有机锡5-甲基/氨基-1H-四唑乙酸酯的合成、结构与抗肿瘤活性
谢运甫 于 洋 唐良富*,
(1天津科技大学理学院,天津 300457)
(2南开大学化学学院,元素有机化学国家重点实验室,天津 300071)
The chemistry of organotin carboxylates has flourished for decades,owing to their remarkable structural diversity[1]and significant biological activity[2-3],for example as antibacterial and antitumor agents,as well as other potential applications in catalysis[4-5].Recently,carboxylic acids with additional donor atoms have proved their values in the capability of affecting the coordination modes of tin atom as well as decent bioactivities,and therefore attracted a great deal of attention.Lots of organotin carboxylates derived from S-or N-functionalized carboxylic acids have been synthesized and characterized in recent years,which displayed fascinating structural features[6-11]and excellent biological activities[12-15].On the other hand,tetrazole has been extensively exploited as ligand in coordination chemistry because of its variable coordination modes,and its derivatives have prominent versatile biological activities[16-17].Our previous work showed that organotin derivatives of tetrazolyl-1-acetic acid exhibited considerable structural diversity and good antifungal activity[12,18].As an extension of our investigations on biologically active organotin derivatives,we herein report the syntheses,structures and antitumor activities in vitro of organotin 5-substituted 1H-tetrazolyl-1-acetates.
1 Experimental
NMR spectra were obtained with a Bruker 400 spectrometer,and the chemical shifts were reported with respect to reference standards(internal SiMe4for1H and13C NMR spectra,external SnMe4for119Sn NMR).IR spectra were obtained from a Tensor 27 spectrometer as KBr discs.Elemental analyses were carried out on an Elementar Vario EL analyzer.Melting points were measured with an X-4 digital micro melting-point apparatus and were uncorrected.5-Methyl-1H-tetrazolyl-1-acetic acid (L1)and 5-amino-1H-tetrazolyl-1-acetic acid (L2)[19]were prepared according to the published methods.Organotin oxide and organotin hydroxide are commercially available and used as received without further purification.
1.1 Synthesis of 1
The mixture of 5-methyl-1H-tetrazolyl-1-acetic acid (0.28 g,2 mmol)and (Ph3Sn)2O (0.72 g,1 mmol)in anhydrous benzene (40 mL)was stirred and heated at reflux for 8 h.The reaction mixture was filtered off while hot,and the filtrate was concentrated to give the crude product,which was recrystallized from benzene/hexane to afford colorless crystals of 1.Yield:80%(0.79 g),m.p.96~98 ℃.1H NMR (CDCl3):δ2.34 (s,3H,CH3),5.08 (s,2H,CH2),7.45~7.55 (m,9H,C6H5),7.60~7.74 (m,6H,C6H5).13CNMR (CDCl3):δ8.8 (CH3),48.5 (CH2),128.5,129.0 (3J(13C-119/117Sn)=64.3Hz),130.9,136.9 (2J(13C-119/117Sn)=48.9Hz)(C6H5),152.6 (CN4),169.9(COO).119Sn NMR (CDCl3):δ-82.7.IR (cm-1):νas(COO)1 674,νs(COO)1 393.Anal.Calcd.for C22H20N4O2Sn(%):C 53.80,H 4.10,N 11.41;Found(%):C 53.99,H4.31,N 11.07.
1.2 Synthesis of 2
This complex was obtained similarly using tricyclohexyltin hydroxide instead of (Ph3Sn)2O as described above for 1,but in a 1∶1 molar ratio.Yield:68%,m.p.133~135 ℃.1H NMR (CDCl3):δ1.24~1.42(m,9H),1.60~1.73 (m,15H),1.81~1.98 (m,9H)(C6H11),2.55 (s,3H,CH3),5.06 (s,2H,CH2).13CNMR(CDCl3): δ8.9 (CH3),26.8,28.8 (3J(13C-119/117Sn)=63.8 Hz),30.9 (2J(13C-119/117Sn)=14.6 Hz),34.5,48.7 (CH2),152.3 (CN4),168.8 (COO).1H NMR (acetone-d6):δ1.11~1.20 (m,9H),1.46~1.58 (m,16H),1.73~1.80 (m,8H)(C6H11),2.41 (s,3H,CH3),5.10 (s,2H,CH2).13CNMR(acetone-d6):δ7.3 (CH3),47.8 (CH2),26.0 (4J(13C-119/117Sn)=7 Hz),28.1,30.1 (2J(13C-119/117Sn)=18 Hz),34.4 (1J(13C-119/117Sn)=359,343 Hz)(C6H11),151.9 (CN4),168.7 (COO).119Sn NMR (acetone-d6):δ17.7.IR (cm-1): νas(COO)1 663,νs(COO)1 351.Anal.Calcd.for C22H38N4O2Sn(%):C 51.88,H 7.52,N 11.00;Found(%):C 51.60,H 7.55,N 10.78.
1.3 Synthesis of 3
The mixture of 5-amino-1H-tetrazolyl-1-acetic acid (0.29 g,2 mmol)and (Ph3Sn)2O (0.72 g,1 mmol)in anhydrous benzene (40 mL)was stirred and heated at reflux for 8 h.After cooling to room temperature,the solid was filtered off and recrystallized from acetone/benzene to afford white crystals of 3.Yield:66% (0.65 g),m.p.165~166℃.1H NMR (DMSO-d6):δ 4.79 (s,2H,CH2),6.59 (s,2H,NH2),7.39~7.48 (m,9H),7.70~7.92 (m,6H) (C6H5).13C NMR (DMSO-d6):δ 47.4 (CH2),128.3 (3J(13C-119/117Sn)=70 Hz),129.0,136.2(2J(13C-119/117Sn)=47 Hz),142.5 (C6H5),155.8 (CN4),168.8(COO).119Sn NMR (DMSO-d6): δ -262.2.IR (cm-1):ν(NH2)3 403 and 3 192,νas(COO)1 624,νs(COO)1 384.Anal.Calcd.for C21H19N5O2Sn(%):C 51.25,H 3.89,N 14.23;Found(%):C 51.13,H 4.14,N 14.18.
1.4 Synthesis of 4
This complex was obtained similarly using 5-amino-1H-tetrazolyl-1-acetic acid and (nBu3Sn)2O instead of 5-methyl-1H-tetrazolyl-1-acetic acid and(Ph3Sn)2O as described above for 1.Yield:69%,m.p.113~116 ℃.1H NMR (acetone-d6):δ0.74 (t,J=7.3 Hz,9H,CH3),1.09~1.23 (m,12H,CH2CH2CH2CH3),1.40~1.56 (m,6H,SnCH2),4.78 (s,2H,CH2),5.98 (s,2H,NH2).13C NMR (acetone-d6):δ13.1 (CH3),17.5 (1J(13C-119/117Sn)=412,394 Hz)(SnCH2),26.7 (3J(13C-119/117Sn)=72 Hz)(CH2CH3),27.7 (2J(13C-119/117Sn)=26 Hz)(SnCH2CH2),47.4(CH2),156.0(CN4),169.7(COO).119Sn NMR (acetone-d6):δ69.9.IR (cm-1):ν(NH2)3 372 and 3 202,νas(COO)1 647, νs(COO)1 376.Anal.Calcd.for C15H31N5O2Sn(%):C 41.69,H 7.23,N 16.21;Found(%):C 41.37,H 6.81,N 16.70.
1.5 Synthesis of 5
This complex was obtained similarly using 5-amino-1H-tetrazolyl-1-acetic acid and tricyclohexyltin hydroxide instead of 5-methyl-1H-tetrazolyl-1-acetic acid and (Ph3Sn)2O as described above for 1,but in a 1:1 molar ratio.Yield:72%,m.p.164~166 ℃.1H NMR (acetone-d6):δ1.11~1.22 (m,9H),1.46~1.61 (m,16H),1.72~1.82 (m,8H)(C6H11),4.86 (s,2H,CH2),5.91 (s,2H,NH2).13C NMR (acetone-d6): δ27.6,29.9,31.6 (2J(13C-119/117Sn)=17 Hz),35.7 (1J(13C-119/117Sn)=357,341 Hz)(C6H11),48.0 (CH2),156.9 (CN4),171.0 (COO).119Sn NMR (DMSO-d6):δ-89.9.IR (cm-1):ν(NH2)3 390 and 3 202, νas(COO)1 644, νs(COO)1 367.Anal.Calcd.for C21H37N5O2Sn (%):C49.43,H 7.31,N 13.73;Found(%):C 49.27,H 7.37,N 14.15.
1.6 Crystal structure determination
Crystals of 2,4 and 5 suitable for X-ray analysis were obtained by slowly cooling their hot acetone/hexane solutions.All intensity data were collected with a Rigaku Saturn diffractometer using Mo Kα radiation (λ=0.071 073 nm).The structureswere solved by direct methods and difference Fourier map using SHELXS of the SHELXTL package and refined with SHELXL[20]by full-matrix least-squares on F2.All nonhydrogen atoms were refined with anisotropic displacement parameters.Hydrogen atoms were added geometrically and refined with riding model position parameters.The partial cyclohexyl carbons in 2 were disordered,and their occupancy factors were refined to 0.457 for C(18),C(19),C(21)and C(22)as well as 0.543 for C(18)′,C(19)′,C(21)′and C(22)′.The butyl carbons of C(4)-C(15)in 4 were also disordered,satisfactory results were obtained when they were given occupancy factor of 0.5.A summary of the fundamental crystal data for these three complexes is listed in Table 1.
CCDC:1854152,2;1854153,4;1854154,5
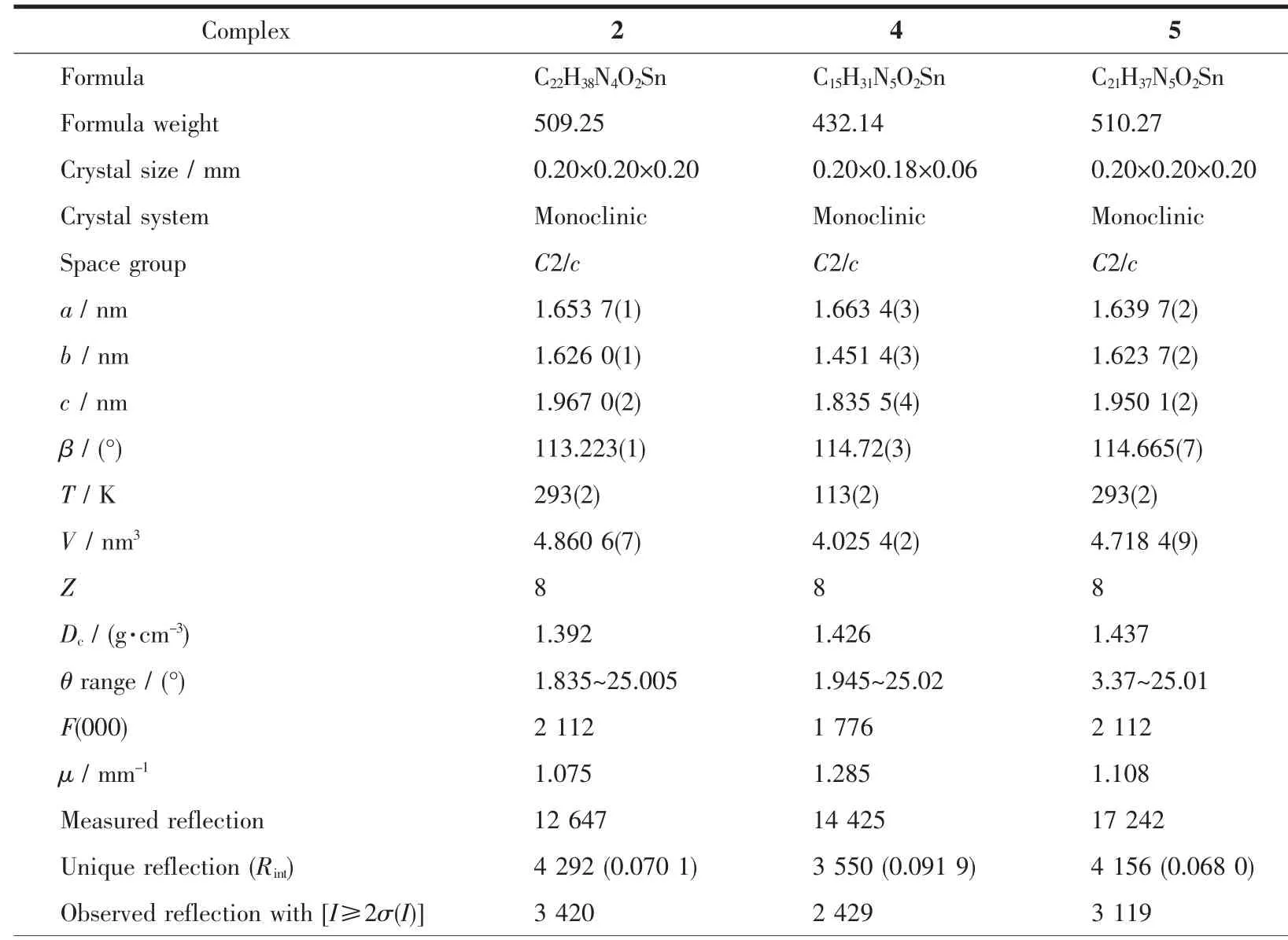
Table 1 Crystallographic data and refinement parameters for complexes 2,4 and 5

Continued Table 1
2 Results and discussion
2.1 Syntheses of complexes 1~5
Triorganotin 5-substituted 1H-tetrazolyl-1-acetates(1~5)were easily obtained by the reaction of organotin oxide or organotin hydroxide with 5-methyl-1H-tetrazolyl-1-acetic acid or 5-amino-1H-tetrazolyl-1-acetic acid in anhydrous benzene (Scheme 1).The complexes showed significantly different solubility in organic solvents.Complexes 1 and 2 were moderate soluble in chlorinated solvents,while complexes 3~5 were slightly soluble in these solvents.All complexes were soluble in acetone,and very soluble in strong polar solvents such as DMFand DMSO.The complexes have been characterized by IR and NMR(1H,13C and119Sn)spectra as well as elemental analyses.

Scheme 1 Syntheses of complexes 1~5
The IR spectra of complexes 1~5 showed the absence of the strong and broad band ascribed to the carboxyl group as well as the low carbonyl stretching frequencies,which indicated the removal of the carboxyl proton and the formation of the Sn-Obonds[21].The values of Δν(νas(COO)-νs(COO)for 1~5 were observed in the region of 240~312 cm-1,larger than those detected in the corresponding sodium salts of acids L1(216 cm-1)and L2(226 cm-1),in which the asymmetric and symmetric stretching vibrations of the carboxylate groups appeared at 1 624 and 1 408 cm-1for L1,as well as 1 632 and 1 406 cm-1for L2,respectively,implying the monodentate manner of the carboxylate groupsin the complexes to the tin atom[22-23].The1H NMR spectroscopic data are also consistent with the suggested structures,exhibiting the expected integral values and chemical shifts.The13C NMR spectra of 1~5 clearly showed the carbonyl carbon resonances atδ168.8~171.1.The119Sn NMR chemical shift of triphenyltin complex 1 (δ-82.7)in CDCl3solution was in the range of those values for fourcoordinated triphenyltin carboxylates[1],suggesting that this complex should be monomeric four-coordinated structure in non-coordinating solvent.Due to the relatively low solubility of other complexes,their satisfactory119Sn and13C NMR signals in CDCl3solution could not be observed.However,the119Sn NMR values of tricyclohexyltin complex 2 (δ17.7)and tributyltin complex 4 (δ69.9)in the weakly coordinating solvent acetone-d6were also compared to those values reported in the corresponding fourcoordinated tricyclohexyltin and tributyltin carboxylates[1].These results show that the polymeric structures in solid as shown in Fig.1~3 break down to monomeric structures in solution possibly owing to the weak Sn…N interactions.
2.2 Crystal structures of complexes 2,4 and 5
The molecular structures of 2,4 and 5 have been confirmed by X-ray crystallography,and presented in Fig.1~3,respectively.The selected bond distances and angles are listed in Table 2.Fig.1~3 show that the carboxylate group exhibits a monodentate coordination mode in the complexes,as above-mentioned by their IR spectra.Furthermore,the complexes form a similar linkage structure through the intermolecular Sn…N interactions.The Sn…N distance is markedly different in these three complexes.The Sn-N distance is 0.257 2(7)nm in 4,slightly shorter than those in triorganotin derivatives of tetrazolylacetic acid,such as triphenyltin 1H-tetrazolyl-1-acetate (0.260 9(6)nm)[12].The Sn…N distances in 2 (0.281 4(4)nm)and 5 (0.298 9(6)nm)are markedly longer than that in 4,but similar to that in tricyclohexyltin 1H-tetrazolyl-1-acetate (0.292 4(9)nm)[12],indicating that the Sn…N interactions are very weak in 2 and 5.The non-bond Sn…O distances are in the range of 0.333 9~0.338 4 nm,markedly longer than the covalent Sn-O bond distances (0.212 7~0.218 5 nm)in these three complexes,but still shorter than the sum of the van der Waals radii for the Sn and O atoms of 0.357 nm[24],suggesting that some weak interactions possibly exist between these two atoms.It is worth pointing out that complexes 4 and 5 form 3D supramolecular architectures through intermolecular N-H…N,N-H…O or C-H …O hydrogen bonds (Supporting Information)owing to the presence of amino group.

Fig.1 Molecular structure of 2 with 30%probability displacement ellipsoids
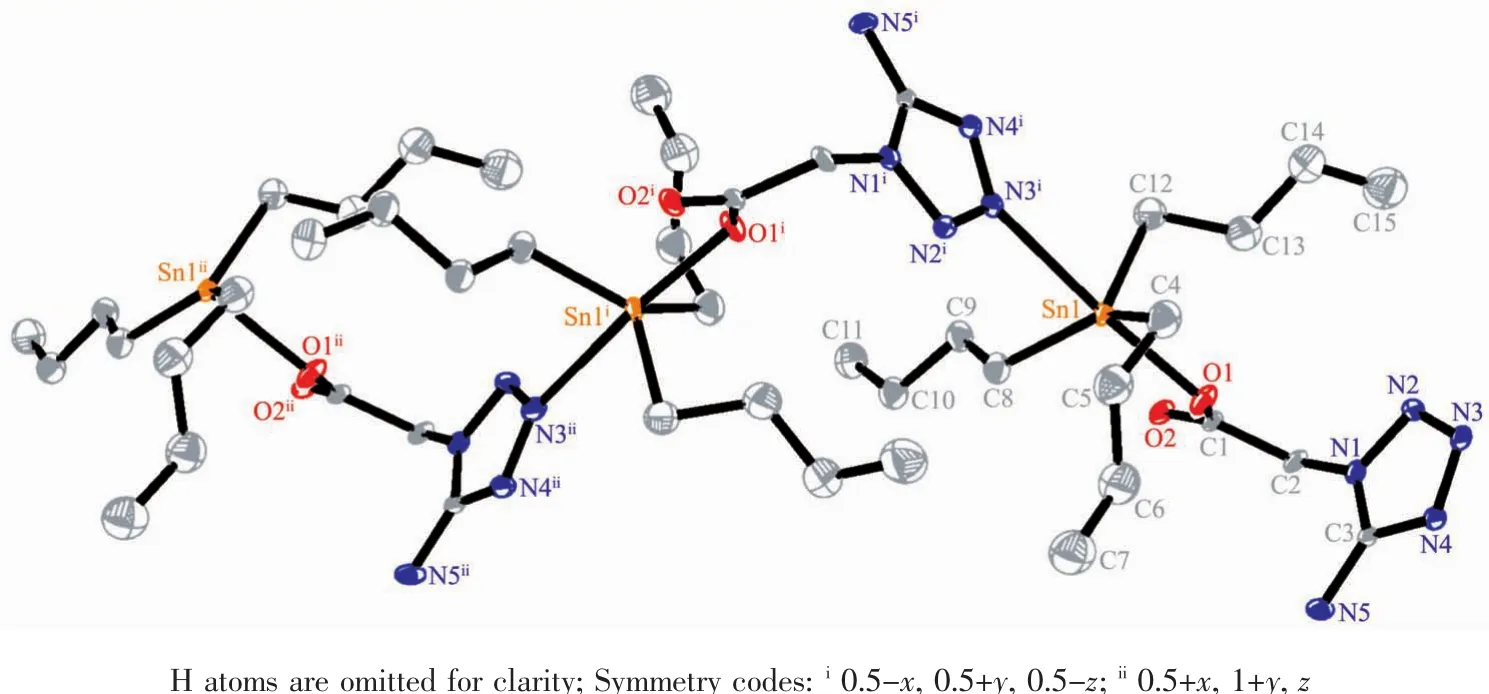
Fig.2 Molecular structure of 4 with 30%probability displacement ellipsoids

Fig.3 Molecular structure of 5 with 30%probability displacement ellipsoids

Table 2 Selected bond distances(nm)and angles(°)for complexes 2,4 and 5
2.3 Cytostatic activity evaluation
The cytotoxic activity of complexes 1~5 and the free acids (L1and L2)for Hela and A549 cells in vitro was assayed by the MTT method[25],and the data of IC50are summarized in Table 3.From these results,it is observed that the free acids have scarcely any activity against Hela and A549 cells,but all complexes except 4 display good activity to these two cells.It is also found that the complexes exhibit higher activity against A549 cells than Hela cells.Moreover,complex 3 exhibits promising result.This complex is even more active against A549 cells than cisplatinsupported by its smaller IC50value compared to that of cisplatin.

Table 3 IC50 values of complexes 1~5 for HeLa and A549 cells μmol·L-1
3 Conclusions
In conclusion,five triorganotin 5-methyl-1H-tetrazolyl-1-acetates and 5-amino-1H-tetrazolyl-1-acetates have been synthesized and characterized.The crystal structural analyses of three of them reveal that the carboxylate group acts as a monodentate ligand in the complexes,and the complexes form linkage coordination polymers through the intermolecular Sn…N interactions in solid state.Most of the complexes display good cytotoxicities for Hela and A549 cells in vitro, especially complex 3 exhibits excellent cytotoxicity for A549 cells.
Supportinginformation isavailableat http://www.wjhxxb.cn
———理学院

