process and is found to be elevated in patients with PDR[14]. Whereas, the pigmented epithelium-derived factor(PEDF) has been shown to be an inhibiting factor in the angiogenesis process, and found at a lower level among patients with PDR[15,16].Retinal pigment epithelial (RPE) cells play an important role in the visual function and DR development[17]. The major functions of RPE cells include nutrients, minerals, water, glucose and ions transport.Moreover, RPE plays a role in the generation of angiogenic markers,such as VEGF, PEDF, insulin growth factor (IGF), and fibroblast growth factor (FGF)[18,19].
Natural sources (i.e. plants, vegetables, fruits) are rich in phytochemicals and bioactive constitutes that are widely reported to have health-promoting activities for human benefits[20]. Of these natural sources, carotenoids-rich fruits play an important role in the management of diabetes-related diseases[21,22]. Gac (Momordica cochinchinensis Spreng) is a tropical fruit, indigenous to Southeast Asian countries and reported to be a rich source of phytochemicals,especially carotenoids[23]. Carotenoids have been linked with the prevention of eyes disorders, such as age-related macular degradation and cataracts[24]. Furthermore, the consumption of carotenoids-rich foods has been associated with a decreased risk of DR development and visual acuity improvement through ROS neutralising,neuroprotective and anti-inflammatory functions[24-27]. Gac fruit extracts were acknowledged to have pharmacological activities via suppressing migration and invasion of breast cancer cells[28],reversing tert-butyl peroxide-induced cell damage[29], and reducing wet tumor weight in vivo[30]. However, limited information about the effect of extracts of Gac peel, pulp, seed and aril on the treatment of hyperglycaemia-related DR disease has been found. Therefore,this study aimed at investigating the impact of Gac fruit parts on the proliferation and angiogenesis activity of human retinal pigment epithelial (ARPE-19) cells in high glucose (HG) conditions.
2. Materials and methods
2.1. Chemicals and reagents
In undertaking this study, the following chemicals and reagents were used; Dulbecco’s Modified Eagle’s Medium (DMEM),fetal bovine serum, phosphate buffer saline (PBS), penicillin and streptomycin, trypsin-EDTA (1×), and Trypan blue dye,dimethyl sulfoxide (DMSO) (Sigma, St. Louis, MO USA), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)(Sigma-Aldrich, Billerica, MA USA), human ROS, modulator 1 ELISA kit (E1924h, EIAab, Wuhan, China), human VEGF and human EDF ELISA kits (CUSABIO, Wuhan, China). Other reagent analytical grade chemicals were also used along with pure distilled water for all experimental steps.
2.2. Collection of Gac fruit
Gac (Momordica cochinchinensis Spreng) fruit Malaysian cultivar was supplied from the International Tropical Fruits Network,Selangor, Malaysia. Eleven ripe fruits were randomly collected during the harvesting season in October of 2017. Collectively, all fruits collected weighed 8 kg, and the average individual weight was about (755 ± 185) g. The fruits were then kept separately in sealed plastic bags and stored in -80 ℃ for a few weeks until they were ready to use.
2.3. Fruit extract preparation
The Gac fruit was removed from storage and exposed to room temperature in order to completely thaw, followed by washing with tap water to remove any debris. The fruit was next separated into four parts; peel, pulp, seed, and aril. It was then cut into small slices and pieces, followed by each fruit part being lyophilised using a freeze dryer at -45 ℃ for 3 d (BT2K, VirTis, Warminster, USA).The freeze-dried parts were then ground, mixed, extracted with 70%ethanol at the ratio of 1:20 (w/v), and vigorously shaken using an orbital shaker (SHO-2D, Daihan Scientific, Seoul, Korea) at 180 rpm, for 2 h and filtered. Next, the obtained extract was evaporated using a rotary evaporator (R-210, Buchi, Flawil, Switzerland). The extract following evaporation (sticky and dark liquid extract) was then dried using a freeze dryer, and finally, the resulting dried extract was stored at a temperature of -20 ℃ for further use.
2.4. Cell culture
Human RPE (ARPE-19) cells were purchased from the American Type Culture Collection (Rockville, MD, USA). In order to grow the cells, ARPE-19 was grown in DMEM complete media supplied in 1% penicillin/streptomycin and 10% fetal bovine serum (v/v). A humidified incubator at a temperature of 37 ℃ in 5% CO2condition was used to incubate the flasks containing the cells. The passage number used during all experiments was between P6 and P12.
2.5. Cell viability under different glucose conditions
ARPE-19 cells were seeded in DMEM with low glucose (LG)(5.5 mmol/L) at a density of 5 000 cells/well using 96-well plate.On the second day, the media were replaced with 100 μL of media containing different glucose concentrations (5.5, 30, 50, 70, 100 mmol/L). After 48 h, the media were removed and 20 μL of MTT(1 mg/mL) was added to each well and incubated for 4 h[31]. Then,the MTT was removed carefully and 100 μL of DMSO was added.The absorbance was read at 570 nm using a microplate reader(VersaMax, Sunnyvale, USA), and 630 nm was used as a reference wavelength. The results were expressed as percentage for three triplicates using the following formula:
Cell viability percentage (%) = OD570-630of each HG (30, 50, 70, 100 mmol/L) /OD570-630of LG (5.5 mmol/L) × 100 Where OD = Optical density.
2.6. Cytotoxicity evaluation
ARPE-19 cells were plated in 96-well plate at 5 000 cell/well density with 100 μL of DMEM with LG (5.5 mmol/L) media and were allowed to attach overnight. ARPE-19 cells were divided into groups including: ARPE-19 with LG (5.5 mmol/L) and HG (30 mmol/L), ARPE-19 with different concentrations (31.2-1 000 μg/mL)of extracts obtained from freeze dried peel, pulp, seed and aril in HG(30 mmol/L). After 48 hours of incubation, all media were removed,20 μL of MTT (1 mg/mL) was added to each well and incubated for 4 h[31]. MTT was then removed and 100 μL of DMSO was added. The absorbance was read at 570 nm using a microplate reader and 630 nm was used as a reference wavelength. The results were expressed as percentage for three measurements using the following formula:
Cell viability percentage (%) = OD570-630of HG groups (treated and non-treated) /OD570-630of LG group × 100 Where OD = Optical density.
2.7. Cell morphology examination
Using 6-well plates, the ARPE-19 cells were seeded at a density of 300 000 cells/well in LG media, allowing to attach overnight. Next,the medium of each well was removed, and the cells were washed twice with PBS (1×). After that, the ARPE-19 cells were exposed to 2 mL of HG (30 mmol/L) media containing different concentrations(62.5-1 000 μg/mL) of the Gac fruit extracts (peel, pulp, seed and aril). In this assay, the concentration of Gac extracts started from 62.5 μg/mL because small concentrations might not be effective to induce morphology changes. The untreated cells with HG and LG media were also considered. Following 48 hours of incubation,a light-inverted microscope was used to observe the normal morphological changes.
2.8. Trypan blue dye assay
For further determining the effects of Gac fruit extracts on ARPE-19 cell viability, 6-well plates were used, and 300 000 ARPE-19 cells were seeded with LG (5.5 mmol/L). After 24 h, the media were removed, then ARPE-19 cells were incubated with 2 mL of LG (5.5 mmol/L) and HG (30 mmol/L), and with different concentrations(31.2-1 000 μg/mL) of extracts obtained from freeze dried peel,pulp, seed and aril dissolved in HG (30 mmol/L) respectively. After 48 hours of incubation, the medium of each group was removed and the cells were detached using Trypsin-EDTA. After entirely detaching, the cells’ suspensions were centrifuged at 1 200 rpm, 4 ℃for 5 min. Next, the supernatant was discarded, and the pellet was suspended with 1 mL of PBS. Finally, 10 μL of the resulting cell suspension was blended with 10 μL of 0.4 % Trypan blue solution,and then the ARPE-19 cells were microscopically counted using a haemocytometer chamber under a light-inverted microscope[32].
2.9. Measurement of ROS level
Doses of Gac extracts used in the previous evaluations were to assess the IC50and toxic doses, resulting in the usage of three (low,medium and high) non-toxic doses of Gac parts extracts in further experiments. To determine the level of ROS, 200 000 cells of ARPE-19 were seeded in 6-well culture plate with LG (5.5 mmol/L). On the second day, all media were discarded and ARPE-19 cells were washed twice with 1×PBS. Next, ARPE-19 cells were incubated with different groups of media including: ARPE-19 with LG (5.5 mmol/L) and HG (30 mmol/L), and ARPE-19 in HG (30 mmol/L)with different concentrations (50, 100 and 250 μg/mL) of extracts from freeze dried Gac peel, pulp, seed and aril. In accordance to the protocol defined by the manufacturer (E1924h, EIAab, Wuhan,China), the medium of each group was collected in test tubes and centrifuged at 1 000 ×g, at 4 ℃ for 10 min. Next, 100 μL of the sample’s supernatants and standards were added to the 96-wells plate coated with a specific antibody for ROS and incubated for 2 h at 37 ℃. Next, the liquid of each well was discarded, and 100 μL of the detection reagent A was added. Following 1 hour of incubation at 37 ℃, the liquid was removed, and the wells were washed 3 times with washing buffer, and 100 μL of detection reagent B was added.After 1 hour of incubation at 37 ℃, the liquid was removed from the wells and washed five times with washing buffer. Then, 90 μL of substrate solution was added to each well, protected from light and incubated at 37 ℃ for 15-30 min. Finally, 50 μL of stop solution was added to each well, and the absorbance was read within 10 min at 450 nm using a microplate reader. The concentration of ROS was then calculated based on the standard curve of ROS.
2.10. Measurement of VEGF and PEDF levels
To determine the VEGF and PEDF levels, ARPE-19 at 200 000 cells/well density was seeded with LG (5.5 mmol/L) using a 6-well plate.After 24 hours of incubation, the media were discarded and the cells were washed twice with 1×PBS. Next, ARPE-19 cells were incubated with different groups of media including: ARPE-19 with LG (5.5 mmol/L) and HG (30 mmol/L); ARPE-19 with HG (30 mmol/L)containing different concentrations (50, 100 and 250 μg/mL) of extracts obtained from freeze dried peel, pulp, seed and aril and incubated for 48 h. VEGF and PEDF levels were measured in each group according to the manufacturer’s protocol (CSB-E11718h VEGF, CSB-E088181h PEDF, Wuhan, China). The media were collected and centrifuged at 1 000 ×g, at 4 ℃ for 10 min. Next, 100 μL of the samples and standards were added to the 96-wells plate that was pre-coated with specific antibodies for VEGF and PEDF. After 2 hours of incubation at 37 ℃,the liquid was removed, and 100 μL of Biotin-antibody (1×) was added to each well. Following 1 hour of incubation at 37 ℃, the liquid was carefully discarded, and the wells were washed 3 times with washing buffer (1×). Next, 100 μL of HRP-avidin (1×) was added to each well and incubated for 1 h at 37 ℃. Then, the liquid was then discarded,and the wells were washed 5 times with washing buffer. After that, 90 μL of TMB substrate was then added to each well, protected from light and incubated at 37 ℃. Finally, 50 μL of stop solution was added, and the absorbance was read at 450 nm using a microplate reader within 5 min. The concentrations were calculated based on the standard curve of VEGF and PEDF of three measurements.
2.11. Statistical analysis
The data was presented as mean ± standard deviation (SD). The analysis of the significance and differences among the means was undertaken through One-way ANOVA and Tukey’s post hoc multiple comparison test. Differences in the means were considered statistically significant at P < 0.05. Notably, all experimental data values were statistically subjected using the GraphPad PRISM program version 6.01.
3. Results
3.1. Cell viability under different glucose concentrations
As illustrated in Figure 1, LG group at 5.5 mmol/L had the lowest ARPE-19 cell viability. Among the groups with different concentrations higher than 5.5 mmol/L, there were no statistically significant differences. However, HG group at 30 mmol/L had the highest ARPE-19 cell viability. For this reason, HG at 30 mmol/L was used for further experiments with LG at 5.5 mmol/L as a control group.

Figure 1. ARPE-19 cell viability incubated with different glucose concentrations (5.5, 30, 50, 70, 100 mmol/L) for 48 h using MTT assay.Results are expressed as mean ± SD of three measurements. Bars having different letters are significantly different at P < 0.05 using Tukey’s test.
3.2. Effect of extracts from Gac fruit parts on ARPE-19 cell viability
Gac fruit extracts (peel, pulp, seed and aril) at different concentrations were evaluated for cell toxicity against ARPE-19 cell line in HG condition at 30 mmol/L. ARPE-19 cell viability significantly increased in HG group (100 ± 9)% compared to LG group (64.3 ± 7.5)% (Figure 2). The effect of Gac extracts on cell viability was dependent on the dose. Pulp, seed and aril at 1 000 μg/mL had the most cytotoxic effect towards ARPE-19 cell viability [(7.5 ± 5.1)%, (2.7 ± 0.5)% and (3.2± 1.1)% respectively]. In contrast, peel at 1 000 μg/mL appeared to have a moderate cytotoxic effect on ARPE-19 cell viability [(41.2 ±7.1)%]. In addition, Gac seed extract had the highest IC50equivalent to 190 μg/mL.

Figure 2. Effects of extracts from Gac fruit parts (peel, pulp, seed and aril) at different concentrations (31.2-1 000 μg/mL) on ARPE-19 cell viability in HG condition after 48 hours of treatment.Results are expressed as mean ± SD of three measurements. Bars not having the same letter are significantly different at P < 0.05. LG: low glucose (5.5 mmol/L); HG: high glucose (30 mmol/L).
3.3. Effect of extracts from Gac fruit parts on ARPE-19 morphological changes
It was noticed through inverted microscope that there were remarkable variations in the cell morphology of treated cells as shown in Figure 3. Elevated concentrations of the fruit peel, pulp,seed and aril extracts led to increasing morphology changes and decreased cell viability as clearly shown in 1 000 μg/mL of ARPE-19 treated groups compared to other doses. These alterations included:cell detachments, floating, cell spherical and rounding shape, and cell shrinkage. Furthermore, the marked morphology chagnes were observed in the ARPE-19 cells treated with seed and pulp extracts,indicating their dramatic impact on the ARPE-19 cell viability and morphology. According to Figure 3, the Gac extracts appeared to arrest and slow the proliferation and duplications of ARPE-19 cells.However, there were no similar changes observed on the untreated cells.
3.4. Trypan blue dye evaluation
To further confirm the effects of Gac parts extract on ARPE-19 cell viability, Trypan blue dye was applied. The results were consistent with the morphological examinations, where the number of ARPE-19 cells decreased when the extract dose increased. As seen in Figure 4, there was a significant increase in the number of ARPE-19 cells in HG [(483 000.0 ± 4.2) cells], higher than that of LG [(345 000.0 ±4.3) cells] groups. Gac seed at 1 000 μg/mL dramatically decreased ARPE-19 cells number [(48 000.0 ± 1.2) cells], followed by(95 000.0 ± 1.2) cells of Gac pulp at the same dose. In contrast,Gac aril and peel at the highest dose showed the lowest reducing ability [(213 000.0 ± 3.5) cells, (168 000.0 ± 2.5) cells, respectively].Collectively, seed and pulp parts at moderate and high doses showed more significant differences (P < 0.05) than Gac peel and aril in reducing ARPE-19 cell viability.
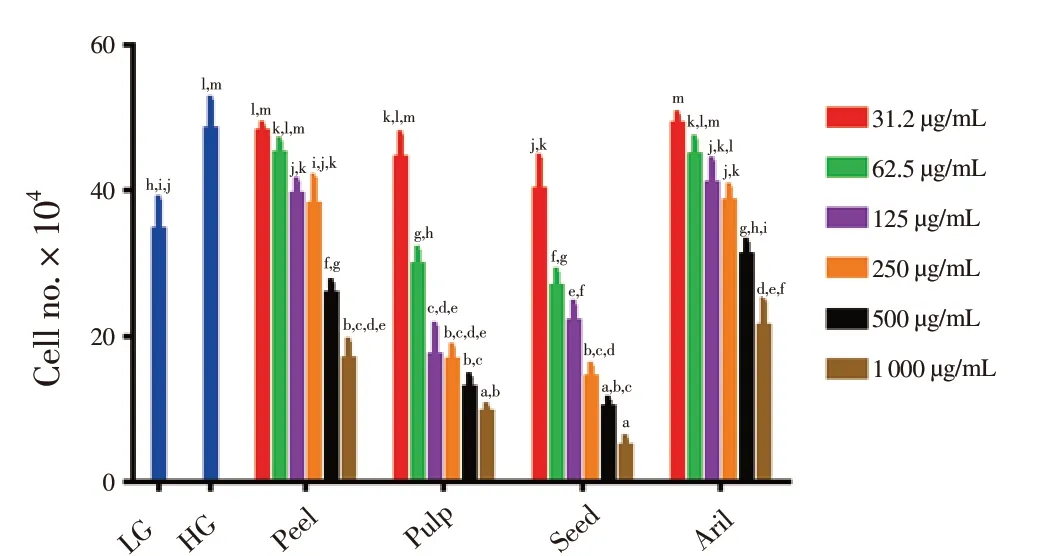
Figure 4. Effects of extracts from Gac fruit parts (peel, pulp, seed and aril)at different concentrations (31.2-1 000 μg/mL) on ARPE-19 cell viability by Trypan blue dye in HG conditions after 48 hours of treatment.Results are expressed as mean ± SD of three measurements. Bars not having the same letters are significantly different at P < 0.05. LG: low glucose (5.5 mmol/L); HG: low glucose (30 mmol/L).
3.5. Effects of extracts from Gac fruit parts on ROS level

Figure 3. Effects of extracts of Gac fruit part (peel, pulp, seed, and aril) at different concentrations (62.5-1 000 μg/mL) on cellular morphology of ARPE-19 cells in HG conditions for 48 h.Cell detachment, abnormal shapes and condensation are pointed with light blue arrows, spherical and rounding cells are pointed with yellow arrows (Magnification 100×) under light-inverted microscope. Experiment was conducted in three independent observations.
Figure 5 shows level of ROS in different groups of ARPE-19 cells. Comparatively, the level of ROS in HG [(71.7 ± 2.9) pg/mL]was significantly higher than in LG [(58.0 ± 4.3) pg/mL] group.Furthermore, the Gac fruit parts were able to reduce the ROS level generated by the ARPE-19 cells in HG conditions, but the results varied concerning the extracts’ ability to minimise the ROS level.Gac aril at 250 μg/mL significantly reduced ROS level [(40.6 ± 3.3)pg/mL], followed by pulp [(49.9 ± 2.7) pg/mL], seed [(51.2 ± 5.7)pg/mL], and lastly peel [(55.3 ± 5.6) pg/mL].
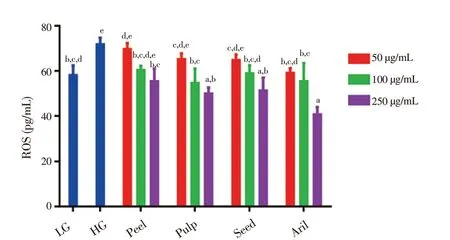
Figure 5. Effects of extracts from Gac fruit parts (peel, pulp, seed and aril) on ROS level of ARPE-19 cells in HG condition treated with different concentrations (50, 100 and 250 μg/mL) for 48 h.Results are expressed as mean ± SD of three measurements. Bars not having the letters are significantly different at P < 0.05. LG: low glucose (5.5 mmol/L); HG: high glucose (30 mmol/L).
3.6. Effects of extracts from Gac fruit parts on VEGF Level
Figure 6 demonstrates the level of VEGF produced by ARPE-19 cells treated with different concentrations of Gac extracts in HG conditions. As illustrated, the level of VEGF produced by the untreated ARPE-19 cells in HG group [(606.9 ± 81.1) pg/mL] was significantly higher (P < 0.05) compared to the VEGF level produced in LG group [(307.9 ± 30.3) pg/mL]. Among the treated groups, Gac aril at 250 μg/mL reduced VEGF level most significantly. Gac pulp at 250 μg/mL and aril at 100 μg/mL also showed significant anti-VEGF activity compared to the HG control.
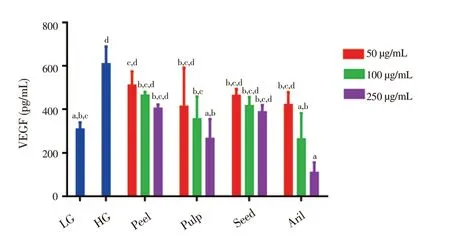
Figure 6. Effects of extracts from Gac fruit parts (peel, pulp, seed and aril)on VEGF level of ARPE-19 cells in HG condition treated with different concentrations (50, 100 and 250 μg/mL) for 48 h.Results are expressed as mean ± SD for three measurements. Bars not having the same letters are significantly different at P < 0.05. LG: low glucose (5.5 mmol/L); HG: high glucose (30 mmol/L).
3.7. Effects of extracts from Gac fruit parts on PEDF level
As seen in Figure 7, there were no significant differences between the level of PEDF among LG and HG cells, even though the level of PEDF in the HG group [(0.48 ± 0.39) ng/mL] was less than that found in the LG group [(1.85 ± 0.21) ng/mL]. Gac peel at 250 μg/mL showed the highest level of PEDF [(18.2 ± 0.3) ng/mL] followed by seed [(13.9± 4.0) ng/mL] at 250 μg/mL. In contrast, Gac peel showed significant increase of PEDF level at all doses, while Gac pulp at both 100 and 250 μg/mL doses as well as Gac seed and aril at only 250 μg/mL demonstrated the markedly increase.
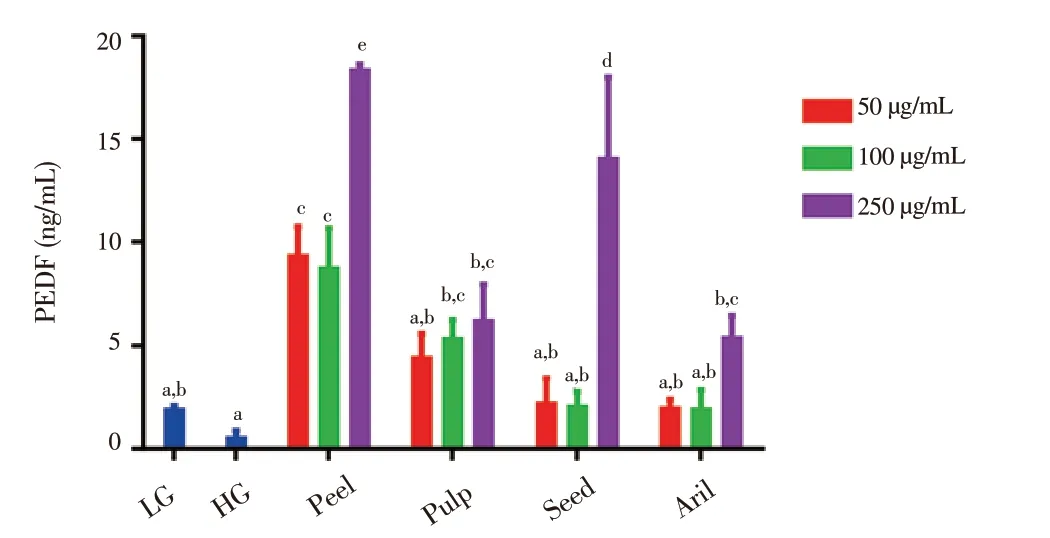
Figure 7. Effects of extracts from Gac fruit parts (peel, pulp, seed and aril)on PEDF level of ARPE-19 cells in HG condition treated with different concentration (50, 100 and 250 μg/mL) for 48 h.Results are expressed as mean ± SD for three measurements. Bars not having the same letters are significantly different at P < 0.05. LG: low glucose (5.5 mmol/L); HG: high glucose (30 mmol/L).
4. Discussion
Gac fruit has been traditionally used in folk and ancestral medicine and treatments for several conditions. Newly, several biological effects and health benefits of Gac fruit have been revealed, such as antioxidant, anti-proliferative, anti-cancer, and antibacterial activities.In the present study, the effects of extracts from Gac fruit parts (peel,pulp, seed, and aril) on ARPE-19 cell viability, cellular morphology and ROS, VEGF, PEDF generations in high glucose conditions were investigated for the first time. The results revealed that Gac fruit parts reduced ARPE-19 cell viability, induced morphological changes, decreased ROS, VEGF productions, and increased PEDF levels.
Chronic exposure to hyperglycaemia by the retina is one of the most threatening issues among diabetes patients which leads to PDR and lastly blindness. PDR is characterized by retinal neovascularization due to hypoxia that produces abnormal blood vessels which bleed and leak into the retina. RPE cells play a crucial role in the progression of such condition. A recently published report showed that HG induced abnormal activation of RPE cells which might be associated with PDR development[33].
The results of this study revealed that HG increased ARPE-19 cell viability when compared to LG, which was consistent with a previous study that reported a 2-fold increase in cell viability in HG than LG condition[34]. Amongst the fruit parts, Gac seed at the highest concentration 1 000 μg/mL had the highest anti-proliferative activity tested by MTT and Trypan blue dye. This was in line with the results of some studies published earlier which demonstrated that Gac seed exhibited considerable suppression activity against the proliferation of breast cancer cells ZR-75-30[28], normal HaCat and melanoma D24[35], and lung cancer cell A549[36]. In this study,Gac seed and pulp induced noticeable morphological changes, such as cells condensation, floating, detachments, spherical and rounding shapes, and these characteristics might be due to the cytotoxic effect of high doses. Recently, studies reported that Gac seed water extract also induced morphological changes and cytotoxic effect against normal HaCat, melanoma D24 and C1 cell lines[35]. However, the molecular mechanisms, such as apoptotic and necrotic pathways underlying Gac extracts-induced ARPE-19 morphological changes,need to be further studied. Gac aril in this study also inhibited ARPE-19 cell viability which was consistent to another study which revealed that water extract taken from Gac aril significantly reduced MCF-7 and melanoma with a rate of 60% and 70%, respectively[37].In addition to the seed and aril parts, this study has also showed that Gac peel and pulp reduced ARPE-19 cell viability, but the biological and anti-proliferative potentiality of Gac pulp and peel has not been studied well.
One of the essential initiators of DR development is ROS, which has been noticed to be elevated by chronic hyperglycaemia[38,39].High concentrations of glucose were found to stimulate ROS production in RPE in vitro[33,40]. This finding was in agreement with the result of this study which revealed that HG (30 mmol/L)led to an increase in ROS level produced by ARPE-19 compared to a lower level in LG (5.5 mmol/L). This increment can be naturally avoided by the antioxidant defence system. However, in some conditions such as chronic hyperglycaemia, the balance between ROS and the antioxidant system is disrupted[41]. Thus, it is necessary to support and recover the balance and decrease the level of ROS in the management of PDR. Furthermore, the results of this study showed the ability of extracts from Gac fruit parts to reduce ROS production. This proved the role of phytochemicals and natural sources as antioxidants and their potential ability in the management of DR[42,43]. Amongst all of the Gac fruit parts, Gac aril exhibited the highest anti-ROS ability which could be due to the rich carotenoids,phenolics and other bioactive compounds found in this part as revealed by previous studies[24,44]. Gac seed and peel were also found to possess anti-oxidant activities which might be due to rich trypsin inhibitors compounds, saponins, and phenolics content[35,45].
Retinal neovascularisation as mentioned earlier is the last stage of DR which is characterized by abnormal proliferation and this process is tightly controlled by inhibitors and stimulators of angiogenic factors[46]. Therefore, one of the main strategies to treat PDR is to modulate angiogenesis process by either suppressing angiogenic stimulators, such as VEGF and/or stimulating angiogenic inhibitors,such as PEDF. Current treatment patterns of this process include anti-VEGF drugs injection in addition to laser photocoagulation and surgery[47,48]. These strategies are extremely expensive and accompanied with undesired results, such as retinal detachments,retinal damage, and vitreous haemorrhage[49,50]. In this study, HGinduced secretions of VEGF were about 2-fold higher than that in LG group which was consistent with the results of previous studies.It was previously shown that VEGF secretion was highly responsive to the change in glucose concentration, which increased when the glucose dose increased[51]. It was also reported that the VEGF level produced by the ARPE-19 cells increased under HG conditions[52,53].HG-stimulated VEGF secretion in ARPE-19 cells as illustrated in this study was reversed when treated with Gac parts extracts.Amongst all of the Gac fruit parts, the aril extract dramatically decreased VEGF secretions, which might be attributed to the rich content of phytochemicals, especially carotenoids. The role of carotenoids in the modulation of angiogenesis markers has been well established[54]. A previous study confirmed the anti-angiogenic activity of lycopene via decreased VEGF production, inhibited tube formation, and migration in human umbilical vein endothelial cells[55].
During this study, the PEDF level was evaluated by using the extracts of Gac fruit parts. The results revealed that the PEDF level of HG group reduced compared to that of LG group, whereas Gac fruit parts boosted the production of PEDF by the ARPE-19 cells. In PDR, the increase in the level of PEDF is essential in order to balance the high concentration of VEGF that is stimulated by HG thus modulating the angiogenesis process. Although one study showed that the PEDF level was enhanced when treated with xanthatin as a medicinal component from Xanthium[56], limited information has been found regarding the role of phytochemicals in PEDF secretions.
To the best of our knowledge, this study is the first to investigate the effects of Gac fruit extracts on HG-induced PDR biomarkers in vitro. The data revealed anti-proliferative, anti-ROS, angiogenesis biomarkers regulating activities of Gac fruit extracts. Therefore, the current findings suggest that Gac fruit could potentially be utilized as a therapeutic agent in the treatment of HG-related eye disease.However, additional studies are highly needed to explore the active compounds and their mechanisms of actions underlying potential of Gac parts extracts.
Conflict of interest statement
The authors have no conflicts of interest to declare related to this study.
Acknowledgements
The authors would like to acknowledge Dr Mohd Esa Hassim from the International Tropical Fruits Networks (TFNet), Malaysia for supplying the studied fruits.
Funding
This project was supported by Research Grant Number: UPM, GPIPS/2017/7956600.
 Asian Pacific Journal of Tropical Biomedicine2018年12期
Asian Pacific Journal of Tropical Biomedicine2018年12期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- A survey of biochemical and acute phase proteins changes in sheep experimentally infected with Anaplasma ovis
- Anti-hemolytic, antibacterial and anti-cancer activities of methanolic extracts from leaves and stems of Polygonum odoratum
- Anti-cancer and anti-inflammatory activities of aronia (Aronia melanocarpa) leaves
- Antidiabetic effects of Tetracarpidium conophorum seed on biomarkers of diabetesinduced nephropathy in rats
- Anti-cancer effects of hydro-alcoholic extract of pericarp of pistachio fruits
- Anti-insulin resistant effect of ferulic acid on high fat diet-induced obese mice
