Circulating microRNAs as a liquid biopsy: a nextgeneration clinical biomarker for diagnosis of gastric cancer
Shuhei Komatsu, Jun Kiuchi, Taisuke Imamura, Daisuke Ichikawa, Eigo Otsuji
1Division of Digestive Surgery, Department of Surgery, Kyoto Prefectural University of Medicine, Kawaramachihirokoji, Kamigyo-ku,Kyoto 602-8566, Japan.
2First Department of Surgery, Faculty of Medicine, University of Yamanashi, Chuo, Yamanashi 409-3898, Japan.
Abstract Accumulating evidence has suggested the potential clinical utility of novel body fluid biomarkers, or “liquid biopsy”,using circulating tumor cells and cell-free nucleic acids from cancer patients. Noninvasive and reproducible, liquid biopsy could provide the basis for individualized therapeutic strategies by identifying genetic and epigenetic aberrations that are closely associated with cancer initiation and progression. MicroRNAs (miRNAs) are short noncoding RNAs that posttranscriptionally regulate gene expression. They also play important roles in various physiological and developmental processes as oncogenic or tumor-suppressive regulators. Specific miRNA expression signatures have been identified in a number of human cancers. Circulating miRNAs have been detected in plasma and serum, and this in blood has attracted the attention of researchers for their potential as noninvasive biomarkers. Circulating miRNAs have emerged as tumorassociated biomarkers that reflect not only the existence of cancer, but also the dynamics, malignant potential, and drug resistance of tumors. Herein, we review the recent biological and clinical research on the circulating miRNAs of gastric cancer and discuss future perspectives for their clinical applications as a liquid biopsy.
Keywords: Liquid biopsy, circulating nucleic acids, circulating microRNA, biomarker, gastric cancer
INTRODUCTION
Gastric cancer is third-leading cause of death among all cancers worldwide[1]. While improved perioperative management and diagnostic techniques have boosted early detection and decreased mortality in recent years,gastric cancer continues to constitute a global health problem as a prevalent form of cancer[1]. Gastric cancer patients at advanced stages of the disease have a very poor prognosis[2]. Despite these continued difficulties,no biomarker molecule has been employed for the early diagnosis of gastric cancer in clinical settings, and researchers have validated only a scant number of molecules as therapeutic targets[3-7]. Therefore, for gastric cancer, identifying novel molecular targets and clinical biomarkers remain vital clinical challenges.
Recently, the concept of a “liquid biopsy” has become widely accepted in the clinical setting. Liquid biopsy is a less approaches for obtaining genetic and epigenetic aberrations that are closely associated with cancer initiation and progression[8]. Moreover, liquid approaches allows for repeated sampling and this makes it possible to evaluate the longitudinal evolution of a tumor and its heterogeneous characteristics, which single sampling may fail to capture[9-13]. Understanding circulating tumor cells and cell-free nucleic acids in cancer patients may bring new insights into prognostic and predictive value of liquid biopsy. In this article, we review recent research on the circulating miRNAs of gastric cancers, and discuss future perspectives on next-generation clinical biomarkers and treatment targets in gastric cancer.
THE MOLECULAR FEATURES AND BIOLOGICAL SIGNIFICANCE OF MICRORNAS
Small noncoding RNAs known as microRNAs (miRNAs) regulate how specific protein-coding genes are translated. After miRNAs were discovered in 1993[14], researchers have correlated changes in miRNA expression with diseases progression in multiple forms of cancer[15-18]. Numerous recent studies have detailed how miRNAs can be detected in plasma/serum while keeping their impressive stability[16,19-22]. Plasma/serum miRNAs resist endogenous ribonuclease activity through binding with plasma proteins such as Argonaute 2 and high-density lipoprotein (HDL)[23,24]or being surrounded by different secretory vesicles, including plasma/serum exosomes and apoptotic bodies[19,25-27]. Thus, miRNAs in peripheral blood are not digested by RNase or damaged by other conditions such as low or high pH, extended storage, boiling, and multiple freeze-thaw cycles. In addition, numerous extracellular miRNAs are made present by active secretion in addition to cell lysis[10,28,29]; such miRNAs are able to play a role as intercellular transmitters[22,28,30,31]. As one possible mechanism, the extracellular miRNAs involved in exosome vesicles has been reported to be released through ceramide-dependent secretory systems and function in recipient cells[29].
CIRCULATING MICRORNAS ARE A PROMISING SOURCE OF DIAGNOSTIC AND PROGNOSITC INFORMATION IN SOLID TUMORS
Mitchell et al.[19]first reported that circulating miRNAs had potential utility as new biomarkers in patients with solid cancers. As noninvasive and reproducible biomarkers in cancer patients, circulating miRNAs have since attracted the attention of researchers. As indicated by the usefulness of cell-free DNA and circulating tumor cells, the concept of “liquid biopsy” through circulating miRNAs may also provide ideal individualized therapeutic strategies for cancer patients and contribute to the development of precision medicine. Indeed,previous studies, including our own, have identified various blood-based miRNA biomarker candidates,which are useful for cancer detection, monitoring tumor dynamics, and predicting malignant potential,prognosis, and chemoresistance in cancer patients[32-45].
HIGH LEVELS OF CIRCULATING MICRORNAS IN PLASMA/SERUM IN GASTRIC CANCER
Various studies have identified circulating miRNAs for use in the diagnosis and prognosis of gastric cancer patients [Table 1]. In 2010, we reported the usefulness of circulating miRNAs and demonstrated their feasibility as biomarkers in the plasma of patients with gastric cancer. We selected four miRNAs (miR-17-5p,21, 106a, and 106b) that has been previously reported as upregulated in gastric cancer tissues, analyzed their levels in plasma using RT-qPCR, and confirmed their utility as diagnostic biomarkers[32]. We then identified plasma miR-451 and miR-486 as novel cancer screening markers using the Toray® 3D-Gene microRNAarray-based approach on pre- and postoperative samples[46]. The area under the curve (AUC) values for these markers were high, at 0.96 and 0.92, respectively for the diagnosis of gastric cancer[46]. Additionally,genome-wide miRNA expression profiles followed by RT-qPCR assays revealed that circulating miR-378 had an AUC of 0.861 with 87.5% sensitivity and 70.73% specificity[47]. As shown in Table 1, many circulating miRNAs have been previously identified (by our group and others) as promising blood biomarker candidates for the detection of gastric cancer: miR-16, miR-17-5p, miR-18a, miR-19b, miR-20a, miR-21, miR-23b, miR-25, miR-92a, miR-92b, miR-93, miR-100, miR-106, miR-106a, miR-106b, miR-107, miR181c, miR-185, miR-191, miR-192, miR-199a-3p, miR-200c, miR-210, miR-221, miR-222, miR-223, miR-331, miR-370, miR-378,miR-421, miR-451, miR-486-5p, and miR-664, all of which are up-regulated in plasma/serum. These are promising diagnostic biomarkers[32,40,46-55,57,58,69-89].

Table 1. High level of circulating microRNAs in plasma/serum in gastric cancer

D: diagnostic value; P: prognostic value; M: monitoring value
LOW LEVEL OF CIRCULATING MICRORNAS IN PLASMA/SERUM IN GASTRIC CANCER
Kosaka et al.[29,59,60]recently suggested that healthy cells secrete some tumor-suppressor miRNAs as a way of slowing aberrant cell growth. We have previously found that blood-borne tumor-suppressor miRNAs, such as let-7a[32]and miR-375[35,45]were significantly downregulated in comparison to those of normal volunteers. Circulating miRNAs are released from both normal and cancer tissues, and the majority of these tumor-suppressor miRNAs are thought to arise from normal tissues. We therefore hypothesize that the progression of cancer causes healthy cells to become depleted of some tumor-suppressor miRNAs. That hypothesis is supported by our previously data that shows that a decrease in the plasma level of the tumor-suppressor miR-375 in esophageal cancer patients[34]and this[61]is correlated with reduced survival. We have also proposed that tumor progression and the resultant poor prognostic outcomes are correlated with the downregulation of tumor-suppressor miRNAs in the bloodstream[34,35]. As shown in Table 2, various circulating tumor-suppressor miRNAs have previously been identified as promising blood biomarker candidates for the detection and diagnosis of gastric cancer. These include miR-15a, miR-17, miR-26a, miR-31, miR-92a, miR-93, miR-106b, miR-122, miR-181b, miR-195-5p, miR-203, miR-204, miR-206, miR-218, miR-375, miR-503, miR-940, and let-7a, which are downregulated in plasma/serum with a great degree of diagnostic ability[32,52,56,62,75,79,84,90-100].
CIRCULATING MICRORNAS RELATED TO MALIGNANT POTENTIAL, TUMOR RECURRENCE,AND PROGNOSIS BIOMARKERS IN PLASMA/SERUM IN GASTRIC CANCER
Wang et al.[70]have reported that high levels of plasma miR-17-5p and miR-20a were significantly correlated with poor overall survival in gastric cancer patients. Valladares-Ayerbes et al.[49]have also reported that higher expression levels of miR-200c in blood are associated with poor overall survival. We demonstrated that the postoperative cause-specific survival was significantly poorer in gastric cancer patients with high plasma miR-21 levels than in those with low levels[54]. Moreover, the incidence of vascular invasion was also slightly higher in gastric cancer patients with high miR-21 levels, and multivariate analysis revealed that the presence of high miR-21 plasma levels was an independent prognostic factor[54]. Therefore, various up-regulated circulating miRNAs have previously been identified as blood-based prognostic biomarkers for gastric cancer: miR-17-5p, miR-20a, miR-21, miR-23b, miR-25, miR-93, miR-106, miR-106b, miR-200c, miR-222, and miR-664 [Table 1][49,53,54,70,74,81,82,84,88].Regarding tumor-suppressor miRNAs, Imaoka et al.[62]reported that serum expression of miR-203 was significantly lower in stage IV than in stages I-III of gastric cancer patients. Serum miR-203 expression was significantly lower in gastric cancer patients with worse malignant potential, as indicated by higher T stage, vessel invasion, and nodal, peritoneal, and distant metastases. Low expression of serum miR-203 was correlated with poor disease-free survival and overall survival. This low expression was an independent predictive marker for metastases, including nodal, peritoneal, and distant metastases, and a poor prognosis in gastric cancer patients[62]. Therefore, various downregulated circulating miRNAs have been identified as blood-based prognostic biomarkers for gastric cancer: miR-15a, miR-93, miR-195-5p, miR-203, miR-204,miR-218 and miR-503 [Table 2][62,84,90,93,95,97,99].
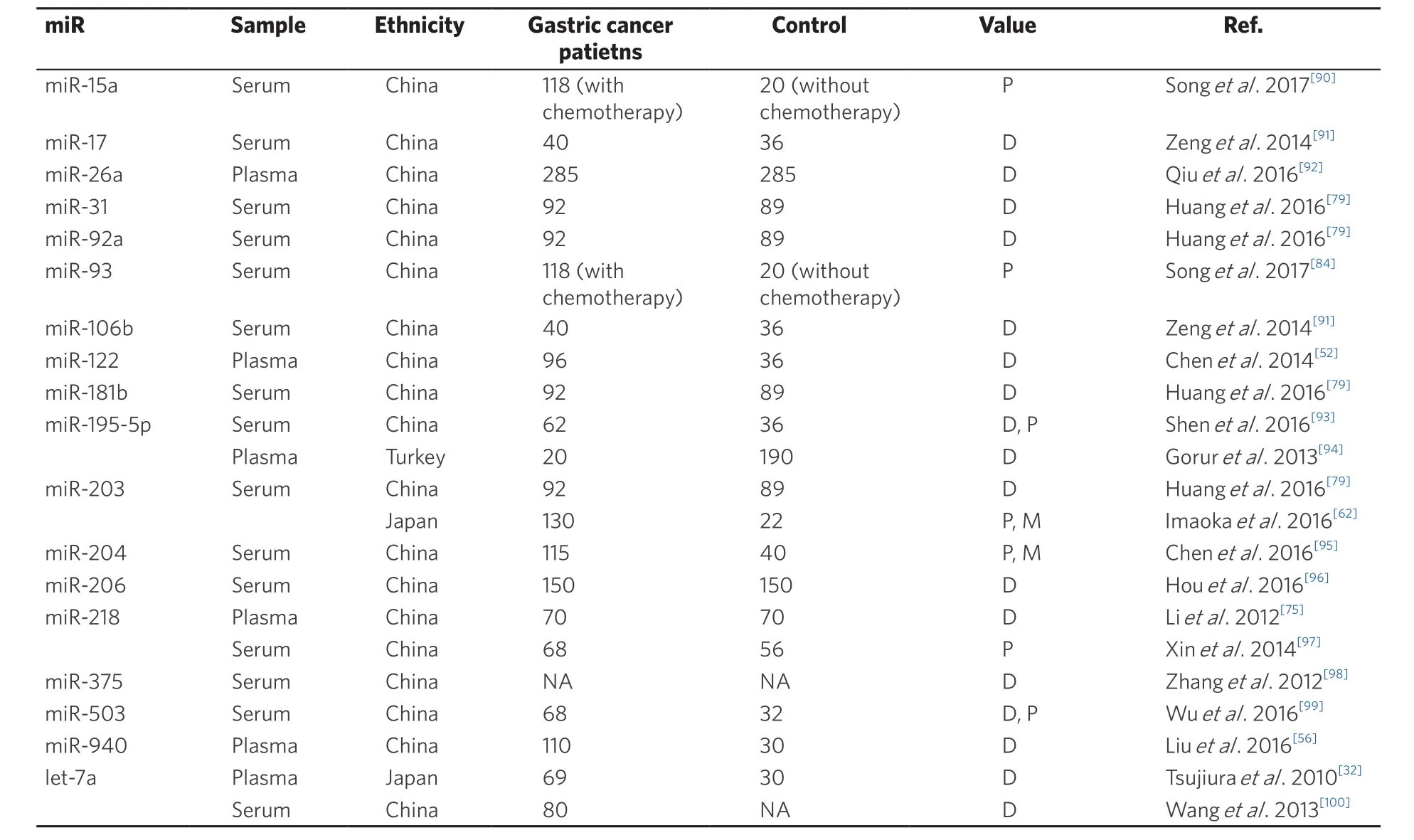
Table 2. Low level of circulating microRNAs in plasma/serum in gastric cancer
DIFFERENT EXPRESSION LEVELS OF SOME CIRCULATING MIRNAS BETWEEN PLASMA AND SERUM IN GASTRIC CANCER
From the viewpoint of liquid biopsy using blood miRNAs, many issues must still be addressed before novelfindings can be translated into clinically useful and noninvasive screening strategies for gastric cancer patients. Because plasma includes more abundant proteins, such as coagulation factors, than does serum,miRNA profiles in the plasma of cancer patients differ considerably from those in the serum[63], as has been shown in esophageal cancer[37,64]and pancreatic cancer[63]. In gastric cancer, the expression levels of some circulating miRNAs, such as miR-17, miR-92a, miR-93, and miR-106b, moved in opposite directions in the plasma and serum [Tables 1 and 2]. Although detailed mechanisms remain unknown, the data strongly suggest that these issues should be considered in future clinical applications of cancer treatments.
FUTURE PERSPECTIVES ON CIRCULATING TUMOR-SUPPRESSOR MICRORNAS FOR TREATMENT TARGETS IN GASTRIC CANCER
Multiple researchers have recently examined therapeutic miRNA-based drugs by using synthetic miRNA mimics[101]. Various efforts have been made to develop miRNA-based therapies in the past several years, and two studies have shown particular promise. The first study focused on the therapeutic silencing of diseaseassociated miRNAs using miRNA inhibitors. Miravirsen (Santaris Pharma) is one of several promising miRNA inhibitors; it can bind to miR-122 and inhibit its biogenesis. Miravirsen was developed for the treatment of hepatitis C and is currently under evaluation in clinical trials[65-67]. The second study examined therapeutic miRNA-based drugs through the use of synthetic miRNA mimics. Recently, a phase I clinical trial using the miRNA mimic MIRX34 (Mirna Therapeutics, Inc.) was performed[68]. MIRX34 is a synthetic miRNA mimic of the tumor suppressor miR-34 and was administered to patients with primary or metastatic liver cancer. This trial was ended because of serious adverse immune-related effects. The administration of tumor-suppressor miRNA mimics continues to bear undesirable risks and negative, unexpected physiological effects because multiple genes, regulating multiple biological functions, can be impacted by miRNAs. Restoring tumor-suppressor miRNAs, which are abundantly detected in the plasma/serum of healthy individuals but lowered in patients with cancer [Table 2], may minimize the physiological risks of systemic administration. We recently reported that restoring and maintaining the miR-107 plasma level significantly inhibited tumor progression in mice[61]. The systemic delivery of tumor-suppressor miRNAs in gastric cancer patients may thus provide significant advantages because effects can be repeatedly examined repeatedly using blood-based miRNA levels.
CONCLUSION
The development of liquid biopsy-based analyses could improve diagnosis and therapy for patients with gastric cancer. As a liquid biopsy, circulating miRNAs have the potential to diagnose gastric cancer at an early stage, predict prognosis and recurrence, evaluate patient status and therapeutic efficacy, and provide optimal, individualized treatment strategies. It should be noted that the present review is limited by examining a relatively small number of retrospective cohort studies. Additional research with large cohorts or prospective clinical trials with longer follow-up periods are therefore necessary to confirm the usefulness of candidate miRNAs. Translation into clinically useful gastric cancer treatments also requires significant additional work. The physiological effects of tumor-suppressor miRNAs must be examined in greater detail before they can be safely administered systemically, and their tumor-suppressive functions must be validated in vivo before clinical use. Delivery systems for miRNAs must be further refined to surmount problems such as cellular uptake and bloodstream stability. Finally, more powerful anticancer tumor-suppressor miRNAs should be found by examining the plasma of patients with different cancers, through methods such as microarray analysis, next-generation sequencing, and digital PCR-based approaches. Currently under evaluation, these strategies will likely provide the future’s next innovations.
DECLARATIONS
Authors’ contributions
Designed the research and wrote the paper: Komatsu S
Collected the data and performed data analyses: Komatsu S, Kiuchi J, Imamura T
Reviewed the paper: Komatsu S, Ichikawa D, Otsuji E
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflict of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2018.
 Journal of Cancer Metastasis and Treatment2018年7期
Journal of Cancer Metastasis and Treatment2018年7期
- Journal of Cancer Metastasis and Treatment的其它文章
- Circulating tumor cells in gastric cancer
- Necrotizing fasciitis as a complication of taxanes: a case report
- Current trends in gastric cancer treatment in Europe
- Surgical treatment of stage IV gastric cancer: is it worthwhile?
- New insights into the role of intra-tumor genetic heterogeneity in carcinogenesis: identification of complex single gene variance within tumors
- Laparoscopic personalized function-preserving gastrectomy with sentinel node mapping for earlystage gastric cancer
