液液萃取-气相色谱法测定发酵液中的有机酸与乙醇
范桂芳,李佩佩,齐立松,李十中
液液萃取-气相色谱法测定发酵液中的有机酸与乙醇
范桂芳,李佩佩,齐立松,李十中※
(清华大学核能与新能源技术研究院;北京市生物燃料工程技术研究中心 北京 100084)
嗜热菌降解纤维素是第二代燃料乙醇发展的重要方向。测定发酵液中乙醇和有机酸组成的变化,对发酵过程控制具有重要意义。该文以正丙醇为内标,建立了发酵液中乙醇、乙酸、丙酸和丁酸的气相色谱检测方法。该方法为:发酵液调节pH值、与内标混合、添加氯化钠至饱和、乙酸乙酯萃取,有机相进气相色谱检测。该方法下几种化合物的检出限范围为10~45 mg/L,低、中和高3个水平的加标回收率范围为80.65%~107.94%,相对标准偏差范围为1.71%~4.98%,该方法能用于纤维素菌群降解体系有机酸与乙醇的检测。该文还测算了20 ℃时乙醇、正丙醇、乙酸、丙酸和丁酸在乙酸乙酯和饱和氯化钠溶液中的分配系数,其值分别为0.28、1.64、1.37、2.51和3.29。该检测方法还可以推广应用于其他水相体系中有机酸和乙醇的检测,如废水厌氧消化液中挥发性脂肪酸的检测以及发酵醪中乙醇的检测。
乙醇;有机酸;发酵液;液液萃取;气相色谱
0 引 言
2017年9月,国家发展和改革委员会、国家能源局等十五部门联合印发《关于扩大生物燃料乙醇生产和推广使用车用乙醇汽油的实施方案》,要求到2020年在全国范围内推广使用车用乙醇汽油,并基本实现全覆盖。利用嗜热菌降解木质纤维素可以同步耦合纤维素预处理工艺,降低纤维素乙醇成本,是第二代燃料乙醇发展的重要方向[1-3]。测定发酵液中乙醇和有机酸组成的变化,对控制发酵过程具有重要意义。
高效液相色谱法[4-13]和气相色谱法[14-29]广泛用于发酵液中醇和酸类物质的检测。相比于液相色谱,气相色谱具有仪器成本更低、分离能力更强、分离时间更短的特点。在气相色谱检测方法开发工作中,顾福权等[14]研究表明溶液的pH值对乙酸的实际检出值有很大影响,应控制溶液的pH值为3.5以下防止有机酸离解;采用合适的极性柱消除拖尾,以及添加甲酸作为吸附占据剂能促进有机酸的检测。Lin等[15]利用等柱温条件下的分离结果建立温度与容量因子及保留时间的关系,预测不同温度下的保留时间与容量因子,在设置升温程序时,遵循每个峰的分辨率满足分析要求和分析时间最短的原则,使分析时间由传统的20 min缩短至10 min。Darwin等[16]开发了一种用无机酸将乳酸衍化为乙醛的预处理方法,使得气相色谱可以检测发酵液中不能挥发的乳酸。上述气相色谱检测方法的不足之处是:发酵液中溶解了一些不能挥发的无机盐和糖,水相直接进样将导致这些物质残留在进样衬管里,衬管需频繁更换。为了克服这个问题,Fu等[25]开发了乙醇、丁醇和丙酮的顶空进样法,一些文献[26-29]采用有机溶剂萃取、气相色谱检测的方法分析了发酵液中的醇、酮和有机酸。针对有机溶剂萃取条件,Liu等[7]考察了不同有机溶剂(氯仿和乙酸乙酯)、萃取剂与溶液的体积比(0.5∶1~2∶1)、旋转速度(80~ 260 r/min)和萃取温度(10~40 ℃)对分析物萃取率的影响,结果表明有机溶剂为乙酸乙酯、萃取剂与溶液的体积比为0.5∶1、旋转速度为260 r/min和萃取温度10 ℃条件下萃取率最高,不同因素的重要次序为萃取温度>体积比>旋转速度>有机溶剂。采用有机溶剂萃取乙醇和有机酸,并对分析方法的精密度及加标回收率进行评价的论文还未检索到。
本文优化了乙酸乙酯液液萃取-气相色谱法检测条件,并对该方法的精密度以及加标回收率进行系统评价,为气相色谱法检测发酵液中的醇和有机酸提供参考。
1 材料与方法
1.1 仪器与试剂
7890A 安捷伦气相色谱仪 (配火焰离子检测器,CP-Wax-57CB型色谱柱);高速冷冻离心机(Legend Mach1. 6R 型,Thermo公司)。乙醇(纯度为99.5%,美国MREDA公司)、乙酸(纯度为99.5%,北京化工厂)、丁酸(纯度为99.0%,北京化工厂)。硫酸(纯度为99.5%,北京化工厂)、乙酸乙酯(纯度为99.9%,美国Sigma公司)、氯化钠(纯度为99.5%,北京化工厂)。
1.2 色谱条件
载气流速:1.6 mL/min;进样口温度:200 ℃;隔垫吹扫:3 mL/min;模式:分流模式;分流比:50∶1;进样量:0.5L;程序升温:40 ℃保持5 min,以10 ℃/min升到210 ℃保持3 min;检测器温度:230 ℃;氢气:氮气:空气为30∶20∶300。
1.3 标准溶液的配制
用超纯水配制了乙醇、乙酸、丙酸、丁酸几种标准物质的混合溶液,各组分的质量浓度分别为0.050、0.100、0.200、0.500、1.000、1.500和2.000 g/L。配制2.000 g/L正丙醇的水溶液做为内标液。加硫酸调节混合标准水溶液的pH值为2~3,按体积比1∶1与内标混合,加入NaCl至饱和,按水相与有机相体积比2∶1混合,漩涡震荡15 s,离心(6 000 r/min,2 min),取上层有机相进色谱检测,数据用于绘制标准曲线。
1.4 样品预处理
取2 mL发酵液,离心(10 000 r/min,5 min)。取1 mL上清液,加入8L浓硫酸调节pH值至2~3使待测液中的有机酸游离出来,按体积比1∶1与内标混合,加入NaCl至饱和,按水相与有机相体积比2∶1混合,漩涡震荡15 s,离心(6 000 r/min,2 min),取上层有机相进色谱检测。
1.5 萃取条件对萃取率与检测精密度的影响
为了对比加盐以及温度对萃取率与萃取标准偏差的影响,考察了3种萃取条件。1)常温约20 ℃,不加盐;2)常温,加盐;3)萃取前后样品置于冰盒中,不加盐。每种条件重复操作6次。
1.6 分配系数计算
配一份含各待测物和内标的饱和氯化钠溶液,分别在水相与有机相体积比为1∶1与2∶1时进行萃取试验,联立方程求各待测物与内标物在乙酸乙酯与饱和盐溶液两相中的分配系数。
1.7 实际样品重复性与加标回收率
取发酵初始液1份,按低、中和高3个水平进行加标回收率试验,加标量分别为0.200,1.000和2.000 g/L,每个加标水平重复6次。
取发酵3 d样品1份,重复测样5次,考察样品检测精密度。
2 结果与分析
2.1 萃取条件优化
表1为3种萃取条件对萃取率与相对标准偏差RSD(relative standard deviation)的影响。由表1数据可知,水相用氯化钠溶解至饱和提高了乙醇在乙酸乙酯中的分配比例,降低了乙酸、丙酸和丁酸在乙酸乙酯中的分配比例。温度为0 存放降低了乙醇在乙酸乙酯中的分配比例,提高了乙酸、丙酸和丁酸在乙酸乙酯中的分配比例。 20 ℃加盐萃取产生了最低的RSD,冰盒存放可能由于操作时需在0和20 ℃下切换引入了更多的不确定性,产生了较大的RSD。乙醇在乙酸乙酯中的溶解度比水小,较有机酸不易被乙酸乙酯萃取,加盐能提高乙醇的萃取率,乙醇是发酵的重要产物,为保证乙醇在实际样品中的检测精度与有机酸一样在可以接受(RSD<5%)的水平上,确定20 ℃加盐萃取为本文的萃取条件。

表1 萃取条件对萃取率与检测精密度的影响
注:RSD为标准偏差,下同。
Note: RSD is standard deviation, same as below.
2.2 分配系数K的计算
分配系数由式(1)~(4)计算。




式中化合物(乙酸乙酯)、化合物(水)分别为化合物在乙酸乙酯、水中的浓度,g/L;化合物为化合物的质量,g;水为水的体积,L;乙酸乙酯为乙酸乙酯的体积,L;化合物2(乙酸乙酯)和化合物1(乙酸乙酯)分别为水相与有机相萃取体积比为2∶1和1∶1时化合物在乙酸乙酯中的浓 度,g/L。因为标准曲线过原点,并且萃取体积比1∶1比2∶1条件下乙酸乙酯中的分析物浓度更小,没有超出标准曲线的浓度范围0.050~2.000g/L,可以认为峰面积比与物质浓度比成正比,以色谱峰面积比代替浓度比进行分配系数的计算。为消除进样体积误差对峰面积的影响,用溶剂乙酸乙酯的峰面积对化合物的峰面积进行校正。将式(1)代入式(2),并联立2种萃取条件下的方程得式(4)。表2显示了水相与有机相萃取体积比为1∶1时化合物与乙酸乙酯的峰面积比1和水相与有机相萃取体积比为2∶1时化合物与乙酸乙酯的峰面积比2,1和2为2次检测的平均值。经计算乙醇、正丙醇、乙酸、丙酸和丁酸在乙酸乙酯与饱和盐溶液中的分配系数分别为0.28、1.64、1.37、2.51和3.29。

表2 化合物在乙酸乙酯与饱和盐溶液中的分配系数
注:1∶1,2∶1为水相与有机相萃取体积比。
Note: 1∶1, 2∶1 refer to volumetric ratio between aqueous phase and organic phase when extracting.
2.3 标准曲线的绘制
以化合物与正丙醇的峰面积比R对化合物与正丙醇的质量浓度比R进行线性回归,结果见表3。以10倍色谱峰信噪比S/N (signal/noise) 确定检出限LOD (limit of detection) ,各组分的检出限见表3。由表3数据可知,乙醇、乙酸、丙酸和丁酸标准曲线的相关系数范围为0.999 0~0.999 9,检出限的范围为10~45 mg/L。标准曲线见图1。
2.4 重复性和回收率
取发酵初始液1份,对其进行低、中、高3个加标水平的回收试验。发酵初始液中乙醇、乙酸、丙酸和丁酸质量浓度分别为0.24、0.17、0和0 g/L,3个加标水平下检测到的回收率与RSD值见表4。由表4可知,3个加标水平下的回收率范围为80.65%~107.94%,RSD值范围为1.71%~4.98%。
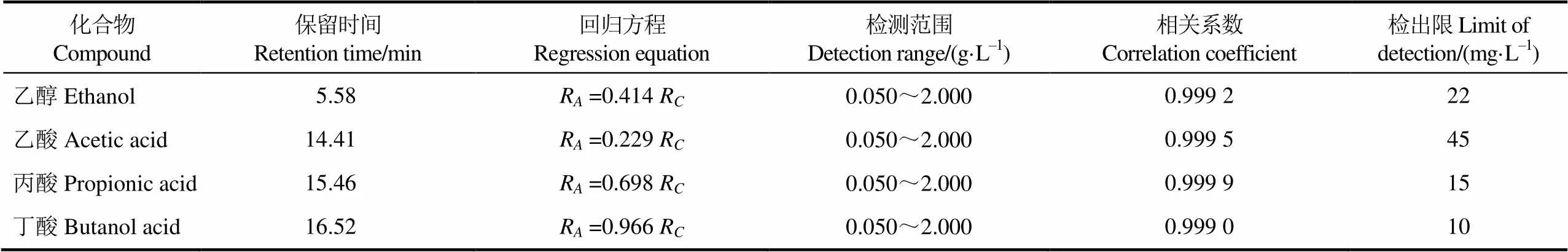
表3 化合物的标准曲线与检出限
注:A为化合物与正丙醇峰面积比,C为化合物与正丙醇质量浓度比。下同。
Note:Aisratio of peak area of compound to n-propyl alcohol,Cis ratio of mass concentration of compound to n-propyl alcohol. Same as below.
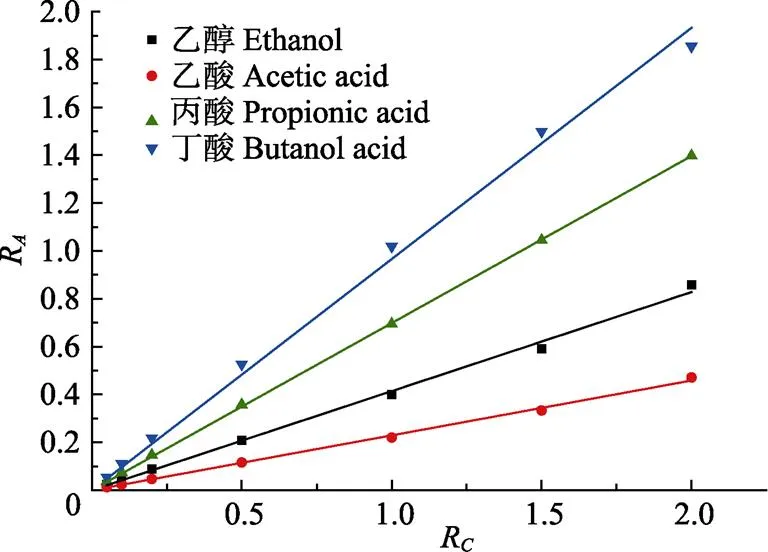
图1 化合物的标准曲线
2.5 实际样品检测结果
取发酵3 d的实际样品,检测得乙醇、乙酸和丁酸质量浓度分别为0.49、0.94和0.06 g/L,对应RSD分别为2.55%、1.69%和4.62%,未检出丙酸。
2.6 本文方法用于甜高粱发酵物料中乙醇检测结果
传统甜高粱发酵物料中乙醇的检测方法为:取50 g固态发酵料于500 mL蒸馏瓶中,加入200 mL去离子水进行简单蒸馏,准确收集前100 mL馏出液,用气相色谱测定乙醇含量[30]。为检测发酵物料中可溶性糖的含量,发酵物料中的可溶性物质已采用固液萃取方法转移至水相,再用该文所述液液萃取-气相色谱法检测乙醇含量。2种方法检测得到的乙醇质量浓度结果见表5。由表5数据可知,2种方法检测所得数据差异较小,小于该文报道方法的相对误差。用液液萃取操作可以省去较为繁琐的蒸馏操作。
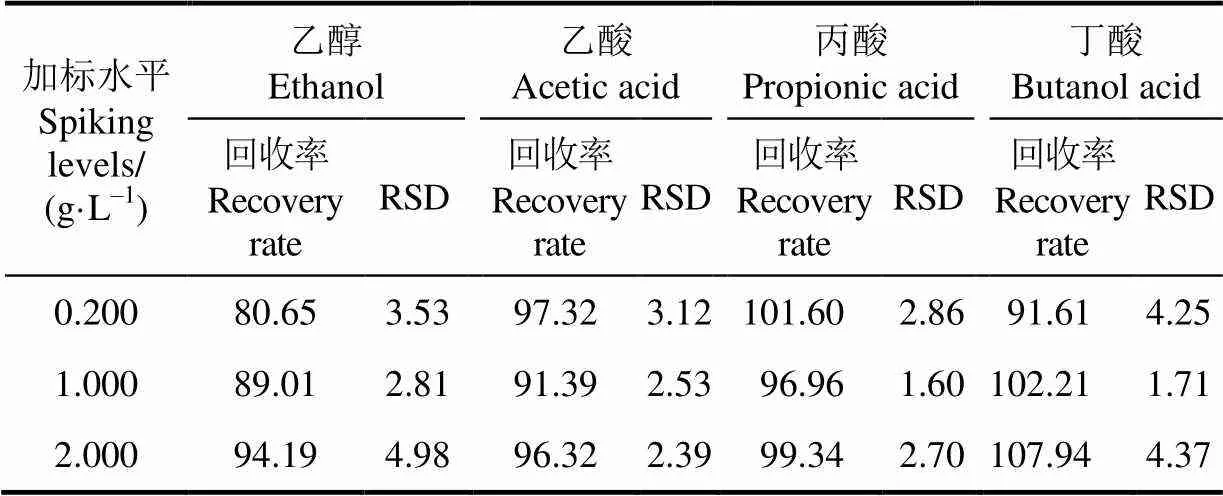
表4 3个加标水平的回收率与标准偏差
液液萃取方法还可以替代蒸馏提取法应用于固体发酵物料[30-31]中乙醇的检测。该检测方法亦可用于废水厌氧发酵[14,24]中有机酸的检测。
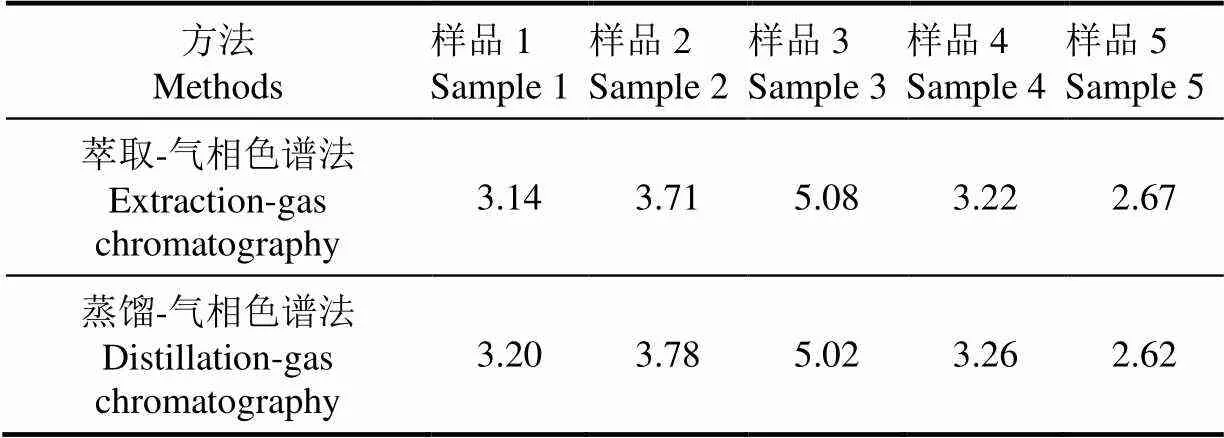
表5 2种方法检测的甜高粱秆渣乙醇质量浓度对比
3 结 论
本文以正丙醇为内标建立了乙醇、乙酸、丙酸和丁酸的气相色谱检测方法。该方法为:发酵液调节pH值、与内标混合、用氯化钠溶解至饱和、乙酸乙酯萃取,有机相进气相色谱检测。该方法下几种化合物的检出限范围为10~45 mg/L,低、中和高3个水平的加标回收率范围为80.65%~107.94%,相对标准偏差范围为1.71%~4.98%,该方法可用于纤维素降解菌群发酵液中乙酸、丙酸、丁酸及乙醇的测定。本文还测算了20 ℃下乙醇、正丙醇、乙酸、丙酸和丁酸在乙酸乙酯和饱和氯化钠溶液中的分配系数,其值分别为0.28、1.64、1.37、2.51和3.29。
使用该方法需要注意的是,溶液的pH值、氯化钠浓度、操作温度都会影响分析物在有机相和水相中的分配系数,检测时应该保持操作条件一致。有机相进样比水相进样的另一个优点是可以防止进样针被酸腐蚀。
[1] Lynd L R, Liang X Y, Biddy M J, et al. Cellulosic ethanol: Status and innovation[J]. Current Opinion in Biotechnology, 2017, 45: 202-211.
[2] Jiang Y J, Xin F X, Lu J S, et al. State of the art review of biofuels production from lignocellulose by thermophilic bacteria[J]. Bioresource Technology, 2017, 245: 1498-1506.
[3] Du R, Yan J B, Li S Z, et al. Cellulosic ethanol production by natural bacterial consortia is enhanced by[J]. Biotechnology for Biofuels, 2015, 8: 10.
[4] 姜艳,范桂芳,杜然,等. 高效液相色谱法测定菌群降解纤维素产物中的糖、有机酸和醇[J]. 色谱,2015,33(8):805-808 Jiang Yan, Fan Guifang, Du Ran, et al. Determination of sugars, organic acids and alcohols in microbial consortium fermentation broth from cellulose using high performance liquid chromatography[J]. Chinese Journal of Chromatography, 2015, 33(8): 805-808. (in Chinese with English abstract).
[5] 姜岷,雷丹,陈可泉,等. 纤维素水解液厌氧发酵产丁二酸发酵液中有机酸和混合单糖的测定[J]. 分析化学,2009,37(4):605-608. Jiang Min, Lei Dan, Chen Kequan, et al. Determination of organic acids and monomeric sugars in fermentation broth of succinic acid production with lignocellulose hydrolysate[J]. Chinese Journal of Analytical Chemistry, 2009, 37(4): 605-608. (in Chinese with English abstract).
[6] Eiteman M A, Chastain M J. Optimization of the ion-exchange analysis of organic acids from fermentation[J]. Analytica Chimica Acta, 1997, 338: 69-75.
[7] Liu Y L, Chen T, Yang M H, et al. Analysis of mixtures of fatty acids and fatty alcohols in fermentation broth[J]. Journal of Chromatography A, 2014, 1323: 66-72.
[8] 袁文杰,孔亮,孜力汗,等. 高效液相色谱法测定克鲁维酵母菊芋发酵液中的乙醇,糖和有机酸类代谢成分[J]. 分析化学,2009,37(6):850-854. Yuan Wenjie, Kong Liang, Zi Lihan, et al. Simultaneous determination of ethanol, sugar and organic acids inbroth by high performance liquid chromatography-ultraviolet/refractive index detector[J]. Chinese Journal of Analytical Chemistry, 2009, 37(6): 850-854. (in Chinese with English abstract).
[9] 王元好,董悦生,修志龙. 梯度洗脱高效液相色谱法快速测定1,3-丙二醇发酵液中有机酸[J]. 食品与发酵工业,2010,36(1):126-130. Wang Yuanhao, Dong Yuesheng, Xiu Zhilong. Rapid determination of organic acids in 1,3-propanediol fermentation broth with gradient elution-high performance liquid chromatography[J]. Food and Fermentation Industries, 2010, 36(1): 126-130. (in Chinese with English abstract).
[10] Kumar M, Saini S, Gayen K. Acetone-butanol-ethanol fermentation analysis using only high performance liquid chromatography[J]. Analytical Methods, 2014, 6(3): 774-781.
[11] Carver S M, Münster U, Tuovinen O H. A solid phase extraction technique for HPLC analysis of short chain fatty acid fluxes during microbial degradation of plant polymers [J]. Journal of Liquid Chromatography & Related Technologies, 2011, 34(15): 1546-55.
[12] Geng X M, Zhang S F, Wang Q, et al. Determination of organic acids in the presence of inorganic anions by ion chromatography with suppressed conductivity detection[J]. Journal of Chromatography A, 2008, 1192(1): 187-190.
[13] Rahayu F, Kawai Y, Iwasaki Y, et al. Thermophilic ethanol fermentation from lignocellulose hydrolysate by genetically engineered[J]. Bioresource Technology, 2017, 245: 1393-1399.
[14] 顾福权,徐红娟,柳展飞,等. 气相色谱法测定废水中6种挥发性脂肪酸含量[J]. 能源环境保护,2014,28(3): 62-64. Gu Fuquan, Xu Hongjuan, Liu Zhanfei, et al. Content of 6 volatile fatty acid of wastewater by gas chromatography[J]. Energy Environmental Protection, 2014, 28(3): 62-64. (in Chinese with English abstract).
[15] Lin X Q, Fan J S, Wen Q S, et al. Optimization and validation of a GC-FID method for the determination of acetone-butanol-ethanol fermentation products[J]. Journal of Chromatographic Science, 2014, 52: 264-270.
[16] Darwin W, Cord-Ruwisch R. Concurrent lactic and volatile fatty acid analysis of microbial fermentation samples by gas chromatography with heat pre-treatment[J]. Journal of Chromatographic Science, 2018, 56: 1-5.
[17] Diamantis V, Melidis P, Aivasidis A. Continuous determinationof volatile products in anaerobic fermenters by on-line capillary gas chromatography[J]. Analytica Chimica Acta, 2006, 573: 189-194.
[18] 朱灵峰,张楠,苏彩丽,等. 气相色谱法测定厌氧发酵产氢过程中产生的挥发性脂肪酸[J]. 江苏农业科学,2012,40(6):296-297.
[19] 赵兴涛,徐桂转,刘杰博,等. 气相色谱法测定厌氧发酵液中挥发性脂肪酸的研究[J]. 河南农业大学学报,2013,47(5):584-586,591. Zhao Xingtao, Xu Guizhuan, Liu Jiebo, et al. Study on VFAs content in the anaerobic fermentation liquid by gas chromatography[J]. Journal of Henan Agricultural University, 2013, 47(5): 584-586, 591. (in Chinese with English abstract).
[20] 马蕙,仪宏,王丽丽. 气相色谱法测定发酵液中丙酸和乙酸的含量[J]. 饲料工业,2008(9):48-50.
[21] 杜然,李十中,章晓庆,等. 兼性厌氧复合菌群H纤维素降解和产乙醇能力及生态组成初探[J]. 生物工程学报,2010,26(7):960-965. Du Ran, Li Shizhong, Zhang Xiaoqing, et al. Cellulose hydrolysis and ethanol production by a facultative anaerobe bacteria consortium H and its identification[J]. Chinese Journal of Biotechnology, 2010, 26(7): 960-965. (in Chinese with English abstract).
[22] Jiang Y J, Guo D, Lu J S, et al. Consolidated bioprocessing of butanol production from xylan by a thermophilic and butanologenicsp. M5[J]. Biotechnology for Biofuels, 2018, 11: 268.
[23] Tsuey L S, Bin Ariff A, Mohamad R, et al. Improvements of GC and HPLC analyses in solvent (acetone-butanol-ethanol) fermentation byusing a mixture of starch and glycerol as carbon source[J]. Biotechnology and Bioprocess Engineering, 2006, 11(4): 293-298.
[24] Chen Y, Jiang X, Xiao K, et al. Enhanced volatile fatty acids (VFAs) production in a thermophilic fermenter with stepwise pH increase-Investigation on dissolved organic matter transformation and microbial community shift[J]. Water Research, 2017, 112: 261-268.
[25] Fu C Y, Liu H, Fu S Y, et al. Rapid and simultaneous determination of acetone, butanol and ethanol in butanol fermentation broth by full evaporation headspace gas chromatography[J]. Cellulose Chemistry and Technology, 2015, 49(9/10), 813-818.
[26] 仪宏,侯建革,张华峰,等. 毛细管气相色谱法测定发酵液中丙酸的含量[J]. 饲料工业,2003(6):37-39.
[27] Dellomonaco C, Clomburg J M, Miller E N, et al. Engineered reversal of the beta-oxidation cycle for the synthesis of fuels and chemicals[J]. Nature, 2011, 476: 355-359.
[28] Steen E J, Kang Y S, Bokinsky G, et al. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass[J]. Nature, 2010, 463: 559-562.
[29] Xiao Z J, Wang X M, Huang Y L, et al. Thermophilic fermentation of acetoin and 2, 3-butanediol by a novelstrain[J]. Biotechnology for Biofuels, 2012, 5: 88. Doi: 10.118611754-6834-5-88.
[30] 韩冰,范桂芳,李十中,等. 不同糖质原料和菌株固态发酵制取乙醇的特性比较[J]. 农业工程学报,2012,28(5):201-206.Han Bing, Fan Guifang, Li Shizhong, et al. Comparison of ethanol production from different sugar feedstocks by solid state fermentation with two yeast strains[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2012, 28(5): 201-206. (in Chinese with English abstract)
[31] 范桂芳,李佩佩,齐立松,等. 固体发酵物料可溶性糖和乙醇检测方法研究进展[J]. 生物产业技术,2018(3):50-54. Fan Guifang, Li Peipei, Qi Lisong, et al. Progress on soluble sugars and ethanol determination in solid state fermentation material[J]. Biotechnology & Business, 2018(3): 50-54. (in Chinese with English abstract).
Determination of organic acids and ethanol in fermentation broth by liquid-liquid extraction and gas chromatography
Fan Guifang, Li Peipei, Qi Lisong, Li Shizhong※
(100084,)
The degradation of cellulose by thermophilic bacteria is a growing trend in the 2nd generation bioethanol. The determination of the contents of ethanol and organic acids in the fermentation broth is of great significance to the control of the fermentation process. A gas chromatographic method for the determination of ethanol, acetic acid, propionic acid, and butyric acid contents in fermentation broth was established, using n-propanol as internal standard. The pH value of the aqueous phase was adjusted to 3 to prevent the dissociation of organic acids. Mixed with internal standard, the compounds were then extracted into the organic phase using ethyl acetate, and the organic phase was injected to the inlet of gas chromatograph. The mass concentration range of the standard curve was 0.050-2.000 g/L, the correlation coefficients were in the range of 0.999 0-0.999 9, the detection limits of analytes in this method were in the range of 10-45 mg/L, the recovery rates at 3 spiking levels were in the range of 80.65%-107.94%, and the relative standard deviation (RSD) of spiked sample were in the range of 1.71%-4.98%, the RSD of the actual sample was less than 5%. The method could be used for the detection of organic acids and ethanol in fermentation broth. The effects of temperature (0 or 20 ℃) and ion intensity (saturated with sodium chloride or not) on the variability of the extraction efficiency were investigated. The results showed that saturated with sodium chloride increased the partition ratio of ethanol in ethyl acetate and decreased the partition ratio of acetic acid, propionic acid and butyric acid in ethyl acetate; lower temperature decreased the partition ratio of ethanol in ethyl acetate and increased the partition ratio of acetic acid, propionic acid and butyric acid in ethyl acetate. The minimum extraction variability was obtained at 20 ℃ and sodium chloride saturated solution, so this condition was set as the extraction condition. The liquid-liquid extraction experiments were carried out under the conditions of the volumetric ratio of aqueous phase to organic phase being 2:1 and 1:1. Ratios of peak area under the two conditions were used to calculate the partition coefficients of the compounds. The partition coefficients of ethanol, n-propanol, acetic acid, propionic acid, and butyric acid in ethyl acetate and sodium chloride saturated solution at 20 ℃ were measured and calculated to be 0.28, 1.64, 1.37, 2.51, and 3.29, respectively. The ethanol content of the extract solution of fermented sweet sorghum bagasse was determined by this method. The detection value was compared to that of the traditional distillation-gas chromatography method. The differences between two methods were less than the RSD of actual samples in this method. The pretreating process could be simplified by using liquid-liquid extraction instead of distillation. The detection method can also be used in the detection of organic acids and ethanol in other aqueous systems, such as detecting volatile fatty acids in anaerobic digestion waste water and detecting ethanol in fermented liquors. The injection of organic phase instead of aqueous phase can prevent the corrosion of the syringe by acid.
ethanol; organic acids; fermentation broth; liquid-liquid extraction; gas chromatography
范桂芳,李佩佩,齐立松,李十中.液液萃取-气相色谱法测定发酵液中的有机酸与乙醇[J]. 农业工程学报,2018,34(23):227-231. doi:10.11975/j.issn.1002-6819.2018.23.029 http://www.tcsae.org
Fan Guifang, Li Peipei, Qi Lisong, Li Shizhong.Determination of organic acids and ethanol in fermentation broth by liquid-liquid extraction and gas chromatography[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2018, 34(23): 227-231. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2018.23.029 http://www.tcsae.org
2018-06-29
2018-10-08
国家重点研发计划项目(No. 2016YFE0108500)和国家自然科学基金项目(No. 31600067)联合资助
范桂芳,工程师。Email:fanguifang@mail.tsinghua.edu.cn
李十中,研究员,博士,博士生导师。Email:szli@tsinghua.edu.cn
10.11975/j.issn.1002-6819.2018.23.029
S216;O661;TQ353
A
1002-6819(2018)-23-0227-05

