Circulating tumor cells in gastric cancer
Kenichi Nakamura, Masaaki Iwatsuki, Junji Kurashige, Takatsugu Ishimoto, Yoshifumi Baba, Yuji Miyamoto, Naoya Yoshida, Masayuki Watanabe, Hideo Baba
1Division of Gastric Surgery, Shizuoka Cancer Center, Shizuoka 411-8777, Japan.
2Department of Gastroenterological Surgery, Graduate School of Medical Sciences, Kumamoto University, Kumamoto 860-8556, Japan.
3Esophageal Cancer Division, Gastroenterological Surgery, Cancer Institute Hospital of Japanese Foundation for Cancer Research, Tokyo 135-8550, Japan.
Abstract Circulating tumor cells (CTCs) have received a lot of attention as a novel biomarker for cancer research in past decades.CTCs infiltrate the bloodstream derived from the primary tumor, and are significantly involved in cancer metastasis and recurrence. Although clinical applications have been challenging owing to the difficulties of CTC identification, recent development of technology for specific enrichment and detection of CTCs contributes to diagnosis and treatment.Furthermore, CTC analyses will shed new light on the biological mechanisms of cancer progression and metastasis. A number of clinical studies have already been carried out on the basis of CTC technology. Nevertheless, the clinical utility of CTCs is still unknown in gastric cancer. In this review, we elaborate on the latest advances of CTC research in gastric cancer.
Keywords: Circulating tumor cells, gastric cancer, cancer progression and metastasis, tumor heterogeneity, epithelial mesenchymal transition, cancer stem cells, immune check point blockade
INTRODUCTION
Gastric cancer is the sixth most common cancer and the third leading cause of cancer death worldwide[1].Although diagnostic and therapeutic modalities for gastric cancer have been developed, it remains difficult to treat and manage patients with gastric cancer owing to the high frequency of metastasis and recurrence even after curative resection. Thus, to improve prognosis of gastric cancer, it is crucial to understand the process of metastasis and recurrence.Cancer metastasis and recurrence have been conventionally diagnosed by imaging test or serum tumor marker; however, these modalities cannot provide a precise and timely assessment of the process of metastasis and recurrence. This process has been interpreted as involving the circulating tumor cells (CTCs),which are infiltrated into the bloodstream. The detection of CTCs was first described in 1869[2], and the “seed and soil” hypothesis was proposed in 1889[3]. This hypothesis suggested that the dissemination of metastatic tumor cells was organ-specific and not simply anatomic. Because the isolation and detection of CTCs in the blood was technically difficult, the critical role of CTCs hasfinally been demonstrated more than a century later[4]. At last, recent technology has contributed to the diagnosis and treatment of various cancers. The utility of CTCs for early diagnosis, prediction of prognosis, monitoring of the response to anticancer drugs,and early detection of recurrence has been demonstrated in several types of human cancer[5-8]. Moreover, it is expected that the research of CTCs elucidates the biological mechanisms of cancer metastasis and leads to better understanding of tumor heterogeneity. However, the clinical significance of CTCs and its biology in gastric cancer remain controversial. In this article, we review the latest progress of CTCs in gastric cancer.
CANCER METASTASIS AND CTCS
Cancer metastasis is composed of several complex and interrelated steps, including transformation, migration,local invasion, intravasation into circulation, detachment, arrest at organs, extravasation, colonization, and proliferation. All steps are absolutely integral to the establishment of metastasis. In these processes, CTCs exhibit phenotypic diversity, such as epithelial mesenchymal transition (EMT) phenotype, and cancer stem cell(CSC) phenotype[9], which facilitates metastasis.
EMT has been shown to play a critical role in metastatic spread by enhancing cancer cell mobility[10]. During EMT, epithelial cells change phenotype, such as reduction of cell-cell contacts, loss of polarity, development of cell mobility and invasiveness, repression of epithelial markers, and acquisition of mesenchymal phenotype. Epithelial markers [e.g., epithelial cells adhesion molecule (EpCAM), cytokeratin (CK), or E-cadherin] downregulate, while mesenchymal markers (e.g., vimentin, or N-cadherin) upregulate through EMT. Cancer cells undergoing EMT may intravasate as CTCs. Iwatsuki et al.[11]suggested that vimentinpositive tumor cells could survive in the peripheral circulation and the bone marrow and that vimentinpositive cancer cells invading intratumoral vessels must have undergone mesenchymal transition in gastric cancer. Furthermore, Wu et al.[12]reported that mesenchymal CTCs detected by using EMT markers were more commonly found in patients with metastatic sites of several types of human cancers.
In the EMT process, cancer cells can acquire stem cell-like properties, such as self-renewal, tumor initiation,undifferentiated status, and treatment resistance[13]. CD44 has been reported as a representative marker of CSCs in gastric cancer[14]. It has been demonstrated that CD44-positive CTCs were associated with cancer progression and recurrence in gastric cancer[15]. A recent study revealed that CD44-positive CTCs decreased after surgery or chemotherapy; therefore, they may be a predictive marker of treatment response in gastric cancer[16].
METHODOLOGY IN CTCS IDENTIFICATION
CTC identification typically undergoes two processes of “enrichment” and “detection”. The enrichment process is needed to detect CTCs efficiently, because CTCs are extremely rare, ranging between 1 to 10 cells per 10 mL in the peripheral blood[17]. CTCs can be enriched based on their biological and physical properties.Then, CTCs are detected using immunological, molecular, and functional assays [Table 1 and Figure 1][18].
Enrichment techniques
Biological property-based techniques
The biological enrichment techniques are based on specific surface makers detected by antibodies. Epithelial
cell markers are present on normal epithelial surface and epithelial tumors (i.e., carcinomas), but absent on normal blood cells; therefore, they can be used to identify the cancer cells in the bloodstream apart from normal blood cells. EpCAM and members of the CKs family (e.g., CK8, CK18, and CK19) are frequently used for positive selection of epithelial CTCs. However, epithelial cells can undergo EMT, resulting in downregulation of epithelial markers. To prevent false-negatives caused by EMT, N-cadherin and vimentin are used for identification of mesenchymal CTCs. In addition, to enrich CTCs specifically, negative selection is performed by using antibodies against CD45. CD45 is specifically expressed on the surface of leukocytes,whereas it is not expressed on carcinoma cells; thus, anti-CD45 antibody can deplete unnecessary leukocytes.
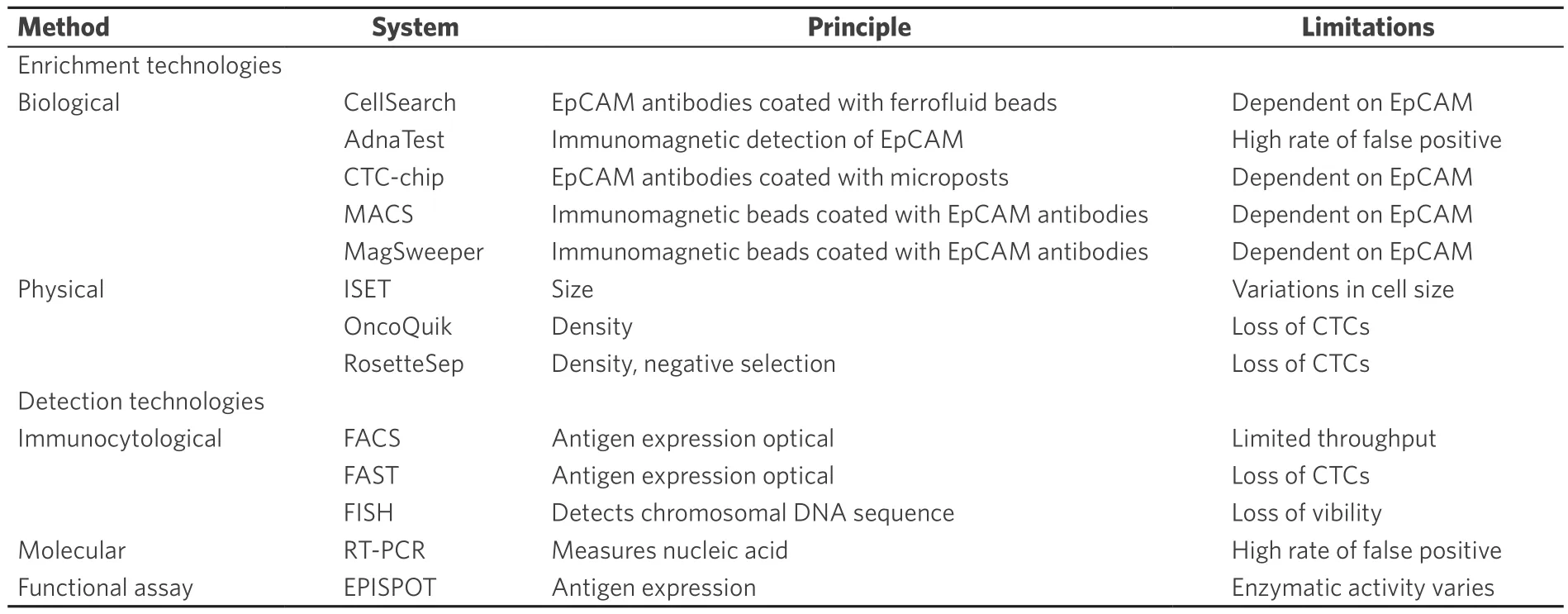
Table 1. CTC enrichment and detection technologies
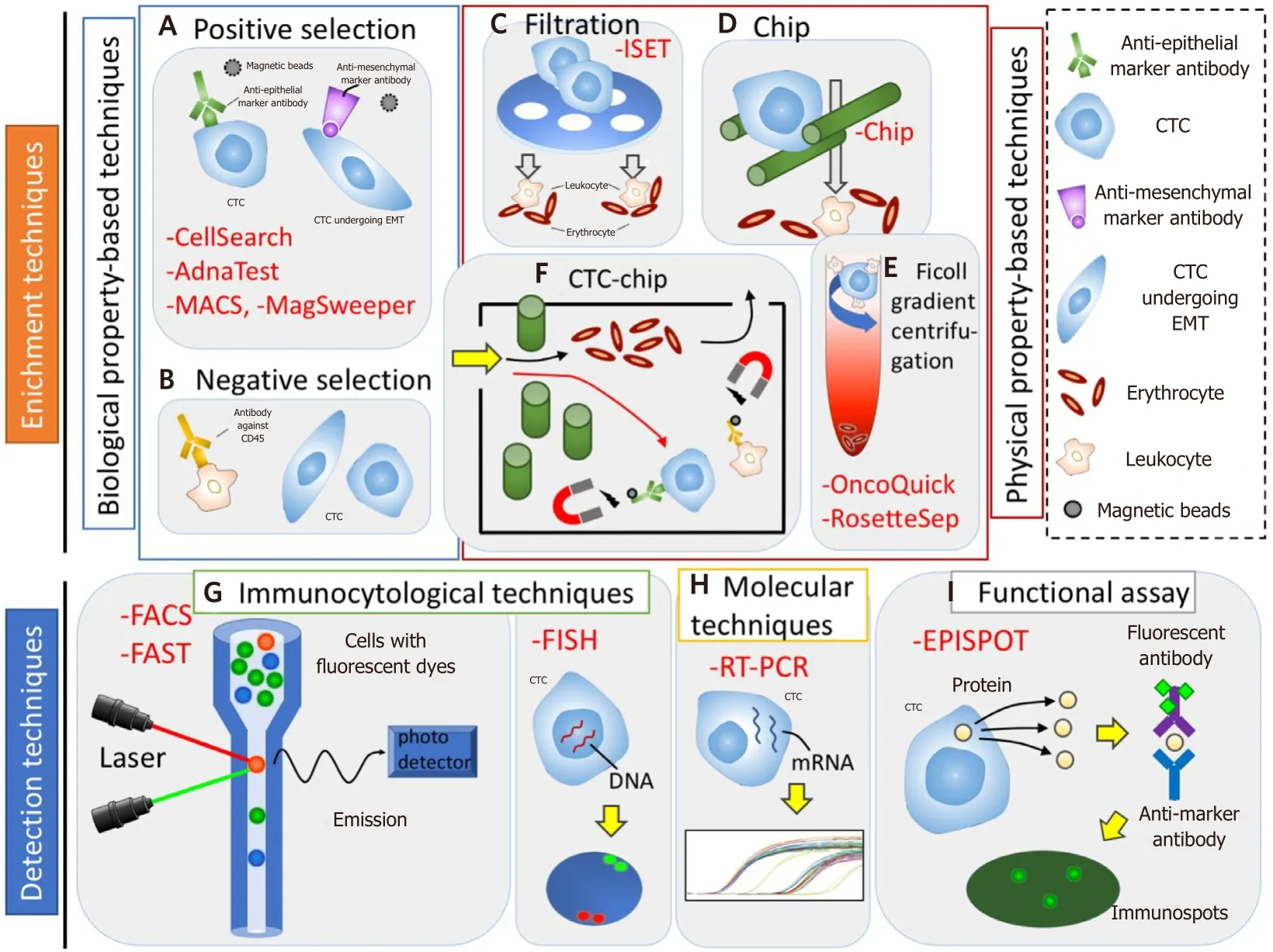
Figure 1. Circulating tumor cells (CTCs) enrichment (A-F) and detection (G-I) technologies. A and B: biological property-based techniques. A: positive selection - CTCs can be positively enriched using anti-epithelial or anti-mesenchymal marker antibody; B:negative selection - CTCs can be negatively enriched by depleting leukocyte using antibody against CD45. C-E: physical property-based techniques. C: filtration - CTCs are filtered using a membrane on the basis of the CTC size; D: chip - CTCs are trapped using microchip on the basis of CTC size and deformability; E: ficoll gradient centrifugation - CTCs are separated through a centrifugation on a ficoll density gradient on the basis of CTC density. F: physical and biological property-based techniques, CTC-chip - firstly, CTCs are selected on the basis of CTCs size, and then CTCs are isolated by magnetic bead-conjugated EpCAM antibodies, while normal hematopoietic cells are depleted by magnetic bead-conjugated antibodies against CD45. G: immunocytologial techniques - CTCs can be detected by using a combination of anti-epithelial, anti-mesenchymal, anti-tissue-specific marker, or anti-tumor-associated antibodies. H: molecular techniques - CTCs can be detected by using RNA-based technologies. I: functional assay - viable CTCs can be isolated by detecting secretion of specific tumor proteins from CTCs. MACS: magnetic activated cell sorting; FACS: fluoroscence-assited cell sorting; FAST:fiber-optic array scanning technology; FISH: fluorescence in situ hybridization; RT-PCR: reverse transcription polymerase chain reaction;EPISPOT: epithelial immunospot; EMT: epithelial mesenchymal transition
Tumor-specific makers, such as carcinoembryonic antigen (CEA), or α-fetoprotein (AFP), are also used for biological CTC enrichment. In particular, human epidermal growth factor 2 (HER2) is suggested to be important biomarkers in the context of recent targeted therapies[19].
On the basis of these techniques, there are various enrichment techniques. Magnetic activated cell sorting(MACS) uses magnetically labeled antibodies to enrich EpCAM-positive CTCs[20]. MagSweeper (Illumina,Hayward, CA, USA) is an automated immunomagnetic cell isolator for separation of rare endothelial cells[21].
CellSearch System® (Veridex) captures CTCs using ferrofluid beads coated with anti-EpCAM antibody.Then, captured EpCAM-positive CTCs are stained with anti-CK and anti-CD45fluorescently-conjugated dyes. Finally, enumeration of EpCAM-positive, CK-positive, and CD45-negative CTCs is completed by immunofluorescence[22]. The US Food and Drug Administration (FDA) has approved CellSearch for clinical use in breast, colorectal, and prostate cancer patients[6,23,24]. However, CTC detection by this system is not suitable for non-epithelial phenotype or EMT phenotype not expressing EpCAM and CK.
AdnaTest (AdnaGen AG, Langenhagen, Germany) is also an assay combining the enrichment and detection processes; that is, enriched by the magnetic procedure and detected by RT-PCR for identification of tumorassociated transcripts[25].
CTC-chip is based on a microfluidic platform that contains an assortment of microposts coated with anti-EpCAM antibodies. Whole blood is pumped through this chip and EpCAM-positive cells are isolated and detected by cameras identifying their morphology, viability and the expression of tumor markers[26].
Physical property-based techniques
Other enrichment techniques depend on physical properties of CTCs, such as size, diameter, density,deformability, and electric charge. The tumor cells were previously thought to be larger (> 8 μm), and less deformable than blood cells. Isolation by size of epithelial tumor cells (ISET; RareCells, Paris, France) isolates epithelial cancer cells by using bloodfiltration with a membrane with 8 μm pores; thus, larger cancer cells arefiltered. ISET can detect a single CTC from 1 mL of peripheral blood[27].
Density-dependent cell separation uses an inert polysucrose called Ficoll (GE Healthcare Bio-Science,Pittsburg, PA; BD Bioscience, San Jose, CA). Ficoll was originally developed to isolate intact mononuclear blood cells from whole blood. Oncoquick™ (Greiner Bio One, Frickenhausen, Germany) based on Ficoll is a density gradient centrifugation system that can separate CTCs from whole blood samples[28].
RosetteSep™ (StemCell Technologies, Vancouver, BC, Canada) is based on negative selection consisting of the depletion of the majority of the leukocytes and erythrocytes. This method employs a complex of antibodytargeted hematopoietic cells in human whole blood and crosslinks them to multiple erythrocytes, which leads to immunorosette formation. A centrifugation over a buoyant density medium such as Ficoll-Paque®allows for the precipitation of immunorosettes and unbound red blood cells, while CTC fractions can be recovered from the medium.
Although cell filtration and centrifugation force have been investigated on the basis of these properties in past decades, it has been demonstrated that variations in CTC size have identified, and CTCs after undergoing EMT could be as deformable as leukocytes[29]. Therefore, new approaches have been developed to improve specificity of CTC enrichment.
Detection techniques
After CTC enrichment, CTCs are detected by many different assays. Recent CTC identification assays combine enrichment and detection processes (e.g., CellSearch System, ISET, AdnaTest, CTC-chip, and EPISPOT). Other detection technologies include immunocytological techniques, molecular techniques, and functional assays.
Immunocytological techniques
Immunocytological techniques detect CTCs using antibodies against various antigens. These provide characteristics with high accuracy and subpopulation quantification with high specificity for simultaneous analysis with multiple parameters. However, the drawback of these techniques is lower sensitivity compared with molecular techniques.
Fluoroscence-assited cell sorting (FACS) is widely used to separate a specific cell population by using antibodies. Since FACS can analyze many parameters simultaneously, it is a versatile method with a wide range of applications. FACS sorts each cell individually, meaning that throughput of FACS is limited.Moreover, sorting conditions may be harmful to certain types of cells[30].
Fiber-optic array scanning technology (FAST; SRI International, Menlo Park, CA) can more efficiently analyze large numbers of immunofluorescent-labeled cells in peripheral blood. FAST applies laser-based techniques to scan broad fields of view, and can detect and characterize CTCs extremely quickly and accurately. As FAST can analyze larger volumes of peripheral blood, it does not require an additional enrichment step and reduces the risk of cell loss[31].
Fluorescence in situ hybridization (FISH) can precisely detect specific DNA sequences within chromosomes by usingfluorescent probes. However, FISH requires high proficiency, and sometimes cannot provide clear results. To overcome these problems, a novel technology named Ikoniscope® (Ikonisis, New Haven, CT)was developed for rare cell detection[32]. This system can detect one CTC per milliliter of peripheral blood.However, cells no longer have viability after FISH; therefore this technology has limited application for analyzing CTC.
Molecular techniques
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) can analyze the expression of specific markers in CTCs. Specificity of qRT-PCR has been reported to be superior to that of immunohistochemistry[33]. Nowadays, a multiplex RT-PCR approach combined with liquid bead array detection has been developed to perform simultaneous amplification and detection of multiple biomarkers.However, there are several limitations, such as the contamination of non-malignant cells, the high rate of false positives, and amplification of cell-free nucleic acids[34]. In addition, once RNA has been collected from cells, the cells cannot undergo advanced analysis.
Functional assay
Epithelial immunospot (EPISPOT) detects specific tumor marker proteins secreted by CTCs[35]. Only viable CTCs are detected by EPISPOT because non-viable CTCs are not enough to detect secretion of proteins.EPISPOT is much more sensitive than ELISA when detecting secretion of CK19 from CTCs[36]. However,because EPISPOT detects only CXCR4-positive CTCs, analysis of the heterogeneity of CTCs captured is limited.
While these developments can make CTC isolation accurate, further research on molecular characterization is necessary to confirm the significance of CTCs. Thus, the number of validation studies focusing on the characterization of CTCs has increased in recent years.
CLINICAL UTILITY OF CTCS IN GASTRIC CANCER
There have been many previous studies of CTCs in gastric cancer, as summarized in Table 2. Although there are various methodologies of CTCs identification (e.g., RT-PCR, FACS, CellSearch System), determining the most appropriate detection method and marker of CTCs in gastric cancer remains controversial. Several meta-analyses have demonstrated that the presence of CTC is associated with advanced clinicopathological features and poor survival in gastric cancer[37,38]. Huang et al.[39]indicated that CTCs was associated with advanced stage, undifferentiated histological type, lymphatic invasion positive, and poorer survival.
Furthermore, CTC detection has been suggested to be a useful biomarker of diagnosis. Although previous meta-analysis showed that CTC cannot be recommended as a screening test of gastric cancer owing to lower and inconsistent sensitivity estimates for CTCs, a recent study demonstrated that CTC detection based on FAST technique, in contrast to previous studies mainly based on RT-PCR, can be an available biomarker for early diagnosis of gastric cancer with high sensitivity and specificity[40]. In addition to diagnosis and prediction of prognosis, recent studies reported that monitoring changes of CTCs during treatment may be a predictive marker of response to treatment. Li et al.[41]demonstrated that elevated CTCs (≥ 3) during treatment were significantly associated with poor response rates and shorter survival. Notably, conversion to CTCs less than 3 after therapy improved the prognosis, while change to CTCs 3 or higher exhibited significantly worse prognosis. Shimazu et al.[42]reported that gastric cancer with diffuse bone metastases might have a very high CTC count (> 200) in a small cohort. In cases with decrease of CTC count after treatment, tumor was sensitive to chemotherapy. They suggested that the change of CTC counts during treatment could be a predictive biomarker[42].
HER2 has become a significant molecule for targeted therapy in gastric cancer. Trastuzumab (anti-HER2 monoclonal antibody) improved survival for patients with HER2 overexpressing gastric cancer. Although the assessment of HER2 status is usually performed on biopsy tissues from primary site, it has been reported that a discrepancy of HER2 status between the primary and the metastatic site was observed in some cases[43]. There has been an attempt to use CTCs for reassessment of HER2 status in recurrence or metastatic sites[44]. Mishima et al.[45]found a number of patients whose primary tumors were HER2 protein negative but who had HER2 gene positive CTCs by using 3D-FISH in gastric cancer. Furthermore, those patients had a favorable response to trastuzumab, and the second stage of the phase 2 trial is ongoing.
FUTURE PERSPECTIVES
Heterogeneity of CTCs
Tumor heterogeneity has been well-known to show genetic and phenotypic diversities between different tumor types, and within the same tumor and the same patient. It has been reported that heterogeneity was associated with the response and resistance to treatment[46]. Since the tumor heterogeneity changes throughout treatment, the serial profiling of disease is needed. However, there have been no diagnostic modalities or biomarkers available for timely and accurate assessment of heterogeneity. Therefore, much attention has been paid to monitoring dynamic changes of tumor heterogeneity during treatment by detecting CTCs, which is a minimally invasive and repeatable procedure, and may allow for reassessing the biology even in recurrence or metastasis. Scher et al.[47]demonstrated that the degree of heterogeneity couldserve as a biomarker of therapy option.
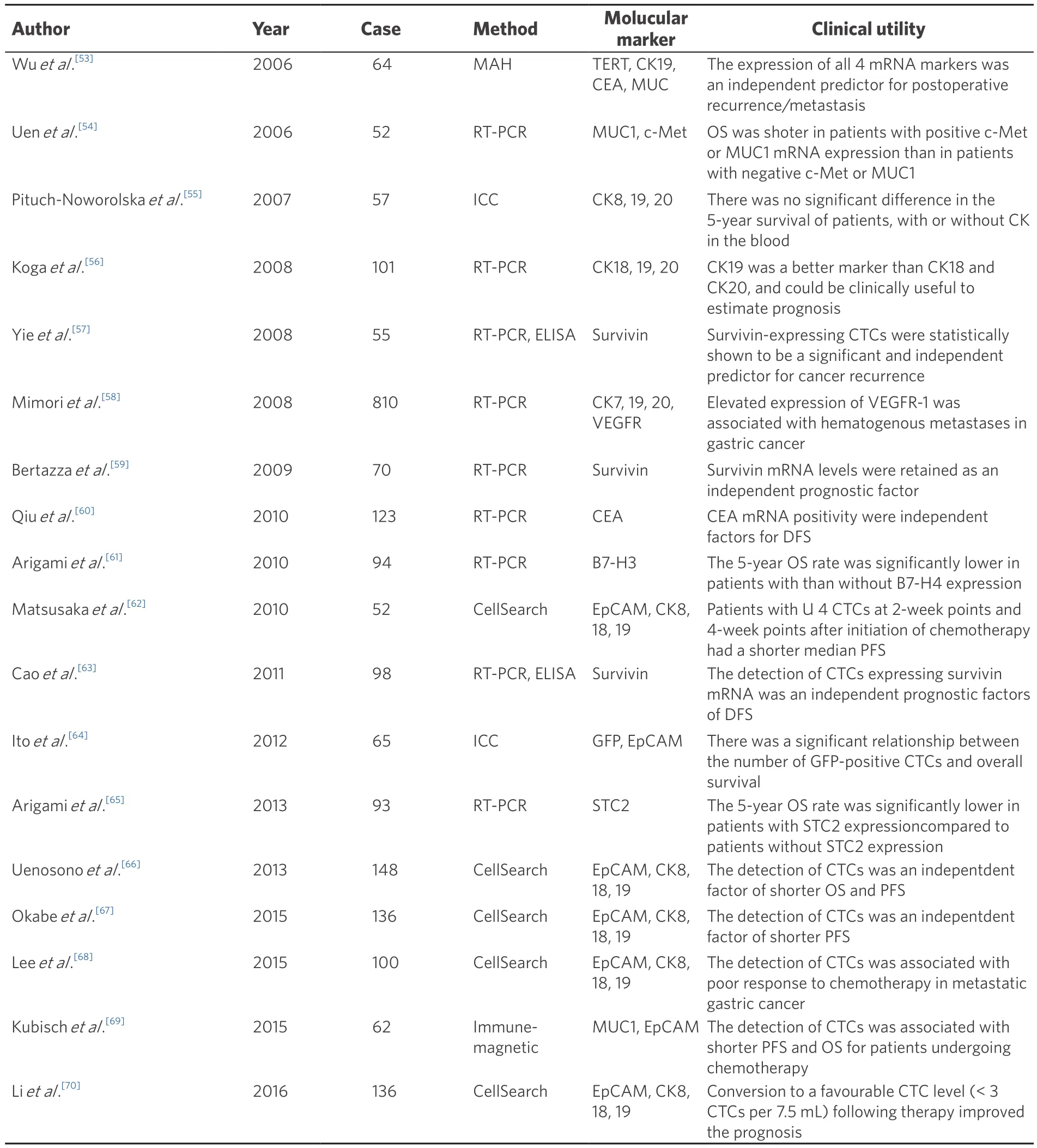
Table 2. Clinical utilities of CTCs in gastric cancer
Furthermore, the advances in single-cell technologies have enabled individual CTC characterization, leading to improved understanding about tumor heterogeneity. Alix-Panabières and Pantel[48]reviewed genomic,transcriptomic, and proteomic characterization of single CTCs in different cancer types, and suggested that analysis of single CTCs may play a key role in understanding the mechanism of resistance to cancer therapy.
PD-L1 expression on CTCs
Immune check point blockade with programmed cell-death protein 1 (PD-L1) inhibitor has recently attracted attention as a novel anticancer approach for treatment of advanced cancers. Overexpression of PDL1 has been considered a potential mechanism of tumor escape immune elimination[49]. PD-L1 inhibitors are currently being most actively investigated for clinical use in various cancers. PD-L1 expression has been evaluated by mainly immunohistochemistry for primary tumor site as a predictive biomarker of response.However, recent studies reported tumor heterogeneity in both primary and distant metastatic site[50].
CTCs survive in the bloodstream by exploiting immune escape mechanisms, including immune check point molecule. Therefore, it is crucial to understand the interaction of CTCs with the immune system to utilize more effective immunotherapies. Mazel et al.[51]demonstrated that PD-L1 frequently upregulated in CTCs of metastatic breast cancer patients. Furthermore, Strati et al.[52]showed that the detection of CTCs overexpressing PD-L1 mRNA at the end of treatment was associated with poor survival, and the absence of PD-L1 overexpression at the end of treatment was related with complete response in head and neck squamous cell carcinoma.
CONCLUSION
Although there are many studies focusing on the utility of CTCs for diagnosis, prediction, monitoring, and choosing therapy, CTCs have not been used yet in clinical practice for gastric cancer. Therefore, further investigation and clinical studies are necessary to achieve clinical utility of CTC in gastric cancer.
DECLARATIONS
Authors’ contributions
Wrote the initial draft of the manuscript: Nakamura K
Contributed to interpretation of data, and assisted in the preparation of the manuscript: Iwatsuki M
Contributed to data collection and interpretation, and critically reviewed the manuscript: Kurashige J,Ishimoto T, Baba Y, Miyamoto Y, Yoshida N, Watanabe M, Baba H
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2018.
 Journal of Cancer Metastasis and Treatment2018年7期
Journal of Cancer Metastasis and Treatment2018年7期
- Journal of Cancer Metastasis and Treatment的其它文章
- Necrotizing fasciitis as a complication of taxanes: a case report
- Current trends in gastric cancer treatment in Europe
- Surgical treatment of stage IV gastric cancer: is it worthwhile?
- New insights into the role of intra-tumor genetic heterogeneity in carcinogenesis: identification of complex single gene variance within tumors
- Laparoscopic personalized function-preserving gastrectomy with sentinel node mapping for earlystage gastric cancer
- Circulating microRNAs as a liquid biopsy: a nextgeneration clinical biomarker for diagnosis of gastric cancer
