Current trends in gastric cancer treatment in Europe
Satoshi Kamiya, Ioannis Rouvelas,2, Mats Lindblad,2, Magnus Nilsson,2
1Department of Surgery, Centre of Digestive Diseases, Karolinska University Hospital, Stockholm SE-14186, Sweden.
2Division of Surgery, Department of Clinical Science, Technology and Intervention (CLINTEC), Karolinska Institutet, Stockhom SE-14186, Sweden.
Abstract Gastric cancer is one of the major causes of cancer-related deaths, despite the gradual decrease of its incidence in the West. Minimally invasive procedures, such as endoscopic resection and laparoscopic gastrectomy, have been successfully introduced in European high-volume centres, in the treatment of early gastric cancer. Regarding advanced,localized gastric cancer a number of prospective trials have been completed in search of better therapeutic options,aiming to optimize the efficacy vs. adverse effect ratio. From the results of these prospective randomized trials, the therapeutic strategy has in the last decades shifted emphasis from adjuvant therapy to neoadjuvant or perioperative chemotherapy, in curatively intended treatment. Moreover, recent studies have shown promising results in the use of molecular targeted agents, both in perioperative and palliative settings. The introduction of molecularly targeted therapy will enable a personalized approach based on each patient’s and tumor’s characteristics, maximizing the benefits from chemotherapy. The present review article focuses on recent therapeutic trends, as well as future perspectives, of surgical and oncological gastric cancer treatment in the Western setting, mainly based on landmark clinical trials.
Keywords: Gastric cancer, surgery, neoadjuvant, adjuvant, perioperative, chemotherapy, Western
INTRODUCTION
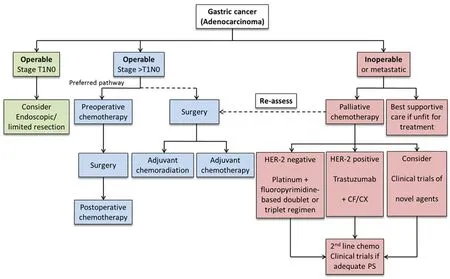
Figure 1. Algorithm for the management of gastric cancer in Europe[15]
Recent evidence provided by clinical trials and modern technical developments have strongly facilitated the employment of a multimodal approach in gastric cancer treatment. Endoscopic resection is now accepted as a curative option for early gastric neoplastic lesions[1,2]. At the same time, laparoscopic gastrectomy has increased in popularity in recent years[3,4]. For locally advanced gastric cancer, radical gastrectomy with D2 lymph node dissection has become the standard surgery in most European high volume centers[5]. In addition, perioperative chemotherapy (CT) is the standard therapy in curatively intended disease in most European countries[6-10], and also molecular targeted therapy has been implemented in human epidermal growth factor receptor (HER)-2 positive tumors in the palliative setting[11]. This review gives an overview of current surgical and perioperative management in curatively intended treatment for localized gastric cancer, as well as palliative management for metastatic disease, in Europe. Furthermore, we discuss recent therapeutic trends and future directions for gastric cancer management in a European setting.
GASTRIC CANCER IN EUROPE
There were 140,000 new cases of gastric cancer diagnosed across all European countries in 2012[12]. Gastric cancer is the sixth most common cancer and the fourth most common cause of cancer related death in Europe, causing 107,000 death annually. The treatment policy in Europe has lately, in several respects, been influenced by the Japanese Guidelines[13,14]and this is reflected in most European professional organization guidelines such as those from ESMO/ESSO/ESTRO[15]. For planning treatment, ESMO/ESSO/ESTRO guidelines require multi-disciplinary team conferences including surgeons, medical oncologists, gastroenterologists, radiologists, pathologists, dieticians and nurse specialists. Figure 1 shows an algorithm for the management of gastric cancer in Europe.
Gastric cancers in the West tend to have a large proportion of the diffuse type histology, often located in the proximal stomach, compared to typical histology and tumor position in the East, which more commonly tend to be of the intestinal type and typically located in the distal stomach. Furthermore, gastric cancer in Europe is more likely to be diagnosed in advanced stages due to the low incidence and consequential lack of screening programs[16,17]. Consequently, due to the difference in disease characteristics, the proportion of total gastrectomies performed is substantially higher in Western treatment populations and studies.
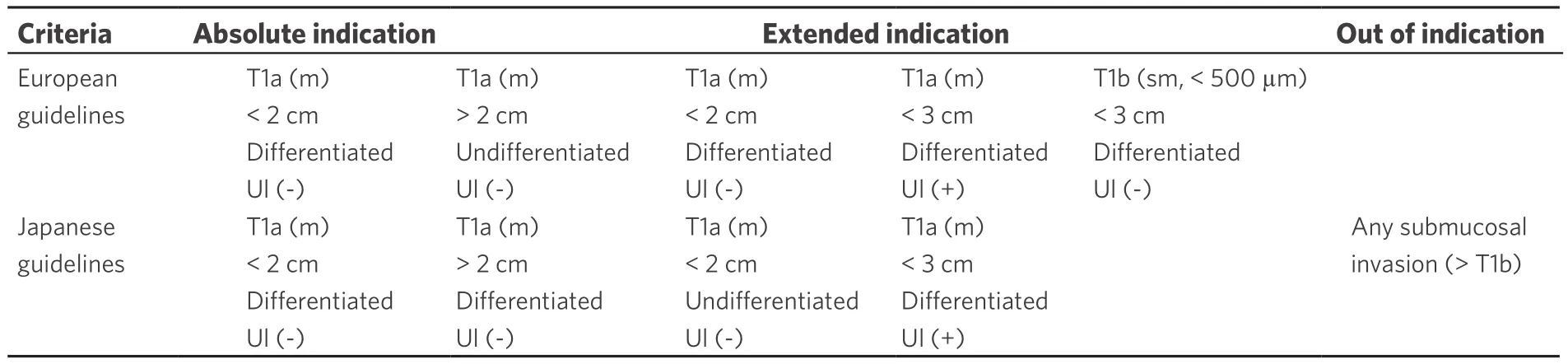
Table 1. Criteria for endoscopic submucosal dissection[19]
MANAGEMENT OF LOCAL/LOCOREGIONAL DISEASE
Endoscopic treatment
Only around 10%-15% of gastric cancers in Europe are diagnosed as early gastric cancers. Although adoption of endoscopic submucosal dissection (ESD) in the West has been slow, due to a lower incidence of early gastric cancer, European Society of Gastrointestinal Endoscopy (ESGE) guidelines recommend ESD as the treatment of choice for most superficial neoplastic gastric lesions[18]. Guidelines from the National Cancer Center in Tokyo have expanded these criteria based on a large number of patients[2,19]. ESD should be considered for lesions with very low risk of lymph node metastasis, no matter if it meets the absolute or expanded indication criteria [Table 1]. Western studies have demonstrated an en-bloc and R0 resection rate of 98.4%and 90.2%, respectively, which are comparable to corresponding results from Eastern Asian institutions[20].The delayed bleeding rate was 6% and perforation rate was 1% which are also equivalent to Eastern Asian rates[21-24]. The potential benefits of ESD are now acknowledged and ESD has become a promising treatment option, alongside conventional endoscopic mucosal resection (EMR), for early gastric cancer in Western countries.
Surgical treatment
Surgical resection remains the only treatment modality that is potentially curative for locally advanced gastric cancer. However, the extent of surgical resection and lymph node dissection is still, to some degree, controversial. Most European guidelines, nevertheless, recommend D2 dissection for stage II and III disease[15].At the same time, minimally invasive gastrectomy is becoming more and more common[25].
Extent of gastric resection
The extent of resection is basically determined by the tumor location as well as the tumor stage, the type and extension of stomach resection has a direct impact on patient’s postoperative quality of life (QOL)[26,27]. In Western, in contrast to Far Eastern countries, most gastric cancers are diagnosed in the proximal stomach as locally advanced tumors, which subsequently usually require total gastrectomy with D2 lymph node dissection for optimized prognosis. Therefore, the number of suitable cases for function preserving surgical techniques, such as proximal and pylorus-preserving gastrectomy, which have been popularized in Eastern Asia due to advantages of improved postoperative QOL, are very few in European populations[28]. The vast majority of diagnosed European gastric cancer cases are instead more suitable for subtotal or total gastrectomy. Several studies have shown some functional advantages and comparable overall survival (OS) rate in subtotal gastrectomy compared with total gastrectomy[26,27,29,30]. ESMO/ESSO/ESTRO guidelines recommend macroscopic proximal margins of 5 cm between the proximal tumor margin and esophagogastric junction(EGJ) for subtotal or distal gastrectomy, and of 8 cm for the diffuse histological type of gastric cancer[15].Nonetheless, some studies reported equivalence regarding oncological outcome with shorter proximal margin[31,32].
Lymph node dissection
Lymph node dissection is an important part of achieving local tumor control in gastric cancer treatment,and there has been much debate over the years on the optimal extent of this dissection. Traditionally, D2 lymph node dissection has been performed in Japan as standard practice since the 1960s, on the basis of excellent long-term outcomes in Japanese case series[13]. In Japan, D2 is the norm, while many surgeons in the West still prefer to perform D1 dissection. One of the reasons is the results of the well-known Dutch randomized clinical trial[33], which compared the survival advantage of D2 lymph node dissection with D1 resection, failing to demonstrate any benefits in D2 group in the main overall survival analysis. However, in this trial the postoperative mortality was very high in the D2 arm, which counterweighed any potential survival advantage of the extended lymph node dissection at 5 years follow-up. A stratified analysis showed that a large proportion of the morbidity and mortality in the D2 group was related to synchronous splenectomy and pancreatectomy while in the subgroup of patients without pancreaticosplenectomy the risk of relapse was significantly lower in the D2 compared to D1 group. However, in 10-year follow-up there was a signi ficant advantage in overall survival for the D2 group[34], despite the great losses in the early postoperative period. This and other publications showing excellent short term outcomes[35]after D2 gastrectomy in Western high volume centres has led to the current Western consensus that D2 dissection should be the standard procedure if carried out in specialized, high-volume centers[5].
Laparoscopic gastrectomy
Laparoscopic gastrectomy was launched in 1991 and the first laparoscopic total gastrectomy with D2 lymphadenectomy for advanced gastric cancer was reported in 2000 in Japan[36,37]. The clinical objective with this technique was to minimize the surgical access trauma while still providing the same oncological operation,in terms of T- and N-radicality, as open gastrectomy. Advantages suggested and to some extent proven with laparoscopic gastrectomy, compared to open surgery, are less postoperative pain, earlier recovery of bowel function, shorter hospital stay and better cosmetic result[37-39]. Furthermore, the concern from sceptics regarding the efficacy of the laparoscopic lymphadenectomy, has been relieved, as the number of harvested lymph nodes has been shown to be comparable to that of open surgery[40]. Although laparoscopic distal gastrectomy for early gastric cancer is gradually accepted as an oncologically safe alternative to open gastrectomy in Europe, laparoscopic total gastrectomy and laparoscopic D2 lymph node dissection for advanced cases are still considered challenging, due to their technical nature. With respect to surgical and oncological safety, these procedures should be carefully implemented in experienced hands at centres with high annual caseloads.
ADJUNCT THERAPY
Many clinical phase III trials on adjunct therapy for gastric cancer have been conducted worldwide. Despite high-level evidence supporting the principle of adjuvant or neoadjuvant treatment, there is no standard of care for adjunct treatment in gastric cancer. Main landmark trials are summarized in Table 2. The two major studies of adjunctive therapy in western populations, the North American Intergroup INT0116 trial[41], the MAGIC trial[42], demonstrated two major directions, postoperative chemoradiotherapy (CRT) and perioperative CT. Through many clinical trials, new regimens such as FLOT[43], enhancement of preoperative treatment, and application of molecular targeted therapeutics are attracting much attention.
Postoperative chemotherapy and chemoradiotherapy
The INT0116 trial, the first randomized study evaluating the benefit of adjuvant CRT[41], and a subsequent retrospective Dutch trial demonstrated that postoperative CRT improved OS and reduced local recurrence rates following D1 lymph node dissection or R1 resection[34]. Also the additional survival benefit of adjuvant CT has shown by Asian phase III ACTS-GC[44]and CLASSIC trial[45]in Asian patients. However, the ARTIST trial[46], a phase III trial from Korea, and the recent Dutch CRITICS trial failed to show a survival advantage of postoperative additional radiation therapy to perioperative CT[47,48]. In the CRITICS trial, only 47%and 52% of patients completed postoperative CT and CRT therapy, to a large extent due to low postoperative treatment tolerance in Western patients. This study suggested that Western adjunct treatment should shift to
preoperative strategies, considering patients’ tolerability for treatment. The subsequent ARTIST-II trial which focused on adjuvant CRT for node-positive patients[49]and CRIRTICS-II trial to evaluate the significance of preoperative CRT strategies for curative gastric cancer are now in progress.
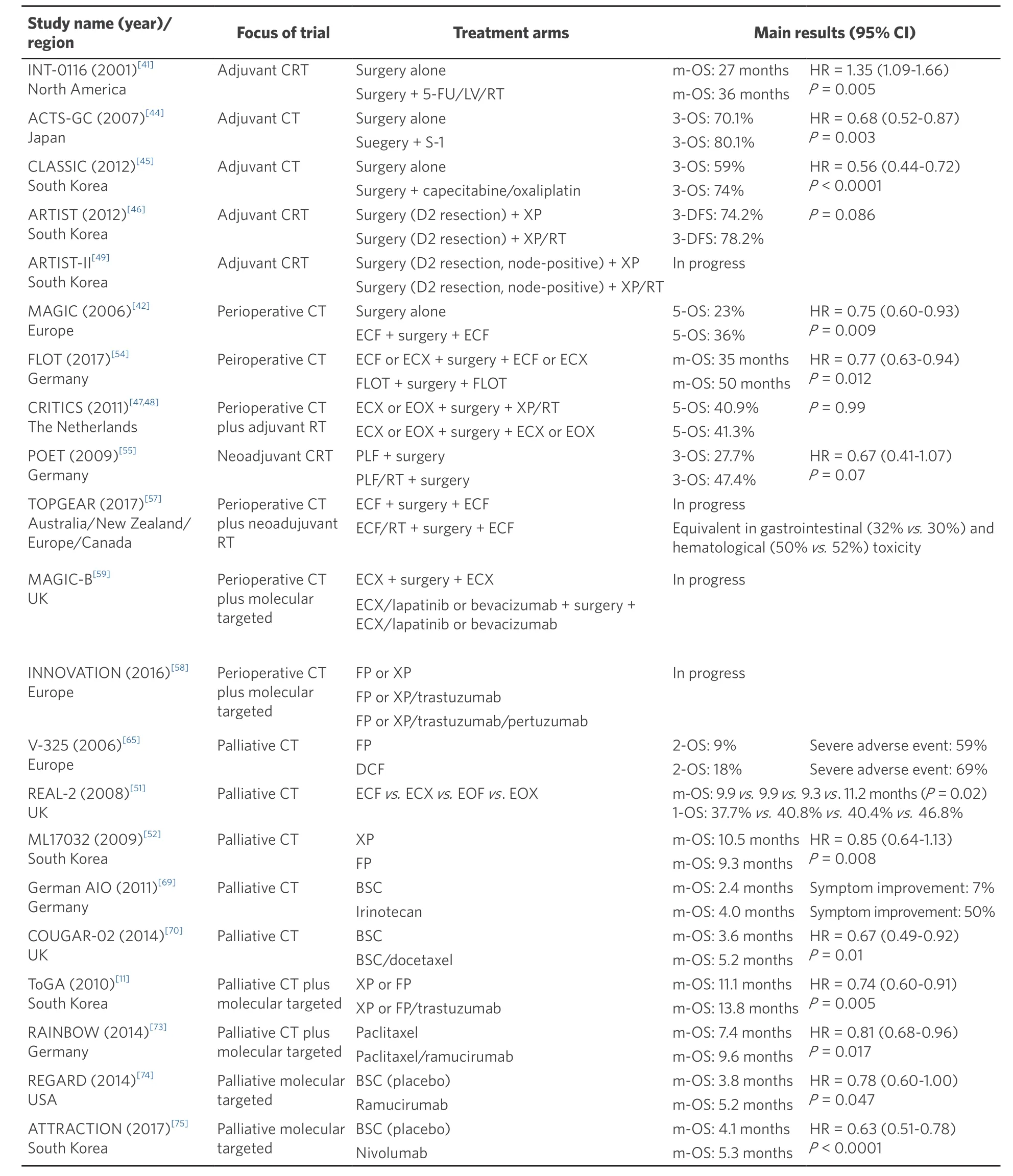
Table 2. Landmark trials of perioperative and palliative chemo/chemoradiotherapy in gastric cancer
Perioperative chemotherapy
In general, prior to surgery, patients usually tolerate adjunct treatment rather well, perhaps due to an intact performance status. Neoadjuvant chemotherapy has not been shown to increase postoperative morbidity or mortality, while neoadjuvant chemoradiotherapy may be associated to increased morbidity, at least for junctional tumors[50]. On this basis, all guidelines recommend this type of down-staging treatment for patients with locally advanced gastric cancer and perioperative therapy has therefore been widely adopted as the standard of care throughout Europe. The MAGIC trial was the first to provide the perioperative therapeutic option for resectable gastric cancer with favorable results of perioperative CT compared with surgery alone[42]. However, the epirubicin, cisplatin andfluorouracil (ECF) protocol used in the MAGIC trial has requirements that limit its use in non-trial situations (e.g., the need for a central line and constant specialized handling) and poor postoperative completion rates (42%). The REAL-2 trial showed that the ECF and epirubicin, oxaliplatin, and capecitabine (EOX) regimens were equally effective for advanced tumors, whereas a meta-analysis of the data from the REAL-2 and ML17032 trials suggested better response rates and OS with capecitabine combinations[51-53]. EOX regimen is now widely accepted as adjunct treatment in the West.
The recent FLOT4-AIO trial offered a new option with favorable results for locally advanced gastric cancer[43]. In this trial, 716 patients who had clinical stage T2 or higher and/or nodal positive disease were randomly assigned to either three pre- and postoperative cycles of epirubicin, cisplatin and either infusion offluorouracil (5-FU) or capecitabine (ECF/ECX group) or four pre-and postoperative cycles of 5-FU/leucovorin, docetaxel and oxaliplatin (FLOT group). Thirty-five percent of patients in the FLOT group had at least one serious adverse event involving a perioperative medical or surgical complication and 51% had grade 3-4 neutropenia, which was higher than 39% in ECF/ECX group. Overall 5-year survival was 45% in FLOT group, significantly better than the 36% in ECX/ECF group with a hazard ratio (HR) of 0.77 (95% confidence interval 0.63-0.94)[54]. FLOT type perioperative chemotherapy can now be considered the Western gold standard regimen in the treatment of locally advanced, non-metastatic gastric cancer.
A number of new clinical trials are in progress to investigate new neoadjuvant and adjuvant regimens to further improve outcomes. The reinforcement of preoperative treatment is one possible future direction.The German POET trial, which aimed to clarify the impact of additional preoperative radiotherapy to neoadjuvant CT for patients with EGJ adenocarcinoma, demonstrated a non-statistically significant improved median survival compared to the CT-alone group[55]. However, it showed a substantially higher rate of pathological complete response[56], in the CRT group (15.6% vs. 2.0%). These results emphasized the importance of strengthening the preoperative therapy, and thus neoadjuvant CRT has been suggested to be effective and beneficial. The TOPGEAR trial is currently evaluating the impact of additional preoperative radiotherapy to perioperative CT[57].
Another option for improving surgical outcomes is molecular targeted therapy, which has been demonstrated in the palliative setting. Some molecular targeted agents, such as trastuzumab and lapatinib, are being introduced into perioperative use. The INNOVATION trial, a 3-arm randomized phase II trial evaluating if neoadjuvant dual HER-2 blockade with CT, may lead to higher pathologic complete response rates than trastuzumab and CT, or CT alone, in resectable gastric cancer[58]. The MAGIC-B trial is also investigating the additional tyrosine kinase inhibitor lapatinib to perioperative ECX in the subset of the patients with HER2 overexpressing tumors[59]. These new studies are expected to provide new, molecularly tailored treatment options.
EUROPEAN TRENDS IN THE MANAGEMENT OF ADVANCED/METASTATIC GASTRIC CANCER
The aim of palliative CT is to increase survival and palliate the clinical symptoms of the disease, with as little toxicity and negative impact on QOL as possible. Available data from randomized clinical trials clearly show a statistically significant advantage of palliative CT, compared with best supportive care (BSC)[60]. To improve the efficacy and to reduce the adverse effects of CT, optimal agents and combinations are currently being sought.
First line
Historically, doublet regimens using platinum andfluoropyrimidine have been frequently used in palliative setting. Alternative to platinum/fluoropyrimidine doublet regimen, taxane-based regimen and irinotecan plus 5-FU are suggested[61]. Irinotecan and oxaliplatin have shown better tolerability and equivalent time-toprogression in comparison to cisplatin, and guidelines suggest that these agents are promising substitutes for cisplatin in combination withfluoropyrimidines as well as capecitabine for 5-FU within doublet and triplet regimen[53,62,63].
A meta-analysis demonstrated that adding anthracycline to platinum andfluoropyrimidine doublet signi ficantly improved survival[64]. Additional docetaxel to 5-FU/CDDP (DCF) is another option to strengthen the CT but careful use is necessary in the palliative setting, due to low margins to toxicity. The V-325 trial and FLOT trial showed the advantage of additional therapeutic effects by taxane-based triplet regimen[65,66]. Although DCF in V-325 trial was superior to CF in response rate (RR) (37% vs. 25%), time-to-progression (5.6 vs. 3.7 months) and 2-year survival rate (18% vs. 9%), the absolute benefit in terms of survival was less than 4 weeks and was counterbalanced by a significant high 3-4 adverse events rate. Similarly, FLOT regimen showed improved response rate (49% vs. 28%), better progression free survival (PFS) (9.0 vs. 7.1 months, P =0.79) and no significant benefit in median OS (17.3 vs. 14.5 months, P = 0.39). Although there were no differences in serious adverse events, QOL was worse in FLOT group.
These triplet regimens have not demonstrated convincing benefits in terms of survival, but instead increased toxicity rates. Therefore, these regimens are not generally accepted in the palliative setting, so far. The clinical question of which subgroups may be suitable for the stronger triplet regimens, for locally advanced or metastatic disease, is currently under investigation.
Second line and more
ESMO/ESSO/ESTRO Guidelines recommend the use of irinotecan, docetaxel and paclitaxel as second line therapy, since these agents have shown to improve OS and QOL compared to BSC in patients with a good performance status[67]. The European guidelines also stress the fact that both paclitaxel and irinotecan have been directly compared in a Japanese Phase III trial showing similar efficacy in median OS for 8 to 9 months[68]. The German AIO phase III study demonstrated superiority of irinotecan compared to BSC in terms of improvement in tumor-related symptoms as second line therapy[69]. The COUGAR-02 trial confi rmed that docetaxel achieved a significant benefit in OS (5.2 vs. 3.6 months, P = 0.01) in patients with a performance status of 0-2 after failure offluoropyrimidine/platinum regimen[70]. In spite of the fact that 21%of patients treated with docetaxel experienced grade 4 toxicities, significantly less pain and a trend towards less dysphagia and nausea, were reported. Based on the results of these well conducted randomized trials, a benefit of irinotecan and docetaxel as second-line treatment was clearly established for patients with good performance status. These treatment options should be offered with close monitoring of potential adverse effects.
Palliative radiation therapy
Guidelines mention that hypo-fractionated radiotherapy is an effective and well-tolerated treatment option for symptomatic locally advanced or recurrent disease[71]. In non-comparative observational studies, the overall response rates for bleeding, pain and obstruction symptoms were 74%, 67% and 68% respectively, low biological equivalent dose of > 39 Gy regimens appear to be adequate for symptom palliation[72].
TARGETED THERAPIES
As in other solid organ tumors, the biological abnormalities triggering the development and progression of gastric cancer are increasingly elucidated through ongoing research. Thesefindings have potentially important implications as investigators attempt to elucidate the key pathways driving the tumor in each individual patient.
Overexpression of the HER2 gene, which is present in approximately 10%-20% of gastric cancers, is more common in intestinal type than diffuse type gastric cancer and more common in EGJ cancer than distal gastric cancer. Following the phase III ToGA trial, which demonstrated statistically significant improvement in PFS and OS with the addition of trastuzumab to a cisplatin/5-FU doublet regimen, trastuzumab was licensed in Europe for use in HER-2 positive disease in combination with capecitabine or 5-FU and cisplatin doublet[11]. This regimen currently represents the standard of care for these palliative patients. Also, the large phase III RAINBOW trial and REGARD trial, have shown the survival benefit of ramucirumab, a monoclonal antibody VEGFR-2 antagonist, as second line in the palliative setting[73,74]. Moreover, nivolumab, a fully human IgG4 monoclonal antibody inhibitor of programmed death-1, significantly improved the median overall survival in patients with advanced gastric cancer, or gastro-esophageal junction cancer, who had been previously treated with two or more chemotherapy regimens (ATTRACTION-2 trial)[75]. Several studies targeting HER2, VEGF, EGFR, T-DM1 are currently ongoing with some potentially favorable results[76-80]. Further developments in molecular subtyping of gastric cancer are likely to offer new possibilities in personalized treatment of gastric cancer in the future[81-84].
CONCLUSION
It is clear that the multimodal therapy encompassing both radical surgical treatment and perioperative CT/CRT offers the best possibility to cure resectable gastric cancer. In Western countries, minimally invasive approaches and D2 dissection have been successfully implemented at some high-volume centers. However,these procedures are still not standardized in the whole population-based case-load of incident cases, due to the low incidence of gastric cancer in Europe and other Western populations. Although the prospective clinical trials performed have achieved clear improvements in the therapeutic outcomes and patients’ prognosis in the last decades, an optimal treatment for advanced gastric cancer has not been established, given the still poor overall survival. Recent advances in molecular tumor biology of adenocarcinoma of the stomach offer us important clues about future tailoring of gastric cancer treatment. Furthermore the rapid developments in sequencing techniques are likely to revolutionize our understanding of disease biology in the next decades. It is very likely that a number of new biomarkers will provide completely new options for personalized therapy,which may realize substantial therapeutic improvements, with excellent efficacy and tolerable adverse effects.
DECLARATIONS
Authors’ contributions
Designed the study, reviewed the literature, and wrote the manuscript: Kamiya S
Contributed to writing the manuscript, drafting, critical revision, editing, andfinal approval of thefinal version: Nilsson M
Contributed to critical reversion of the manuscript andfinal approval of thefinal version: Rouvelas I, Lindblad M
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declare that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2018.
 Journal of Cancer Metastasis and Treatment2018年7期
Journal of Cancer Metastasis and Treatment2018年7期
- Journal of Cancer Metastasis and Treatment的其它文章
- Circulating tumor cells in gastric cancer
- Necrotizing fasciitis as a complication of taxanes: a case report
- Surgical treatment of stage IV gastric cancer: is it worthwhile?
- New insights into the role of intra-tumor genetic heterogeneity in carcinogenesis: identification of complex single gene variance within tumors
- Laparoscopic personalized function-preserving gastrectomy with sentinel node mapping for earlystage gastric cancer
- Circulating microRNAs as a liquid biopsy: a nextgeneration clinical biomarker for diagnosis of gastric cancer
