近年来出现的几类新概念动物疫苗
张腾腾,罗玉子,孙元,李太元,仇华吉
近年来出现的几类新概念动物疫苗
张腾腾1,2,罗玉子1,孙元1,李太元2,仇华吉1
1 中国农业科学院哈尔滨兽医研究所 兽医生物技术国家重点实验室,黑龙江 哈尔滨 150069 2 延边大学 农学院,吉林 延吉 133002

张腾腾, 罗玉子, 孙元, 等. 近年来出现的几类新概念动物疫苗. 生物工程学报, 2018, 34(12): 1963–1973.Zhang TT, Luo YZ, Sun Y, et al. New-concept animal vaccines emerging in recent years. Chin J Biotech, 2018, 34(12): 1963–1973.
动物疫病流行广泛、传播迅速,严重危害养殖业的发展。疫苗接种是预防和控制动物传染病最有效的策略之一。目前,随着生物技术的发展和疫病防控的需要,安全、高效、广谱、用量少、具有标记特征的新型疫苗成为研发重点。文中就近年来出现的黏膜疫苗、长效与速效疫苗、嵌合疫苗、纳米颗粒疫苗等新概念动物疫苗的发展、应用及优缺点进行了评述,并提出了其发展方向,以期为动物疫苗的研发提供借鉴。
新概念疫苗,动物疫病,研究进展
动物疫病危害严重,不仅引起大批动物发病和死亡,而且影响动物及其产品的安全和国际贸易[1],甚至对人类健康造成威胁,据报道约60%–80%的人类新发传染病 (Emerging infectious diseases, EIDs) 来源于动物[2]。接种疫苗是预防和控制动物传染病最有效、最经济的策略之一[3-4]。通过接种疫苗可以降低疫病的发病率、并发症和动物的死亡率,减少经济损失[5]。
疫苗最基本的要求是安全、有效、质量可控。目前使用的动物疫苗是以减毒活疫苗和灭活疫苗为主的传统疫苗。减毒活疫苗保持了良好的抗原性,能有效地刺激机体产生保护性抗体,但该类疫苗因其能够在体内复制,因此存在毒力返强的风险[6]。灭活疫苗保持了完整的病毒结构和抗原表位,能刺激机体产生抗体,因其已失去感染性,所以具有较高的安全性。但是灭活疫苗通常需要与佐剂配伍使用,且免疫剂量高[7]、接种次数多、免疫应答持续时间短,同时还存在灭活不彻底带来的风险。这类传统疫苗缺乏鉴别诊断标记,无区分野毒感染和疫苗免疫动物 (Differentiating infected from vaccinated animals,DIVA) 的特征,不利于疫病的净化和根除。因此,研制安全、高效、广谱、用量少、具有标记特征的新型疫苗极为必要。
随着分子生物学和基因工程技术的发展,传统疫苗逐步发展到编码无毒力抗原蛋白的病毒核酸疫苗,主要包括基因缺失疫苗、重组活载体疫苗等。基因缺失疫苗是将病毒或细菌的致病性基因缺失而获得的弱毒株活疫苗,具有较好的免疫原性。利用基因工程技术手段将外源基因插入至病毒或细菌载体,从而得到重组活载体疫苗。重组活载体疫苗接种与自然感染相似,可诱导广谱的免疫应答、免疫保护力强、具有DIVA特征,因此具有较好的应用前景。但由于机体对载体的反应特性,可能会影响重组活载体疫苗再次免疫的效果。同时质粒DNA可能会插到染色体上引起突变、体内持续产生抗原蛋白易引起免疫耐受等问题也不容忽视。
目前,基因组学和结构生物学技术正被广泛应用于新型疫苗的研究。新概念疫苗多为基于保护性抗原或表位优化设计的安全、高效、广谱、用量少、具有标记特性的新型疫苗,其可以刺激机体产生高水平的体液和细胞免疫应答。基因缺失疫苗、嵌合疫苗、亚单位疫苗、纳米颗粒疫苗、部分口服疫苗和部分长效与速效疫苗均属于标记疫苗的范畴。文中对近年来新出现的黏膜疫苗、长效与速效疫苗、嵌合疫苗、纳米颗粒疫苗 (表1) 等新概念动物疫苗的发展及应用进行综述,以期为新型动物疫苗的研究提供一定的思路和策略。
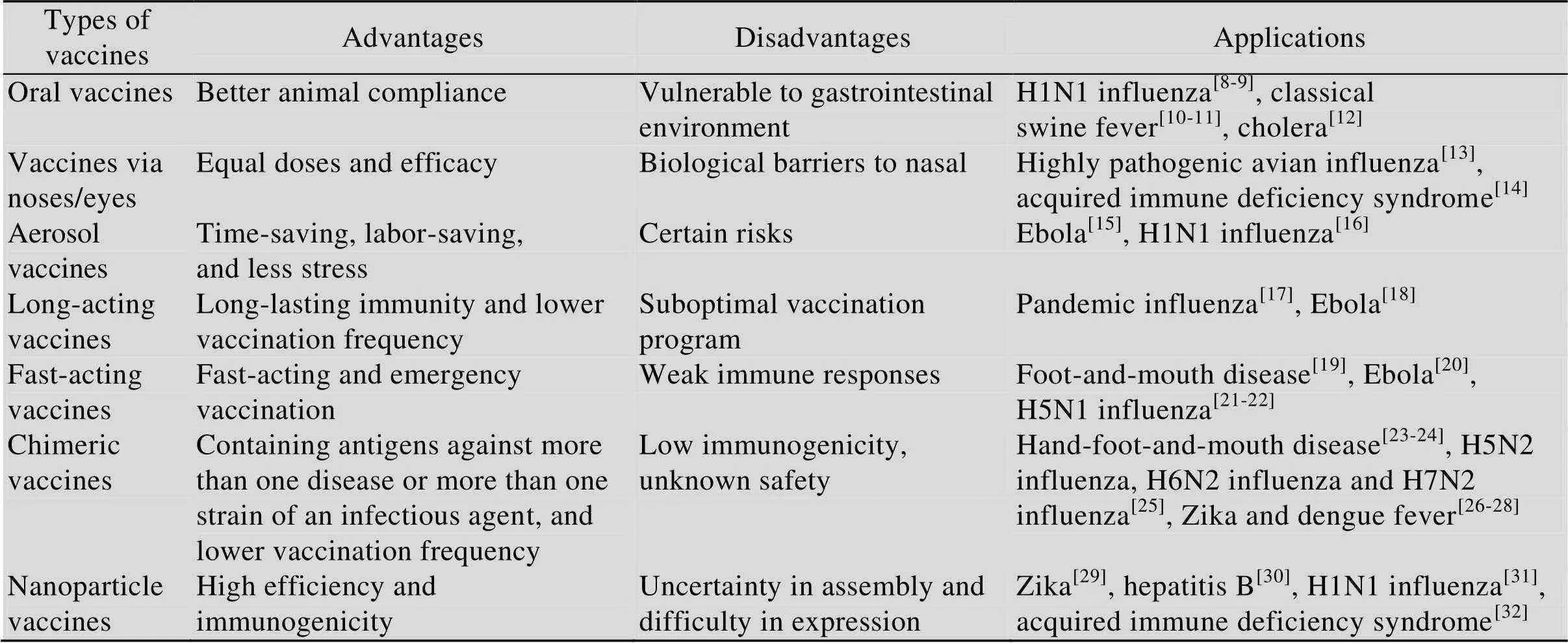
表1 几类新概念动物疫苗的利弊分析及应用
1 黏膜疫苗
黏膜覆盖了机体最大的表面积,包括口腔、呼吸道、消化道、眼腔、耳腔和泌尿生殖道等,其构成了机体与外部环境相隔的屏障。黏膜免疫是针对各种感染的第一道防御机制[33]。
黏膜疫苗通常是指通过黏膜途径接种,对机体产生保护作用的疫苗。黏膜疫苗抗原先后被粘膜上皮中的微皱褶细胞 (M细胞) 和粘膜上皮下的树突状细胞 (Dendritic cells,DCs) 所捕获,然后被递呈给CD4+T细胞和CD8+T细胞启动抗原特异性免疫应答。通过DC细胞和CD4+T细胞之间的相互作用,诱导IgA型B细胞和IgA相关细胞因子的产生,进而产生分泌型IgA (sIgA) (图1)。黏膜疫苗具有一定的生理和应用价值,如可减少针刺伤、降低成本和血源性疾病传播的风险,无痛苦,减小动物应激反应。通过口、鼻或气管内途径递送疫苗不仅可以避免已存在的免疫力对疫苗保护效果的影响,而且可以提高T细胞免疫反应[34-37]。与肠胃外免疫途径不同,黏膜免疫往往需要适当的接种途径和特定的佐剂和/或递送系统才能诱导产生有效的黏膜免疫应答[38]。
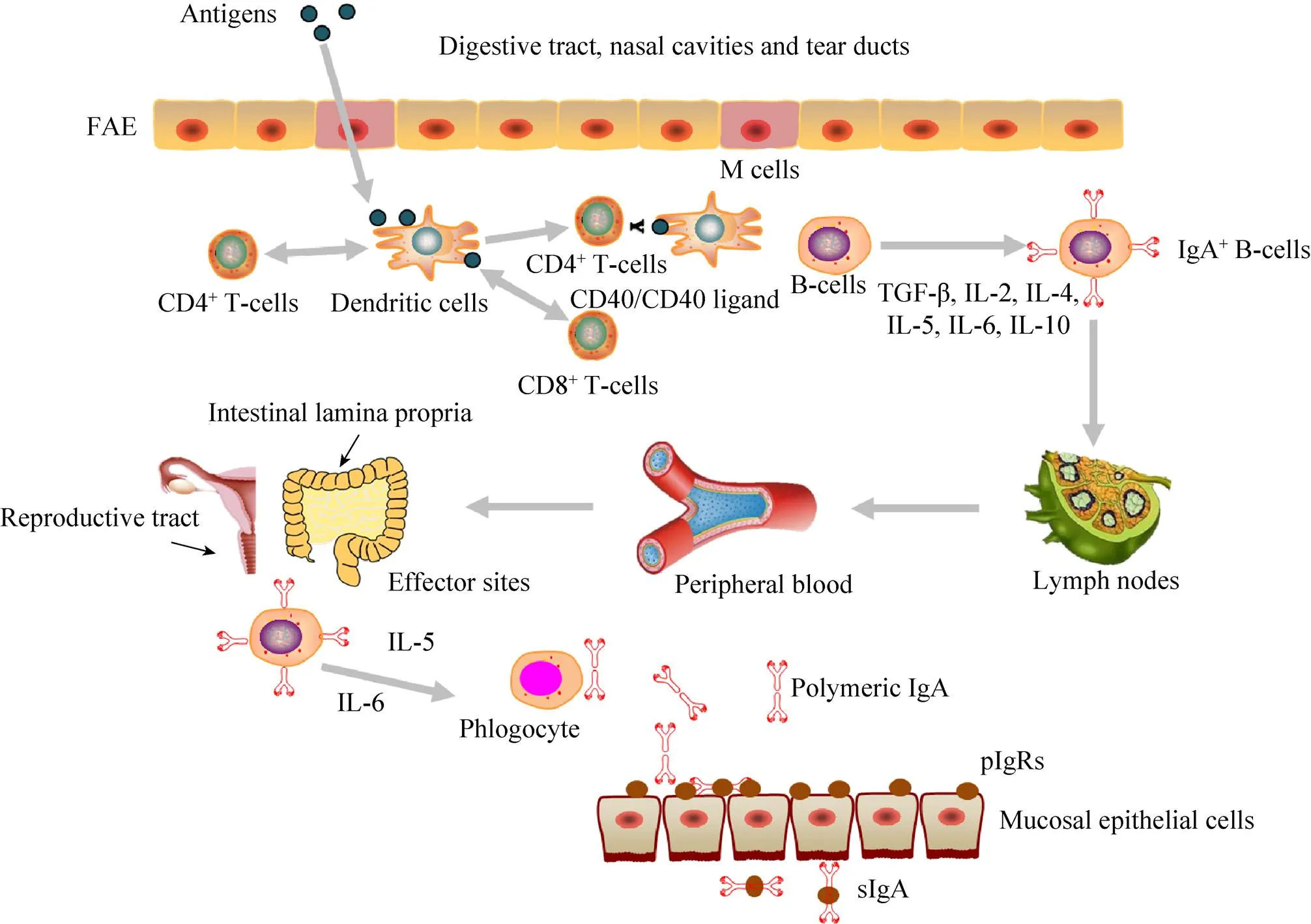
图1 黏膜免疫系统示意图(Reproductive tract改编自https://www.vcg.com/creative/811627697,Lymph nodes改编自http://www.sohu.com/a/113216020_377348)
1.1 口服疫苗
口服疫苗通过胃肠黏膜进行抗原递呈,产生与常规注射类似的免疫反应。口服疫苗能有效地诱导黏膜免疫、体液免疫和细胞免疫应答,消除注射带来的疼痛和不适以及针头相关的生物安全风险[39]。Shastri等的研究表明,口服免疫2次Eudragit S100和海藻糖组成的包含灭活A/PR/34/8 H1N1流感病毒的微粒,能够提高抗原特异性IgG、IgG1和IgG2a抗体滴度和对小鼠的保护水平[8]。小鼠口服免疫重组狂犬病病毒 (ERAG3G株) 可以得到较好的免疫保护,此外,该疫苗还具有DIVA特性,利于狂犬病的净化[40]。动物口服猪瘟疫苗包括C株疫苗、重组DNA疫苗等,均获得了良好的免疫效果[10-11]。目前,已有3种霍乱口服疫苗通过WHO认证,这3种疫苗均可提供长达5年的免疫保护作用[12]。另外,食用益生菌也可提高免疫或自然感染后的黏膜sIgA和IgG应答[41],且益生菌可刺激免疫系统,减少过敏反应。乳酸菌 (Lactic acid bacteria,LAB) 就是其中一种较为常见的益生菌。LAB作为疫苗载体能为动物提供更好的保护。小鼠口服免疫重组NC8-pSIP409-HA菌株,可产生sIgA、IgG和HI抗体,诱导CD8+T细胞免疫应答,并对H9N2流感病毒攻击提供完全的保护作用[9]。口服疫苗通过动物胃肠道时,易受胃肠道酸碱度、蛋白酶及微生物的影响,从而限制了其免疫效果,因此需要良好的投递系统才能发挥口服疫苗最优的免疫效果。已有研究表明,包被在植物细胞壁内的抗原能免受胃内酸和酶的破坏[42-45]。口服包被在植物细胞中的胰岛素或类胰岛素能够在30 min内降低血糖水平[42-43]。相信在不久的将来,口服疫苗会在动物疫病防控中发挥重大作用。
1.2 滴鼻/点眼疫苗
滴鼻/点眼疫苗通过逐只接种,能保证每只动物得到有效接种。眼内接种灭活的高致病性禽流感病毒 (Highly pathogenic avian influenza virus,HPAIV) 疫苗不仅诱导全身性系统免疫而且诱导黏膜系统产生抗体反应,保护鸡只抵抗HPAIV的攻击[13],然而其存活率仅为75%。因此,Takaki等将Pam2脂肽 (一种TLR2配体) 和双链RNA模拟poly(I:C) (一种TLR3配体) 作为点眼佐剂,在鸡结膜上应用这些TLR激动剂能引起IL-1β表达上调[46]。与其他黏膜给药途径相比,鼻腔给药能诱导更有效、更广泛的黏膜免疫反应[47]。鼻内接种疫苗在较远的黏膜部位,如女性生殖道,也能有效地激发黏膜免疫反应。但鼻内接种存在鼻腔上皮细胞生物屏障。Li等为了克服鼻腔上皮细胞阻止抗原至淋巴细胞的生物屏障作用,将编码人免疫缺陷病毒 (Human immunodeficiency virus,HIV) gp120蛋白的阴离子mRNA与阳离子环糊精-聚乙烯亚胺2k (Cyclodextrin-polyethylenimine 2k,CP 2k) 结合研制出用于鼻内接种治疗艾滋病 (Acquired immune deficiency syndrome,AIDS) 的强效聚合物,此含有CP 2k的复合物克服了上皮细胞屏障,增强了mRNA的细胞外递送效率,延长了疫苗在鼻腔内停留时间,获得较强的全身性和粘膜系统抗HIV免疫应答,产生更多的细胞因子[14],而且该复合物还具有标记特征。
1.3 气雾疫苗
气雾疫苗能刺激机体产生黏膜和全身性系统免疫应答,且省时、省力、应激小,适于大群体动物的免疫。气雾疫苗能引起比其他鼻内接种疫苗更好的免疫反应[48]。表达埃博拉病毒糖蛋白的重组人副流感病毒3型载体气雾疫苗 (HPIV3/EboGP),能够诱导产生比液体疫苗更高 (或相近) 的特异性IgG、IgA和中和抗体水平,诱导更强的细胞免疫和体液免疫反应,尤其在肺部产生更多的CD8+T细胞和CD4+T细胞[15]。抑制血细胞凝集素信号序列 (S-FLU) 的H1N1流感病毒作为气雾疫苗可诱导猪产生CD4+T和CD8+T细胞免疫应答,攻毒后产生较低的病毒滴度 (相比静脉注射)[16]。但接种气雾疫苗具有一定风险,可能会对鼻腔造成损害,而且鼻腔细胞与脑部有间接的联系,是否会对脑造成不良影响尚不明确。气雾麻疹疫苗有很好的免疫原性,但其产生的抗体阳性率低于皮下接种[49],是否是气雾疫苗未充分吸收所致也是未知的。
2 长效与速效疫苗
2.1 长效疫苗
长效疫苗可减少接种次数,减少动物的应激反应,降低免疫干扰。通过引入外源免疫增强基因、缓释型胶囊的包裹等方式研究的疫苗可为机体提供长久的免疫保护。世界卫生组织将利用微胶囊化技术来简化免疫程序,进而将使单剂量疫苗接种即获长期免疫保护作为疫苗发展的主要目标之一。
在此形势下,各国纷纷开展了长效疫苗的研究。我国科学家为了克服埃博拉病毒候选疫苗保护期短的缺点,使用表达埃博拉病毒GP蛋白的黑猩猩7型腺病毒 (AdC7-GP) 免疫小鼠,并用去除跨膜区的GP1重组蛋白 (GP1t) 作为异源增强剂以诱导长期的免疫应答。该免疫策略持久地诱导保护性抗体的产生,且在免疫后126 d血清中和抗体仍然维持较高水平,具有开发为埃博拉长效标记疫苗的潜力,对埃博拉病毒的防控具有重大意义[18]。接种埃博拉病毒糖蛋白重组水疱性口炎病毒 (Vesicular stomatitis viruses,VSV) 疫苗 (rVSV-ZEBOV),能够诱导持续1–2年之久的病毒特异性抗体应答[50],而一般的埃博拉疫苗仅能提供1–6个月的保护。长效疫苗不仅应用于埃博拉的防治,在其他疫病的防控方面也具有重大意义。利用聚乳酸-乙交酯 (PLGA) 微粒缓释外源膜囊泡基因的重组甲型流感疫苗 (M2e-rOMV) 能够为机体提供持久、高效的免疫保护,在接种后6个月仍然能维持较高的抗体滴度[17]。Liu等研制出融合潜伏相关抗原Rv2626c的分枝杆菌亚单位疫苗 (LT70),与卡介苗相比,该疫苗能够对结核分枝杆菌感染提供更长时间 (至少30 w) 的保护作用[51]。利用重组蛋白和亚单位疫苗研制的长效疫苗,均具有标记特征,有利于疫病的鉴别诊断。但是,目前对长效疫苗的研究不是特别完善,尤其是免疫程序还需要进一步的摸索。
2.2 速效疫苗
通过引入外源基因、佐剂或病毒载体研制的速效疫苗能刺激机体迅速产生免疫应答,抵抗野毒攻击。DeBuysscher等以VSV为载体设计了表达尼帕病毒 (Nipah virus,NiV) 糖蛋白 (G或F) 的速效疫苗,单剂量接种后5 d就能保护仓鼠免受致死性NiV的攻击[52],并且此类疫苗具有标记特征,可诱导有效的体液和细胞免疫应答[53]。You等将共表达猪干扰素α和干扰素γ的重组腺病毒和靶向口蹄疫病毒 (Foot-and-mouth disease virus,FMDV) 非结构蛋白区域的多种小干扰RNA (Small interfering RNA, siRNA) 组合作为疫苗,能在免疫后对FMDV的攻击提供有效保护,并且将此组合与口蹄疫疫苗共同施用,在免疫后1–3 d可对小鼠和猪提供保护作用[19]。利用人腺病毒 (HAd5) 作为载体的速效疫苗已经应用于Ebola[20]、炭疽[54]和H5N1亚型禽流感[21-22]等疫苗的研制。虽然速效疫苗能够刺激机体迅速产生免疫反应,但目前速效疫苗的免疫效果还有待进一步提高。例如,Ebola速效疫苗能更快诱导免疫应答,但诱导的应答反应低于传统疫苗,该速效疫苗可用于应对急性暴发。
3 嵌合疫苗
嵌合疫苗是应用基因工程手段构建的能同时表达两种或两种以上病原体抗原的一类新概念疫苗,应用嵌合疫苗可解决多次接种问题。嵌合疫苗通常具有DIVA特征,有利于疫病的净化和根除。嵌合肠病毒71型 (Enterovirus 71,EV-A71) 和柯萨奇病毒A16型 (Coxsackievirus A16,CVA16) 的重组病毒 (ChiEV-A71) 能诱导有效的体液和细胞免疫应答,并赋予小鼠抵抗EV-A71和CVA16攻击的能力,表明该嵌合疫苗具有潜在的预防手足口病的能力[23-24]。在DENV (Dengue virus)-2 PDK-53基因中嵌合DENV-1、DENV-3和DENV-4的prM和E基因的四价登革热嵌合疫苗能保护猴子和小鼠免受野生型DENV-1、DENV-2、DENV-3和DENV-4的攻击[27-28]。Xie等构建了DENV-2 prM-E嵌合的ZIKV和ZIKV prM-E嵌合的DENV-2,使用这些嵌合病毒免疫小鼠能够保护小鼠免受野生型DENV和ZIKV的攻击[26]。在猪瘟疫苗的研究中,Sun等[55]利用人5型复制缺陷性腺病毒载体和甲病毒复制子作为载体,成功构建了表达猪瘟病毒E2蛋白的标记疫苗 (rAdV-SFV-E2),该疫苗将腺病毒载体的高效递送能力和甲病毒复制子载体的高表达效率集于一身,可诱导比传统载体疫苗更强的免疫反应。进一步研究表明,该疫苗对小鼠、家兔和猪只安全[56],与猪蓝耳病活疫苗和伪狂犬病活疫苗同时免疫互不干扰[57]。Xia等[58]对该疫苗进一步研究证实,rAdV-SFV-E2或C株的母源抗体都不会干扰rAdV-SFV-E2的免疫效力,该疫苗已获得临床试验批复,将会对猪瘟的防控和净化具有重要价值。同样,Elaish等构建的包含流感病毒基质蛋白2胞外区的诺瓦克病毒(Norwalk virus,NV) P粒子 (M2e-PP) 能够对不同亚型HA的流感病毒攻击提供交叉保护[25]。嵌合重组DIVA疫苗H9/H5N2能够保护免疫小鼠抵抗鼠源H9N2和野生型H5N1亚型HPAIV的攻击[59]。但是表达抗原成分不完整、免疫原性较低及嵌合病毒载体的安全性等因素制约了嵌合疫苗的发展和应用。
4 纳米颗粒疫苗
纳米颗粒与细胞成分大小相似[60],很容易进入细胞。因此,不论用于疫病的预防和治疗,还是作为递送系统,或是作为一种免疫佐剂激活或增强免疫,纳米颗粒都得到了广泛的应用[61]。目前已开发出许多纳米颗粒材料作为疫苗载体 (图2),包括人造颗粒 (如金、聚合物或脂质胶束) 和生物颗粒 (如核酸、蛋白质或病毒)[61-62]。
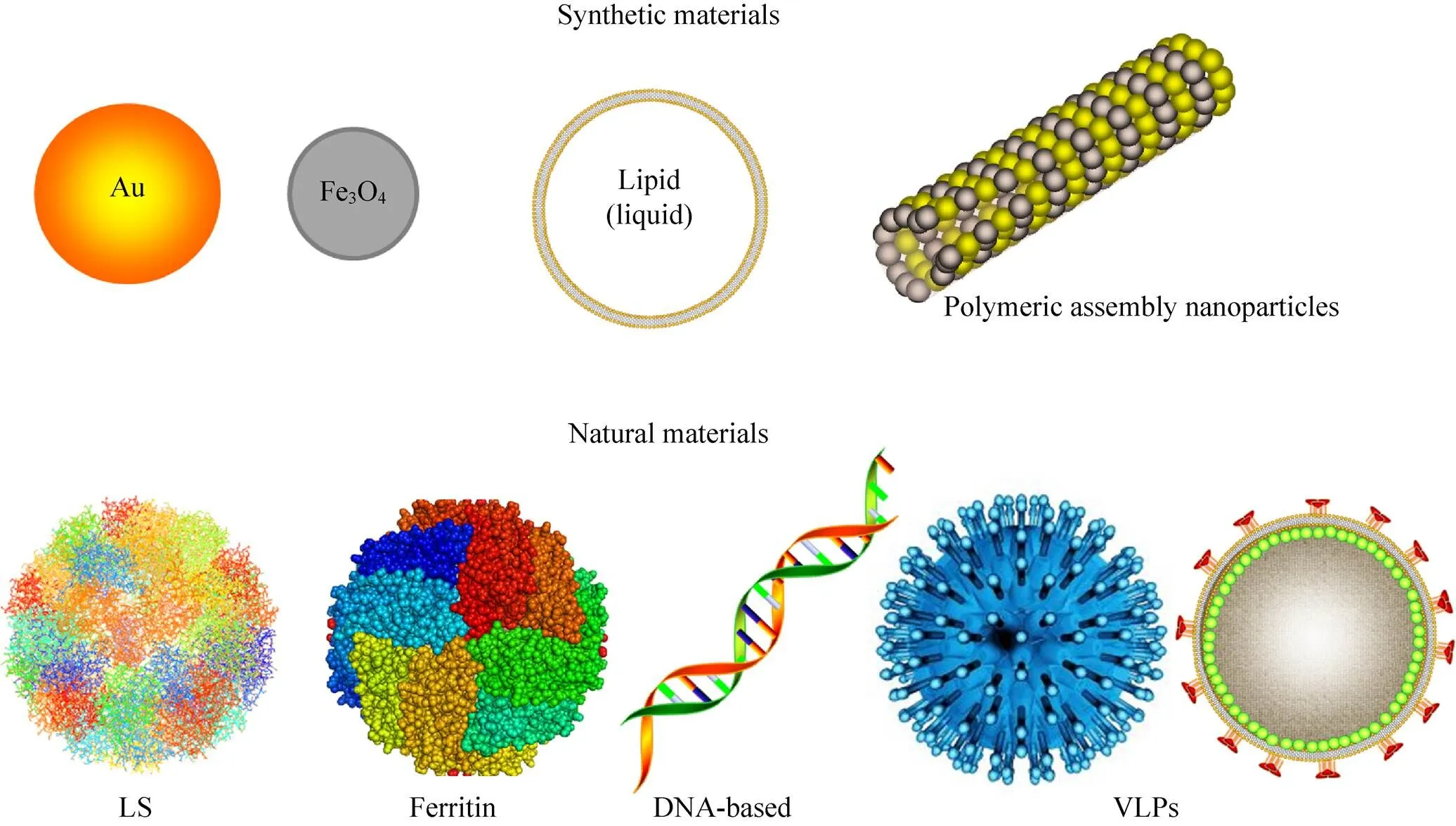
图2 纳米颗粒疫苗平台
当前纳米颗粒疫苗研究中,常用的是病毒样颗粒 (Virus-like particles,VLPs) 和自组装纳米颗粒。VLPs疫苗和自组装纳米颗粒疫苗通常仅表达病毒的主要抗原区,因此具有DIVA特征,从而有助于疫病的净化和根除。
VLPs缺乏感染性核酸,其通过衣壳蛋白自组装而形成。VLPs与天然病毒颗粒的大小和生物结构相似,这就决定了其能够诱导有效的免疫反应[63]。VLPs具有高度的免疫原性,接种VLPs能诱导啮齿动物、猕猴和黑猩猩的体液和细胞免疫应答[64-65]。舌下注射冻干的A型链球菌M蛋白J8区的VLPs (J8-VLPs) 能够诱导较强的粘膜和全身性免疫应答,产生更高水平的唾液IgA和血清IgG抗体[66]。Espinosa等研制的寨卡病毒 (Zika virus,ZIKV) VLPs疫苗能有效诱导中和抗体的产生,为动物提供保护[29]。目前已有几种商品化的VLPs疫苗,包括人乳头瘤病毒 (Human papillomavirus,HPV) 疫苗 (例如Cervarix和Gardasil) 和乙型肝炎病毒 (Hepatitis B virus,HBV) 疫苗 (Sci-B-VacTM)。此外,欧洲监管机构最近批准第一个基于VLPs的疟疾疫苗MosquirixTM。其他几种基于VLP的疫苗正在进行临床前和临床开发[30]。
铁蛋白和二氧四氢喋啶合酶 (Lumazine synthase,LS) 是自组装纳米颗粒疫苗研究中广泛使用的展示平台。铁蛋白能够自组装成直径为10 nm左右的纳米颗粒,其与流感病毒血凝素 (HA) 基因融合形成的重组蛋白可提供比三价灭活流感疫苗更优的免疫应答[31]。LS能够自组装成内径9 nm、外径15 nm左右的二十面体纳米颗粒[67]。LS纳米颗粒在AIDS的治疗[32]、DC疫苗[68]、蓖麻毒素疫苗[69]等抗原展示方面均取得了较好的效果。但是有些病毒难以正确组装成VLPs[70],例如,插入抗原表位的大小影响其组装。另外,纳米颗粒疫苗的表达系统选择较难,这些因素均制约了纳米颗粒疫苗的发展。因此,需要进一步探究纳米颗粒疫苗的表达系统、组装机制,以便研制纳米颗粒疫苗抵抗病毒感染。
5 问题与展望
基于保护性抗原或表位优化设计的新概念疫苗具有安全、高效、广谱、用量少等优点,且一般具有DIVA特征,有利于疫病净化。尽管新概念疫苗优势明显,但各有其局限性。黏膜疫苗给药方便,能刺激肠黏膜内的淋巴细胞产生大量的IgA,但其需要良好的投递系统支持;口服疫苗能增大动物的顺从性,减小应激反应,但同时易受到胃肠道酸碱度、蛋白酶及微生物的影响而降低疫苗的免疫效果;滴鼻/点眼疫苗可保证每只动物都得到相当的免疫,气雾疫苗则不能保证免疫的剂量;长效疫苗可以持续诱导免疫应答,利用微胶囊制备的缓释疫苗可将抗原递送至指定部位,防止疫苗在其他无关环节发生降解,发挥更有效的、长期的免疫作用,但其释放时间、免疫程序有待进一步研究;速效疫苗能迅速诱导免疫应答,可应用于紧急免疫接种,但其诱导的免疫应答弱于传统疫苗;嵌合疫苗可以同时表达两种或两种以上的抗原,产生更好的免疫效果,但其免疫原性低、嵌合病毒载体的安全性等问题也不容忽视;纳米颗粒疫苗能提高抗原密度并有效地进入细胞,诱导更高滴度的抗体水平,并且可以同时展示多种抗原,为研制多价、多联疫苗提供了较好的平台,但其组装仍是一个技术难题。
新概念疫苗在动物疫病的预防和治疗方面显示了较好的应用价值,临床实验也没有出现不良症状。新概念动物疫苗发展历史较短,有些疫苗的免疫效果尚未达到理想状态,而且免疫程序尚不成熟,人们对其安全性也充满担忧,因此还有较长的路要走。新概念疫苗的设计、表达系统、生产工艺、质检、免疫程序和佐剂使用等均有别于传统疫苗,仍需进一步探索。当下应该加强对疫病免疫、新概念疫苗免疫保护机制的研究,同时提高新概念疫苗的免疫效果。可采用颗粒型抗原、蛋白质工程抗原 (如表位串联、胞内表达蛋白改造等) 等,增强新概念疫苗的免疫原性;使用多抗原表达、载体系统等方式提高其免疫效率;使用免疫佐剂和免疫增强剂 (淋巴因子、微胶囊等) 等进一步提高新概念疫苗的免疫效果;探究动物天然免疫和获得性免疫的机制,了解动物的抗病毒反应机制,寻找效果更好的抗病毒分子/药物,以便为动物提供更好的保护;探索更完善的免疫程序为动物提供更全面的保护。为研制安全、高效、速效、广谱、单剂量、用量少、具有标记特征的新型疫苗,目前尚有许多工作要做。相信在不久的将来,新型疫苗会在动物疫病防治中发挥重大作用。
[1] Stephen C, Berezowski J, Misra V. Surprise is a neglected aspect of emerging infectious disease. Ecohealth, 2015, 12(2): 208–211.
[2] Wu ZQ, Li Y, Ren XW, et al. Deciphering the bat virome catalog to better understand the ecological diversity of bat viruses and the bat origin of emerging infectious diseases. ISME J, 2015, 10(3): 609–620.
[3] Pattanayak S. Use of ‘vaccine vial monitor’ in all animal and bird vaccines – a demand of the day. Explor Anim Med Res, 2013, 3(1): 5–6.
[4] Bárcena J, Blanco E. Design of novel vaccines based on virus-like particles or chimeric virions//Mateu M, Ed. Structure and Physics of Viruses. Dordrecht: Springer, 2013, 68: 631–665.
[5] Bojanić J. New achievements in vaccine development. Scr Med, 2015, 46(2): 137–142.
[6] Minor PD. Live attenuated vaccines: historical successes and current challenges. Virology, 2015, 479–480: 379–392.
[7] Zhang J, Chen XW, Tong TZ, et al. BacMam virus-based surface display of the infectious bronchitis virus (IBV) S1 glycoprotein confers strong protection against virulent IBV challenge in chickens. Vaccine, 2014, 32(6): 664–670.
[8] Shastri PN, Kim MC, Quan FS, et al. Immunogenicity and protection of oral influenza vaccines formulated into microparticles. J Pharm Sci, 2012, 101(10): 3623–3635.
[9] Shi SH, Yang WT, Yang GL, et al. Immunoprotection against influenza virus H9N2 by the oral administration of recombinantNC8 expressing hemagglutinin in BALB/c mice. Virology, 2014, 464–465: 166–176.
[10] Chenut G, Saintilan AF, Burger C, et al. Oral immunisation of swine with a classical swine fever vaccine (Chinese strain) and transmission studies in rabbits and sheep. Vet Microbiol, 1999, 64(4): 265–276.
[11] Renson P, Le Dimna M, Keranflech A, et al. CP7_E2alf oral vaccination confers partial protection against early classical swine fever virus challenge and interferes with pathogeny-related cytokine responses. Vet Res, 2013, 44(1): 9.
[12] Azman AS, Parker LA, Rumunu J, et al. Effectiveness of one dose of oral cholera vaccine in response to an outbreak: a case-cohort study. Lancet Glob Health, 2016, 4(11): e856–e863.
[13] Hikono H, Mase M, Matsuu A, et al. Intraocular vaccination with an inactivated highly pathogenic avian influenza virus induces protective antibody responses in chickens. Vet Immunol Immunopathol, 2013, 151(1/2): 83–89.
[14] Li M, Zhao MN, Fu Y, et al. Enhanced intranasal delivery of mRNA vaccine by overcoming the nasal epithelial barrierintra- and paracellular pathways. J Control Release, 2016, 228: 9–19.
[15] Meyer M, Garron T, Lubaki NM, et al. Aerosolized Ebola vaccine protects primates and elicits lung-resident T cell responses. J Clin Invest, 2015, 125(8): 3241–3255.
[16] Morgan SB, Hemmink JD, Porter E, et al. Aerosol delivery of a candidate universal influenza vaccine reduces viral load in pigs challenged with pandemic H1N1 virus. J Immunol, 2016, 196(12): 5014–5023.
[17] Watkins HC, Pagan CL, Childs HR, et al. A single dose and long lasting vaccine against pandemic influenza through the controlled release of a heterospecies tandem M2 sequence embedded within detoxified bacterial outer membrane vesicles. Vaccine, 2017, 35(40): 5373–5380.
[18] Chen T, Li DP, Song YF, et al. A heterologous prime-boost Ebola virus vaccine regimen induces durable neutralizing antibody response and prevents Ebola virus-like particle entry in mice. Antiviral Res, 2017, 145: 54–59.
[19] You SH, Kim T, Choi JH, et al. Coinjection of a vaccine and anti-viral agents can provide fast-acting protection from foot-and-mouth disease. Antiviral Res, 2017, 143: 195–204.
[20] Li JX, Hou LH, Meng FY, et al. Immunity duration of a recombinant adenovirus type-5 vector-based Ebola vaccine and a homologous prime-boost immunisation in healthy adults in China: final report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Glob Health, 2017, 5(3): e324–e334.
[21] Vemula SV, Amen O, Katz JM, et al. Beta-defensin 2 enhances immunogenicity and protection of an adenovirus-based H5N1 influenza vaccine at an early time. Virus Res, 2013, 178(2): 398–403.
[22] Scallan CD, Tingley DW, Lindbloom JD, et al. An adenovirus-based vaccine with a double-stranded RNA adjuvant protects mice and ferrets against H5N1 avian influenza in oral delivery models. Clin Vaccine Immunol, 2013, 20(1): 85–94.
[23] Zhao H, Li HY, Han JF, et al. Novel recombinant chimeric virus-like particle is immunogenic and protective against both enterovirus 71 and coxsackievirus A16 in mice. Sci Rep, 2015, 5: 7878.
[24] Lyu K, He YL, Li HY, et al. Crystal structures of yeast-produced enterovirus 71 and enterovirus 71/coxsackievirus A16 chimeric virus-like particles provide the structural basis for novel vaccine design against hand-foot-and-mouth disease. J Virol, 2015, 89(12): 6196–6208.
[25] Elaish M, Kang KI, Xia M, et al. Immunogenicity and protective efficacy of the norovirus P particle-M2e chimeric vaccine in chickens. Vaccine, 2015, 33(38): 4901–4909.
[26] Xie X, Yang Y, Muruato AE, et al. Understanding Zika virus stability and developing a chimeric vaccine through functional analysis. mBio, 2017, 8(1): e02134–16.
[27] Osorio JE, Brewoo J, Silengo SJ, et al. Efficacy of a tetravalent chimeric dengue vaccine (DENVax) in. Am J Trop Med Hyg, 2011, 84(6): 978–987.
[28] Osorio JE, Huang CYH, Kinney RM, et al. Development of DENVax: a chimeric dengue-2 PDK-53-based tetravalent vaccine for protection against dengue fever. Vaccine, 2011, 29(42): 7251–7260.
[29] Espinosa D, Mendy J, Manayani D, et al. Passive transfer of immune sera induced by a Zika virus-like particle vaccine protects AG129 mice against lethal Zika virus challenge. EBioMedicine, 2018, 27: 61–70.
[30] Mohsen MO, Zha LS, Cabral-Miranda G, et al. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin Immunol, 2017, 34: 123–132.
[31] Kanekiyo M, Wei CJ, Yassine HM, et al. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature, 2013, 499(7456): 102–106.
[32] Tian M, Cheng C, Chen XJ, et al. Induction of HIV neutralizing antibody lineages in mice with diverse precursor repertoires. Cell, 2016, 166(6): 1471–1484.
[33] Srivastava A, Gowda DV, Madhunapantula SV, et al. Mucosal vaccines: a paradigm shift in the development of mucosal adjuvants and delivery vehicles. APMIS, 2015, 123(4): 275–288.
[34] Richardson JS, Abou MC, Tran KN, et al. Impact of systemic or mucosal immunity to adenovirus on Ad-based Ebola virus vaccine efficacy in guinea pigs. J Infect Dis, 2011, 204 (Suppl 3): S1032–S1042.
[35] Bernasconi V, Norling K, Bally M, et al. Mucosal vaccine development based on liposome technology. J Immunol Res, 2016, 2016: 5482087.
[36] Richardson JS, Pillet S, Bello AJ, et al. Airway delivery of an adenovirus-based Ebola virus vaccine bypasses existing immunity to homologous adenovirus in nonhuman primates. J Virol, 2013, 87(7): 3668–3677.
[37] Choi JH, Schafer SC, Zhang LH, et al. A single sublingual dose of an adenovirus-based vaccine protects against lethal Ebola challenge in mice and guinea pigs. Mol Pharm, 2012, 9(1): 156–167.
[38] Yang KJ, Varga SM. Mucosal vaccines against respiratory syncytial virus. Curr Opin Virol, 2014, 6: 78–84.
[39] Kwon KC, Verma D, Singh ND, et al. Oral delivery of human biopharmaceuticals, autoantigens and vaccine antigens bioencapsulated in plant cells. Adv Drug Deliv Rev, 2013, 65(6): 782–799.
[40] Yang DK, Kim HH, Choi SS, et al. Oral immunization of mice with recombinant rabies vaccine strain (ERAG3G) induces complete protection. Clin Exp Vaccine Res, 2015, 4(1): 107–113.
[41] Licciardi PV, Tang ML. Vaccine adjuvant properties of probiotic bacteria. Discov Med, 2011, 12(67): 525–533.
[42] Boyhan D, Daniell H. Low-cost production of proinsulin in tobacco and lettuce chloroplasts for injectable or oral delivery of functional insulin and C-peptide. Plant Biotechnol J, 2011, 9(5): 585–598.
[43] Kwon KC, Nityanandam R, New JS, et al. Oral delivery of bioencapsulated exendin-4 expressed in chloroplasts lowers blood glucose level in mice and stimulates insulin secretion in beta-TC6 cells. Plant Biotechnol J, 2013, 11(1): 77–86.
[44] Shenoy V, Kwon KC, Rathinasabapathy A, et al. Oral delivery of Angiotensin-converting enzyme 2 and Angiotensin-(1-7) bioencapsulated in plant cells attenuates pulmonary hypertension. Hypertension, 2014, 64(6): 1248–1259.
[45] Shil PK, Kwon KC, Zhu P, et al. Oral delivery of ACE2/Ang-(1-7) bioencapsulated in plant cells protects against experimental uveitis and autoimmune uveoretinitis. Mol Ther, 2014, 22(12): 2069–2082.
[46] Takaki H, Sato H, Kurata R, et al. Cytokine responses to eye spray adjuvants for enhancing vaccine-induced immunity in chickens. Microbiol Immunol, 2016, 60(7): 511–515.
[47] Rose MA, Zielen S, Baumann U. Mucosal immunity and nasal influenza vaccination. Expert Rev Vaccines, 2012, 11(5): 595–607.
[48] Bolton DL, Song KM, Tomaras GD, et al. Unique cellular and humoral immunogenicity profiles generated by aerosol, intranasal, or parenteral vaccination in rhesus macaques. Vaccine, 2017, 35(4): 639–646.
[49] Low N, Bavdekar A, Jeyaseelan L, et al. A randomized, controlled trial of an aerosolized vaccine against measles. N Engl J Med, 2015, 372(16): 1519–1529.
[50] Huttner A, Agnandji ST, Combescure C, et al. Determinants of antibody persistence across doses and continents after single-dose rVSV-ZEBOV vaccination for Ebola virus disease: an observational cohort study. Lancet Infect Dis, 2018,18(7):738–748.
[51] Liu X, Peng JX, Hu L, et al. A multistage mycobacterium tuberculosis subunit vaccine LT70 including latency antigen Rv2626c induces long-term protection against tuberculosis. Hum Vaccin Immunother, 2016, 12(7): 1670–1677.
[52] DeBuysscher BL, Scott D, Marzi A, et al. Single-dose live-attenuated Nipah virus vaccines confer complete protection by eliciting antibodies directed against surface glycoproteins. Vaccine, 2014, 32(22): 2637–2644.
[53] Geisbert TW, Feldmann H. Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections. J Infect Dis, 2011, 204(Suppl 3): S1075–S1081.
[54] Wu SP, Zhang Z, Yu R, et al. Intramuscular delivery of adenovirus serotype 5 vector expressing humanized protective antigen induces rapid protection against anthrax that may bypass intranasally originated preexisting adenovirus immunity. Clin Vaccine Immunol, 2014, 21(2): 156–164.
[55] Sun Y, Li HY, Tian DY, et al. A novel alphavirus replicon-vectored vaccine delivered by adenovirus induces sterile immunity against classical swine fever. Vaccine, 2011, 29(46): 8364–8372.
[56] Sun Y, Tian DY, Li S, et al. Comprehensive evaluation of the adenovirus/alphavirus-replicon chimeric vector-based vaccine rAdV-SFV-E2 against classical swine fever. Vaccine, 2013, 31(3): 538–544.
[57] Xia SL, Sun Y, Lei JL, et al. Effects of co-administered pseudorabies Bartha-K61 vaccine on the efficacy of an adenovirus/alphavirus replicon chimeric vectored vaccine rAdV-SFV-E2. Chin J Prevent Vet Med, 2016, 38(8): 642–645 (in Chinese). 夏水利, 孙元, 雷建林, 等. 同时接种伪狂犬病活疫苗Bartha-K61株对嵌合载体猪瘟疫苗rAdV-SFV-E2免疫效力的影响. 中国预防兽医学报, 2016, 38(8): 642–645.
[58] Xia SL, Xiang GT, Lei JL, et al. Efficacy of the marker vaccine rAdV-SFV-E2 against classical swine fever in the presence of maternally derived antibodies to rAdV-SFV-E2 or C-strain. Vet Microbiol, 2016, 196: 50–54.
[59] Kim SM, Kim YI, Park SJ, et al. Vaccine efficacy of inactivated, chimeric hemagglutinin H9/H5N2 avian influenza virus and its suitability for the marker vaccine strategy. J Virol, 2017, 91(6): e01693–16.
[60] Treuel L, Jiang X, Nienhaus GU. New views on cellular uptake and trafficking of manufactured nanoparticles. J R Soc Interface, 2013, 10(82): 20120939.
[61] Zhao L, Seth A, Wibowo N, et al. Nanoparticle vaccines. Vaccine, 2014, 32(3): 327–337.
[62] Gregory AE, Titball R, Williamson D. Vaccine delivery using nanoparticles. Front Cell Infect Microbiol, 2013, 3: 13.
[63] Gai WW, Zheng XX, Wang C, et al. Marburg virus-like particles produced in insect cells induce neutralizing antibodies in rhesus macaques. J Med Virol, 2017, 89(12): 2069–2074.
[64] Warfield KL, Aman MJ. Advances in virus-like particle vaccines for filoviruses. J Infect Dis, 2011, 204(Suppl 3): S1053–S1059.
[65] Warfield KL, Goetzmann JE, Biggins JE, et al. Vaccinating captive chimpanzees to save wild chimpanzees. Proc Natl Acad Sci USA, 2014, 111(24): 8873–8876.
[66] Seth A, Kong IG, Lee SH, et al. Modular virus-like particles for sublingual vaccination against group A streptococcus. Vaccine, 2016, 34(51): 6472–6480.
[67] Zhang XF, Meining W, Fischer M, et al. X-ray structure analysis and crystallographic refinement of lumazine synthase from the hyperthermophileat 1.6 Å resolution: determinants of thermostability revealed from structural comparisons. J Mol Biol, 2001, 306(5): 1099–1114.
[68] Ra JS, Shin HH, Kang S, et al. Lumazine synthase protein cage nanoparticles as antigen delivery nanoplatforms for dendritic cell-based vaccine development. Clin Exp Vaccine Res, 2014, 3(2): 227–234.
[69] Wei YJ, Wahome N, VanSlyke G, et al. Evaluation of lumazine synthase fromas a presentation platform for polyvalent antigen display. Protein Sci, 2017, 26(10): 2059–2072.
[70] Martinez-Torrecuadrada JL, Castón JR, Castro M, et al. Different architectures in the assembly of infectious bursal disease virus capsid proteins expressed in insect cells. Virology, 2000, 278(2): 322–331.
(本文责编 郝丽芳)
New-concept animal vaccines emerging in recent years
Tengteng Zhang1,2, Yuzi Luo1, Yuan Sun1, Taiyuan Li2, and Hua-Ji Qiu1
1 State Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Harbin 150069, Heilongjiang, China 2 Agricultural College of Yanbian University, Yanji 133002, Jilin, China
Animal infectious diseases pose a serious and continuing threat to the animal health and cause huge economic losses throughout the world. Vaccination is one of the most effective solutions to prevent and control animal infectious diseases. With the development of biotechnologies and the need for disease prevention and control, the focus of vaccine research has been shifted to the development of safe, efficient, broad-spectrum, low-dose and marker vaccines. Novel vaccines capable of inducing high levels of both humoral and cellular immune responses are promising to provide more efficient protection against animal infectious diseases. This minireview summarizes the development, applications, advantages and disadvantages of new-concept animal vaccines emerging in recent years, including mucosal vaccines, long-acting and fast-acting vaccines, chimeric vaccines, nanoparticle vaccines, and so on. Furthermore, we discuss future directions of the vaccines, in order to provide new insights for animal vaccine development.
new-concept vaccines, animal infectious diseases, research advances
March 10, 2018;
May 31, 2018
National Key Research and Development Program of China (No. 2017YFD0501105).
Hua-Ji Qiu. Tel/Fax: +86-451-51051708; E-mail: qiuhuaji@caas.cn
国家重点研发计划项目 (No. 2017YFD0501105) 资助。
2018-06-25
10.13345/j.cjb.180078
http://kns.cnki.net/kcms/detail/11.1998.Q.20180622.1436.003.html

