Pyrene-functionalized Core-shell Magnetic Silica Nanospheres for Optical Sensing and Removal of Hg2+ in Aqueous Solution
WANG Yan-yan, TANG Mei-yao, SHN He, CH Guang-bo , SU Bin (1. , , , 130103, ; 2. , , 136000, ; 3. , , , 130103, ; 4. , , 136000, ; . , , 136000, ) * , -: 861206@163.
Abstract: A recyclable mercury(Ⅱ) optical sensor based on pyrene-functionalized core-shell magnetic silica nanosphereswas developed and demonstrated by sol-gel grafting reaction. The obtained multifunctional microspheres displayed excellent fluorescence sensitivity and selectivity towards Hg2+ over other competing metal ions. A good linearity Stern-Volmer working plot (R2=0.998 3) between the fluorescence intensity of multifunctional microspheres and the concentration of Hg2+ was constructed with a satisfactory detection limit of 2.3 × 10-8 mol·L-1. Their fluorescence response in the presence of Hg2+ is found to be almost reversible when treated by EDTA solution. Moreover, these pyrene-functionalized magnetic silica nanospheres can efficiently remove Hg2+ in aqueous solution and easily separated by appling an external magnetic field. These results indicate that functionalized core-shell magnetic silica microspheres are potentially promising materials for simultaneously detecting and removing environmental pollutants.
Key words: optical sensor; magnetic silica nanospheres; recyclable; mercury removal
1 Introduction
Mercury contamination has received considerable attention owing to their potential implications in human health and natural ecosystem[1-3]. Even at very low concentration, it causes severe problems because marine aquatic organisms convert inorganic mercury into neurotoxic methylmercury which bioaccumulates through the food chain[4-6]. Concerns over the toxicity of mercury have extensively pushed explorations aimed at developing selective and efficient methods for detection and removal of Hg2+.
Among various detection techniques, optical sensors are particularly attractive for monitoring Hg2+in environmental analysis since they do not consume analytes, and allow on site and real-time detection without the use of expensive or complicated instrumentations[7-11]. As for optical sensors, suitable fluorescence indicators need to be selected as molecular recognition materials. Pyrene derivatives are preferable as fluorescence indicators in mercury optical sensors owing to some important advantages, such as a large Stokes shift, high quantum yield, excellent photostability, and are relatively nontoxic[12-15]. However, there are some inevitable limitations for their practical applications, such as poor solubility in aqueous solution and difficult to be separated and recovered, as well as removal of mercury ion from the sample.
In recent years, the integration of the fluorescence indicators onto nanomaterials offers a promising approach for simple and efficient separation and detection of toxic metal ions in environmental, biological, and toxicological areas[16-20]. As an important family of advanced nanomaterials, core-shell magnetic silica nanoparticles are of great interest for biomedical and environmental research applications because they are biocompatible, easily renewable, and are stable against degradation[21-25]. The good optical transparency in visible region and the favorable biocompatibility make magnetic silica nanoparticles match the desirable of optical sensors. Furthermore, their low-cost, easy recyclabilityviaexternal magneticeld and suitable chemical treatments make them convenient to remove pollutants and toxic metal ions from samples. In this context, the use of core-shell magnetic silica nanoparticles as solid supports coupled with pyrene derivatives are considered to be a feasible way for fabricating both a chemosensor and adsorbent for Hg2+in heterogeneous solid-liquid phases.
Herein, we report the preparation of pyrene-functionalized core-shell magnetic nanoparticles (denoted as Py-Fe3O4@SiO2), and their applications for the detection and separation of mercury ions. The obtained core-shell nanoparticle is a highly sensitive and selective chemosensor for mercury ions over other competing metal ions. The Py-Fe3O4@SiO2exhibit a good linearity Stern-Volmer characteristics and a satisfactory detection limit. Their fluorescence responses are stable over a broad pH range. Separation experiments further established that it can be used to separate and remove Hg2+from real sample. More importantly, the resultant nanostructured optical sensor could be easily collected and separated by applying an external magnetic field and reused at least five times.
2 Experiments
2.1 Materials
Ferric trichloride, anhydrous sodium acetate, sodium citrate, ammonia solution (28%, mass fraction), ethylene glycol, concentrated HCl, mercury nitrate and the other inorganic metal salts were purchased from Shanghai Chemical Company. Tetraethoxysilane(TEOS, Tianjin Chemicals Co.), 3-(triethoxysilyl)-propyl isocyanate(TESPIC, Aldrich), 4-hydroxy-benzaldehyde(Aldrich) and 1-pyrenecarboxaldehyde(Aldrich) were used as received. 1-(4′-hydroxyphenyl)-4-pyrenyl-2,3-diaza-1,3-butadiene(Py-OH) was synthesized and purified according to reported procedure[26-27]. The solvent toluene and tetrahydrofuran were used after desiccation with anhydrous sodium sulfate. Analytical grade solvents and compounds were used without further purifications for preparation.
2.2 Synthesis of Magnetic Sillia Nanoparticles (Fe3O4@SiO2)
First, the spherical magnetic particles were prepared through asolvothermal reaction. Typically, 1.35 g of FeCl3·6H2O, 3.6 g of NaAc, and 0.40 g of sodium citrate were dissolved in 40 mL of ethylene glycol. The mixture were stirred vigorously for 0.5 h to form a homogeneous yellow solution and then transferred into a 50 mL Teflon-lined stainless-steel autoclave. The autoclave was heated at 200 ℃ and maintained for 24 h. After that, it was cooled to room temperature. The product was centrifuged, washed repeatedly with ethanol and distilled water, and then dried in vacuum at 60 ℃ overnight. Then, the Fe3O4@SiO2were synthesized as follows: Fe3O4nanoparticles(0.05 g) were treated with a mixture of ethanol(40 mL), deionised water(10 mL) and concentrated ammonia solution(0.5 mL, 28%, mass fraction) under ultrasonication for 0.5 h. TEOS(0.03 g) was added dropwise to the above solution. After being mechanically stirred for 6 h at room temperature, the products were separated, washed repeatedly with ethanol and distilled water, and then dried in vacuum at 80 ℃ for 24 h.
2.3 Synthesis of Pyrene-functionalized Magnetic Sillia Nanoparticles(Py-Fe3O4@SiO2)
First, pyrene-based receptor Py-Si was prepared by treating Py-OH with TESPIC according to our previous reported procedure[28]. Py-OH(0.348 g, 1.00 mmol) was dissolved in 50 mL chloroform and excess TESPIC(2 mL, 6.37 mmol) was added dropwise to the solution. After the solution was heated at 60 ℃ for 48 h, cold dried hexane was added to precipitate a yellow solid from the mixture. The final filtered-off precipitate was washed with cold dried hexane for several times and dried in vacuum. Then, equal mass of Py-Si and activated Fe3O4@SiO2were suspended in anhydrous toluene(50 mL) and stirred under reux in N2atmosphere for 48 h. Then, the precipitate wasltered, and adequately washed several times with toluene and THF to rinse away any surplus Py. The final product was produced and denoted as Py-Fe3O4@SiO2.
2.4 Characterization
Transmission electron microscopy(TEM) images were taken with a JEM-2010 transmission election microscope made by Japanese JEOL Company. Scanning electronic microscopy(SEM) images were recorded on a Hitachi S-4800 microscope. Fourier-transform infrared(FT-IR) spectra were collected on PerkinElmer Frontier using KBr pellets. Powder X-ray diffraction(XRD) patterns were recorded on a Bruker D4 X-ray diffractometer(Germany) with Ni-ltered Cu K radiation(40 kV, 40 mA). Magnetization measurements were performed on a MPM5-XL-5 superconducting quantum interference device (SQUID) magnetometer at 300 K. The removal ability of Py-Fe3O4@SiO2for Hg2+in water was measured using an inductively coupled plasma(ICP) spectrometer(Perkin Elmer). All spectrophotometric spectra of the hybrid material were performed with a suspension of sample dispersed in deionized water and then brought into a quartz cell for measurement. All photoluminescence(PL) spectra were measured with a Hitachi F-4600uorescence spectrophotometer. All spectroscopic measurements were performed at least triplicate.
3 Results and Discussion
3.1 Synthesis and Characterization of Py-Fe3O4@SiO2

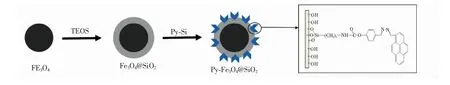
Fig.1 Synthesis procedures for the functionalized nanomaterial Py-Fe3O4@SiO2
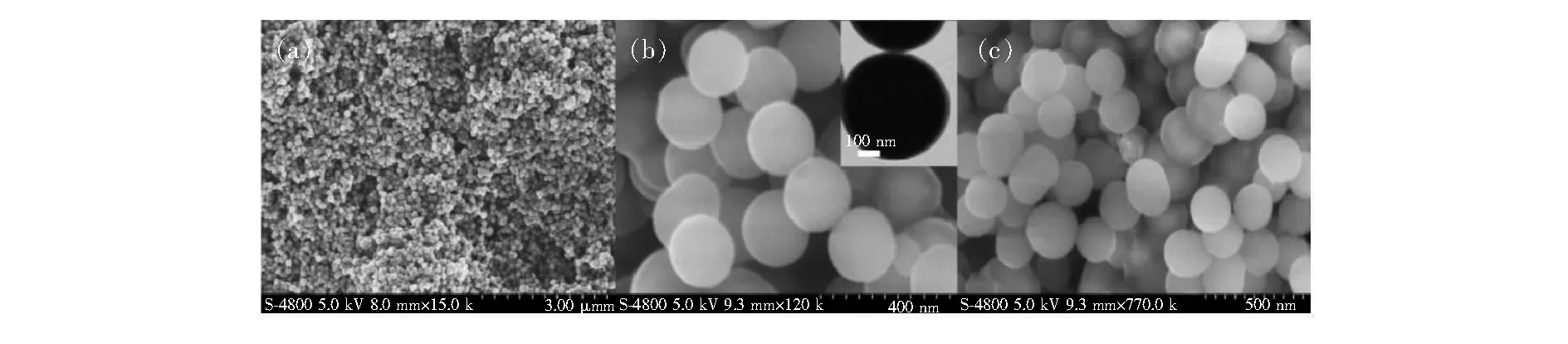
Fig.2 SEM images of the Fe3O4 particles (a), Fe3O4@SiO2 (b), and Py-Fe3O4@SiO2 (c), and TEM images of Fe3O4@SiO2 (inset up of (b)).
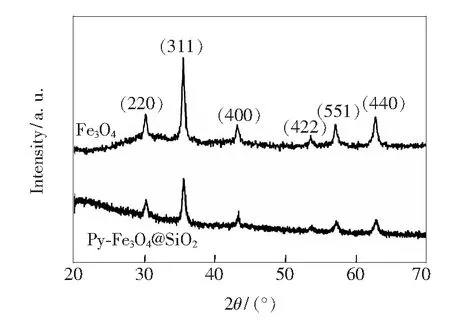
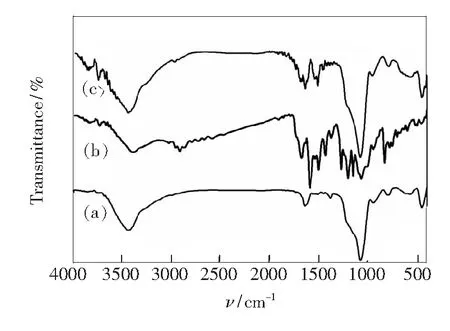
Fig.3 Wide-angle XRD patterns of Fe3O4 and Py-Fe3O4@SiO2 Fig.4 FT-IR spectra of Fe3O4@SiO2 (a), Py-Si(b), and Py-Fe3O4@SiO2 (c).
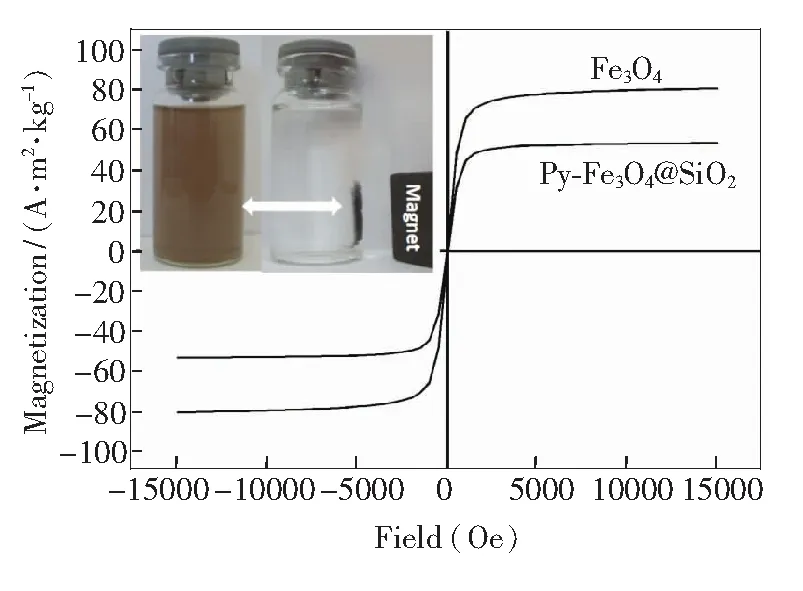
Fig.5 Magnetic hysteresis loops of pure Fe3O4 and Py-Fe3O4@SiO2, and the magnetic separation-redispersion process of Py-Fe3O4@SiO2 (inset down).
3.2 Optical Sensing Performance of Py-Fe3O4@SiO2 for Hg2+
To access the optical sensing performance of Py-Fe3O4@SiO2, we have measured its fluorescence in aqueous solution as function of Hg2+concentration. In the fluorescence spectrum, the Py-Fe3O4@SiO2exhibits a weak emission at 459 nm(λex=365 nm) in the free state. Upon addition of increasing concentrations of mercury ions, the fluorescence intensity is gradually enhanced, as shown in Fig.6. The quenching of pyrene moiety could be ascribed to a photo-induced electron transfer(PET) mechanism by the lone pair of nitrogen atoms in the free state[33-35]. After complexation with Hg2+, the lone pairs no longer participate in the quenching process, thus causing the recovery of pyrene fluorescence. We have recorded the working curve by plotting the ratio of the emission intensities(F/F0)versusmercury ion concentration. A good linearity betweenF/F0and Hg2+concentration(0.1 μmol/L to 10 μmol/L) is constructed with a linearly dependent coeffcientR2of 0.998 3(Fig.6, inset). The detection limit of Py-Fe3O4@SiO2for monitoring Hg2+ion is estimated to be about 2.3 × 10-8mol/L from the titration results under optimized conditions. To get a view on the selectivity of Py-Fe3O4@SiO2towards Hg2+over other common competitive ionic species, we have tested theuorescence spectra upon the addition of these metal ions(at 10-3mol/L) to a suspension of Py-Fe3O4@SiO2with and without Hg2+. Fig.7 illustrates the fluorescence responses of hybrid materials to various metal ions and its selectivity toward Hg2+. No significant spectral changes of the suspension solution are observed in the presence of these metal ions. Only with the addition of Hg2+(10-6mol/L) ions can produce a dramatic increasing in fluorescence intensity, indicating that the sensing of Hg2+is not disturbed by the competitive ions. Moreover, we examine the fluorescence emission intensities of Py-Fe3O4@SiO2with and without Hg2+as a function of pH, as shown in Fig.8. The suitable range of pH for Hg2+determination is found to be between pH 4.0 and 9.0, suggesting that Py-Fe3O4@SiO2is suitable and qualified enough for practical applications.
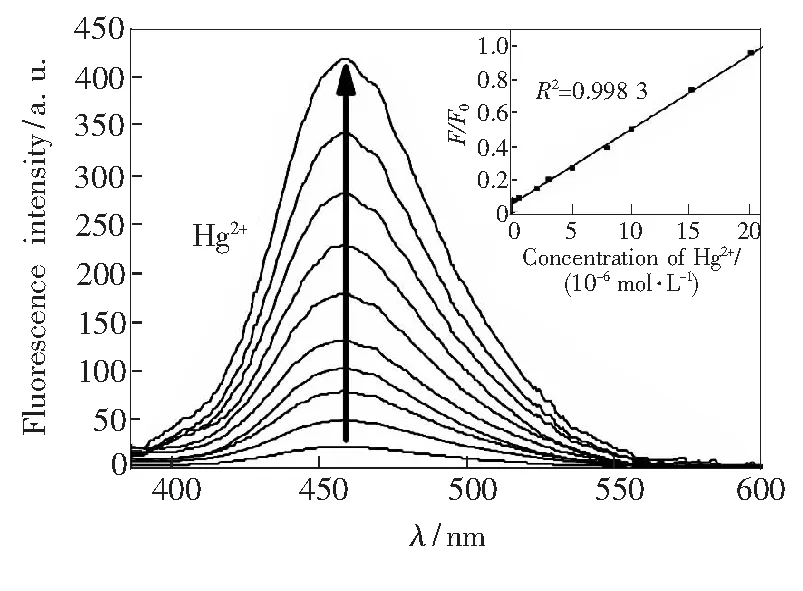
Fig.6 Fluorescence spectra of Py-Fe3O4@ SiO2 (20 mg·L-1) in the presence of various amounts of Hg2+ in aqueous solution. Inset: the plot of normalized fluorescence intensity F/F0 of Py-SBA-15(20 mg·L-1) as a function of the Hg2+ from 0.5 to 20 μmol/L in aqueous solution. Excitation at 365 nm, and emission intensities at 459 nm.
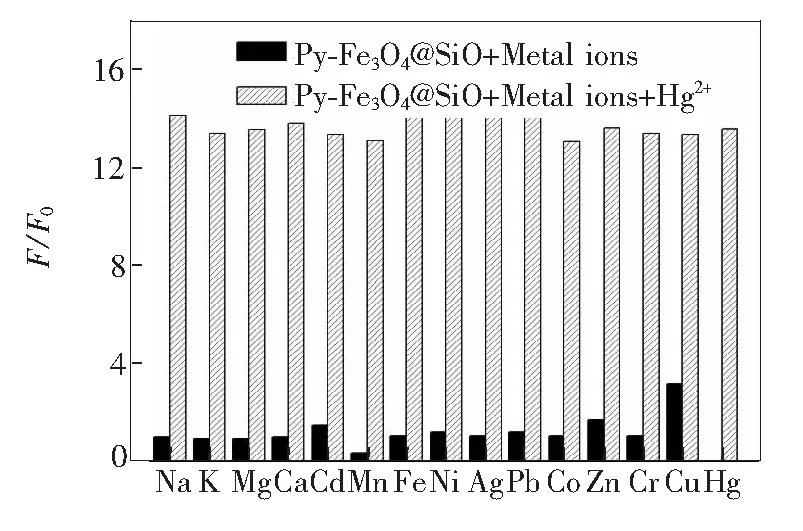
Fig.7 Normalized fluorescence responses of Py-Fe3O4@SiO2 (20 mg·L-1) to common competitive ionic species in aqueous solution. the black bars stand for the responses of Py-Fe3O4@SiO2 upon the addition of various interfering ions (all at 10-3 mol/L). The white bars stand for the responses that occurs once the subsequent addition of 10-6 mol/L of Hg2+ to the above solution. The intensities were recorded at 459 nm, excitation at 365 nm.

Fig.8 Fluorescence intensity changes of Py-Fe3O4@SiO2 (20 mg·L-1) in aqueous solution with and without 10-6 mol·L-1 Hg2+ as a function of pH. Excitation at 365 nm, and emission intensities at 459 nm.
3.3 Recyclable and Removal Abilities of Py-Fe3O4@SiO2
The recyclable ability is an important characteristic feature and strongly desirable for practical applications. The reusability of Py-Fe3O4@ SiO2is clearly demonstrated in Fig.9. Once adding specific concentration of EDTA, its fluorescence intensity was found to be fully recovered since EDTA can remove the Hg2+, which further affirming the complexation sensor mechanism. Py-Fe3O4@ SiO2exhibits good regenerative ability since almost no loss in sensitivity is observed after 5 repeated cycles. More importantly, this fluorescent change being complete within a few seconds allows for rapid recovery of sensing function. Next, we evaluated the effectiveness of Py-Fe3O4@SiO2in the removal of Hg2+. An aliquot (1 mL) of tap water containing Hg2+(10×10-8) was treated with 20 mg of Py-Fe3O4@SiO2. To determine the amount of Hg2+separated by the magnetic silica nanoparticles, the amount of Hg2+left in theltrate was analyzed by ICP-MS. It is found that no residual levels of Hg2+were detectable after treatments, revealing that the designed magnetic silica nanoparticle removed almost all of Hg2+. Thus, it can be a potentially useful and effective agent for the selective separation and rapid removal of Hg2+.
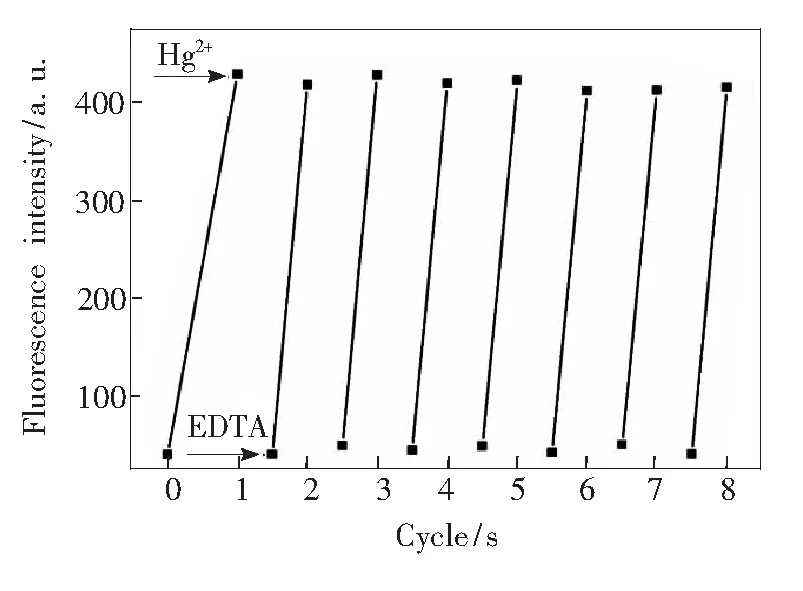
Fig.9 Fluorescence responses of Py-Fe3O4@SiO2 (20 mg·L-1) by alternated addition of 50 ml of Hg2+ (1.0 mmol·L-1), followed by magnetic separation and addition of 100 mmol·L-1 of EDTA (sodium salt). Excitation at 365 nm, and emission intensities at 459 nm. The cyclic index is the number of alternating cycles.
4 Conclusion
In conclusion, we have demonstrated pyrene-functionalized Fe3O4@SiO2core-shell magnetic nanoparticles that can act as a reproducible optical sensor and reusable absorbent for efficient detection and separation of Hg2+in aqueous solutions over the pH range of 4-9. The hybrid material exhibits a high affinity and selectivity for Hg2+over the other competing metal ions, and can successfully remove Hg2+in tap water. These results indicate considerable promise for the combination of well-dened inorganic nanomaterials and organic receptors can play a vital role for study of the environmental function of toxic metal contaminants.

