Phytochemical composition of Caesalpinia crista extract as potential source for inhibiting cholinesterase and β-amyloid aggregation: Significance to Alzheimer's disease
Kolambe Rajappa Chethana, Balappa Somappa Sasidhar, Mahadeva Naika, Rangappa Sangappa Keri✉
1Centre for Nano and Material Sciences, Jain University, Jain Global Campus, Kanakapura, Ramanagaram, Bangalore-562112, India
2Chemical Sciences and Technology Division, CSIR-National Institute for Interdisciplinary, Science and Technology (CSIR-NIIST), Kerala,Trivandrum-695019, India
3Applied Nutrition Division, Defence Food Research Laboratory, Mysore - 570011, India
Keywords:Alzheimer’s disease Caesalpinia crista Acetylcholinesterase Butyrylcholinesterase β-Amyloid
ABSTRACT Objective: To screen plant extract fractions and elucidate the components present in Caesalpinia crista (C. crista) leaves for cholinergic and anti-amyloidogenic activities for the treatment of Alzheimer’s diseases. Methods: This work has been carried out to study the action of C. crista extracts from nonpolar to polar solvents toward inhibition of oxidative stress,cholinergic and amyloidosis. The antioxidant activity was studied using DPPH total antioxidant assay; cholinergic assay by Ellman’s method and anti-amyloidogenic assay by thioflavin-T fluorescence and transmission electron microscopy. Results: The quantification of polyphenols was carried out following C. crista methanolic extract (CCMeOH) HPLC fingerprinting,along with LC-MS and elucidated by MS LAMPS database. GC-MS of CCMeOH was screened for potential moieties. In vitro experimental results showed that the CCMeOH was potential extract that exhibited active inhibition of antioxidant property, cholinergic enzymes acetylcholinesterase and butyrylcholinesterase. For anti-amyloidogenic evaluations, among all the extracts, the CCMeOH was found to have the potential toward inhibiting the oligomers,fibrillation of Aβ42 with good defibrillation of amyloid cascading properties. Conclusions:These results are also supported by the presence of polyphenols as the active ingredients.Multi-potent target drug therapy is a promising option in treating the Alzheimer’s diseases.Methanolic extract of C. crista shows potential activity against cholinergic enzymes, Aβ42 aggregation with antioxidant activity.
1. Introduction
Alzheimer’s disease (AD) is considered as the neurodegenerative disease that contributes to dementia-related brain disorders. The most probable hypothesis on AD is the amyloid hypothesis, inclusive of pathogenic hallmarks such as over expression of the protein,pathogenic mutation, aging, oxidative stress, impaired autophagy,proteasome impairment cumulative chairing in triggering of oligomerization of protein thus stimulating the neuroinflammatory complex and final staging for neurodegeneration. In the pathway leading to amyloidogenesis, the amyloid precursor protein is an integral membrane protein encoded by autosomal dominant familial AD gene. The amyloid precursor protein is cleaved by β-secretase and subsequent snipping by γ-secretase releasing amyloid peptides
into extracellular space. The cleaved protein fragment amyloid beta is of 4 kDa, extracellular assembly of these monomers with intermediates composed into oligomers and protofibrils, which has a stellar role in the formation of insoluble senile plaques which disrupts the neuronal network. Subverting of neuronal cells ensues in the diminishing of the cholinergic network with higher activity of acetylcholinesterase (AChE)[1]. Neurotransmitter acetylcholine is hydrolyzed by the catalytic action of AChE, which belongs to serine hydrolases enzyme classification. The sister enzyme butyrylcholinesterase (BChE) is also well studied in drug inhibition that is said to have a similar role as that of AChE, which is present in glial cells. These two enzymes have been studied extensively as cholinergic neurons in the central nervous system of the brain and are studied for AD drug inhibition. Cholinergic hypothesis is the first ever hypothesis considered for therapeutic approaches for AD[2]. According to Alzheimer report, people living with dementia estimated to be 46.8 million worldwide in 2015 and predicted to be doubled every 20 years, which will be increased to 74.7 million in 2030 and 131.5 million in 2050. Several generic drugs such as donepezil (Aricept), rivastigmine (Exelon), galantamine (Razadyne),memantine (Namenda) found to be symptomatic cure towards AD,where the first three are cholinergic drugs and latter acts as NMDA receptor antagonist[3]. In the past decade, researchers have came up with several drugs to treat AD that only slightly delay the inevitable symptomatic progression of the disease and they do not affect the main neuropathological hallmarks.
Therefore, there is an enormous medical need for new and more effective therapies and drugs, which are essentially free from deleterious side effects for the treatment of AD. Thus, this could add weight to the argument that the natural products are inherently better tolerated in the body than that of the synthetic chemicals and they have higher chances to be approved as new drugs for AD.Caesalpinia crista (C. crista) Linn. [syn Caesalpinia bonducella (L.)Roxb.] which belongs to Fabaceae family is a woody vine that is scandent and straggling. It is also a prickly shrub growing up to 5-10 m tall, which is widely distributed across India growing mainly in marshy and plain terrains, and the habitat of tropical and subtropical regions of Southeast Asia. It is one of the major herbal plants found in Western Ghats region of Karnataka, India locally called as Gajjiga. The Sanskrit name is Kuberakshi or Latakaranj[4,5] and it is also called as fever nut. Many traditional uses of C. crista have been reported for various ailments such as vomiting, leprosy, disorders of the blood, fever and abdominal related problems[6]. Plant parts such as seed, bark, root, flower, leaves, and nuts are used to treat various disorders. Seeds are used to treat malarial fever, skin disorders like leprosy, inflammation, and used as anthelminthic and one of the ayurveda compositions for snake bite, convulsions, and paralysis.Leucorrhea is treated by the skin of the seeds. Fresh leaves juice is used to treat mucous secretion, while oil from leaves is considered as nervine tonic[7,8].
Each and every part of the C. crista plant is claimed to possess some therapeutic properties. However, the seed kernel is the most widely used part all over the world in various systems of medicine.Pharmacological investigations revealed the medicinal properties of C. crista such as antinociceptive, antimalarial, antimicrobial,antifungal, larvicidal, muscle contractile, hepatoprotective,anticonvulsant, anti-filarial, antitumor, antipsoriatic, antiestrofenic,adaptogenic, anthelmentic, antidiabetic and anti-inflammatory[9].Recent studies indicated that the plants belonging to the genus Caesalpinia are rich in cassane- and norcassane-type diterpenoids,flavonoids and peltogynoids[10]. These scientific studies essentially shed insights on the effective usage of C. crista leaves in exploring their potential properties towards treating AD.
Several contemporary pharmaceuticals that are being used in conventional medicine, have natural plant origins, while they have been derived directly or indirectly from plants. The ethnopharmacological approach provided several paths to identify potential new drugs from plant sources. This information may also be useful as a starting point for the discovery of new drug molecules for the treatment of AD and cognitive disorders. Considering the importance of plant sources and continuation of our work towards the treatment of AD[11,12] herein, we wish to report the application of C. crista leaves extract as an anti-AD agent. The present study focused on the screening of plant extracts to evaluate their AChE and BChE inhibitory activities, antioxidant assay and amyloidogenic inhibition supported by thioflavin-T (ThT)assay and morphological properties using transmission electron microscopy (TEM) technique.
2. Materials and methods
2.1. Samples and reagents
2,2-Diphenyl-1-picrylhydrazyl (DPPH), gallic acid,protocateccheuic acid, catechin, chlorogenic acid, epicatechin,caffeic acid, vanillin, p-coumaric acid, sinapic acid, rutin hydrate,myrcitin, cinamic acid, quercetin, kaempferol, Electric eel acetylcholinesterase (Type-VI-S, EC 3.1.1.7AChE) 500 U, 5,5-dithiobis (2-nitrobenzoic acid) (DTNB), ThT-T, 1,1,1,3,3,3-hexafluro-2-propanol (HFIP), uranyl acetate, acetylcholine iodide were purchased from Sigma-Aldrich, St. Louis, MO, USA. Amyloid beta 42 was acquired from EZ biolabs, butyrylcholine iodide from TCI, carbon coated copper grids from Grid tech. Besides,methanol, aluminum chloride, potassium acetate, sodium carbonate,ascorbic acid Tris (hydroxymethyl) aminomethane (Tris buffer),and dimethylsulfoxide (DMSO) were purchased from Merck,USA; Folin and Ciocalteu’s reagent from Thomas Baker Limited;Galanthamine from Medchem; Express LLC and horse serum BChE from MyBioSource. All chemicals and reagents used in the experiment were of analytical grade.
2.2. Collection of plant material
The fresh leaves of C. crista were collected in June to July (2013)from the region near to Suntikoppa, Kanbile village located in Kodagu district, Karnataka, India. The plant material was identified by Botanist Dr. Naresh, Professor at Cauvery College,Gonikoppa, Kodagu, Karnataka, India. Voucher specimen was deposited at Regional Ayurveda Research Institute for Metabolic Disorder, CCRAS, Ministry of Ayush, Government of India, and the authentication number is RRCBI-6244.
2.3. Preparation of plant extracts
Fresh leaves were collected and washed thoroughly to remove dust and left for drying under shade for over a week at room temperature in moist free condition. The dried leaves were subjected to mechanical shear to obtain a coarse powder. About 40 g of coarse powder was subjected to extraction by different solvent systems such as n-hexane, petroleum ether, and methanol with a volume of 200 mL by soxhlet extractor for a period of 72 h and solvent was removed by rotary evaporation. Aqueous extraction was carried out by steam boiling until water condensed to one-third of the initial volume and, allowed to cool and lyophilized to obtain a dried extract.The total extracts were stored at 4 ℃ for further use.
2.4. Determination of plant extract yield
Yield of plant subjected to subsequent solvent extraction was expressed as percentage yield that was calculated by
Yield (%) = (W2/W1) ×100
Where, W2is the dried extract weight and W1weight of the original sample.
2.5. Determination of total polyphenols content and HPLC profiling, liquid chromatography mass spectrometry (LC-MS)and gas chromatography mass spectrometry (GC-MS)
2.5.1. Total phenolic quantification
Total phenol content of the plant extracts was determined using Folin-Ciocalteu reagent. Different concentration aliquots of the samples were taken and volume was made up to 750 µL using distilled water and 0.125 mL of Folin-Ciocalteu reagent was added.Then, the mixture was shaken well and allowed to stand for 6 min before adding 1.2 mL of 7% Na2CO3and the final volume was adjusted to 3 mL and mixed thoroughly and incubated in dark for 90 min. The optical absorbance of the obtained extract was read at 760 nm. Gallic acid was considered as a standard to obtain the calibration curve. Average of three tests was considered and total phenolic content of all plant extracts was expressed as mg of gallic acid equivalents (GAE) per gram of the dried extract (mg GAE/g dry extract)[13].
2.5.2. Total flavonoid quantification
Total flavonoid content was measured by preparing different aliquots of the plant extracts. In this procedure, 0.1 mL 10%aluminum chloride and 0.1 mL potassium acetate (1 M) were added and final volume was made up to 3 mL by adding the distilled water.Then, the samples were incubated for 30 min at room temperature.Absorbance was read at 415 nm and quercetin was considered as a standard to obtain the calibration curve. Triplicate of each tests was used and results were expressed in milligrams of quercetin equivalents (QE) per gram of dry extract (mg QE/g of dry extract)to quantify the total flavonoid content using the standard curve of quercetin[14].
2.5.3. HPLC fingerprinting of C. crista methanolic extract(CCMeOH)
Phenolic acid profiling was carried on RPC18 column (150 mm×4.5 mm) and detected with diode array detector (JASCO HPLC system; Easton, PA, USA) for the MeOH extract of C. crista. The mobile phase used was 0.1% formic acid (solvent A) and methanol(solvent B). The solvent gradient elution program was used as per the reported method with minor modification[15]. The CCMeOH extract and all standards were dissolved in methanol to get concentration of 1 mg/mL solution and filtered through 0.22 µm Merck syringe filters. A total of 20 µL of the sample and standards, such as gallic acid (1), protocatecheuic acid (2), catechin (3), chlorogenic acid (4),epicatechin (5), caffeic acid (6), vanillin (7), p-coumaric acid (8),sinapic acid (9), rutin hydrate (10), myricitin (11), cinamic acid (12),quercetin (13), and kaempferol (14) were injected into the column and the phenolic acids were detected at 270 nm. Triplicate injections were carried for each of standards and the sample. Retention time and UV-visible spectra of the area under peaks that were compared with those of the standards and samples were expressed as mean ±SEM.
2.5.4. TLC profiling of CCMeOH
Preliminary phytochemicals were further confirmed using TLC.Aluminum sheet with silica gel 60 F254 plate was used as stationary phase and solvent system n-hexane:ethyl acetate (4:2) used as a mobile phase for the development of chromatogram. Further, the CCMeOH extract was subjected to silica 60-120 mesh column chromatography with n-hexane:ethyl acetate (4:2) solvent system.All seventeen fractions were collected and out of which only sixteenth and seventeenth fractions were solvent dried and subjected to LC-MS along with whole CCMeOH extract[16].
2.5.5. Identification and quantification analysis of CCMeOH constituents by LC-MS
HPLC was performed using Waters Acquity liquid chromatograph system with column Acquity UPLC BEH C18 1.7 µm×1.0 mm×50 mm and higher pressure limit upto 600 bar. Coupled with Waters AcquityAutosampler Electro Spray Ionization system. Quadru Pole Time of Flight (Waters, USA) fitted with the liquid chromatography system coupled on-line with an Electro Spray Ionization source and MassLynx software was used for data acquisition. Analytical conditions were: positive ion mass spectra and mass were recorded in the range m/z 100-800. Capillary temperature was kept at 250 ℃and voltage 2.8 kV and Sheath gas flow rate was kept at 350 L/h. The resulting total ion chromatogram is a plot of time vs area showing the total response of each constituent on the basis of its molecular ion abundance. The mobile phase was composed of solvent A: 0.1% of formic acid in water, and solvent B: acetonitrile. The flow rate was 0.4 mL/min. The elution was performed starting at 10% of solvent which increased up to 95% solvent B after 4.5 min. The injection volume was 10 µL for the measurement in positive mode with total run time of 8 min. Column temperature was 50 ℃ and sample temperature 20 ℃. The library search was performed by using the database MSLAMPS[17].
2.5.6. Identification and quantification analysis of CCMeOH constituents by GC-MS
GC-MS analysis of CCMeOH extract was performed on a Shimadzu(Tokyo, Japan) Make GCMS-TQ8030 with nonpolar Rxi 5Sil MS capillary column, full scan mode, injector mode-splitless, quadra pole mass selective detector, injection temperature 250 ℃, GC-MS interface temperature was 250 ℃, and the injection volume was 1 µL. Helium was employed as carrier gas, at a pressure of 57.5 kPa; flow rate was 1 mL/min. Mass spectra were detected at 70 eV. Temperature programming was set as follows: column temperature was started from 60 ℃ (held for 2 min) and linearly increased by 5 ℃/min to 200 ℃ (held for 2 min); after that it was increased by 3 ℃/min to 220 ℃ (held for 1 min);further it was increased by 6 ℃/min to 250 ℃ (held for 20 min). Total GC running time was 64.67 min and the results were compared by using Wiley Spectral library search programme[18].
2.6. Antioxidant assay
The antioxidant activity was measured by the DPPH method[19]. The free DPPH in purple color changes into yellow color upon reaction with hydrogen donors, This is because of the reaction between hydrogen donors and DPPH free radical that leads to its reduction to corresponding hydrazine. Sample stock solution (10 mg/mL)was diluted with various concentrations with methanol and 1 mL of DPPH (0.04 mg/mL), and 3 mL of methanol were added into the samples, which were incubated for 30 min at room temperature. The absorbance was measured at 517 nm against the corresponding blank(methanol). All plant samples were dissolved in methanol. The IC50values were calculated by linear regression of plot of standard curve where the abscissa represents the concentration of tested plant extracts and the ordinate the average percentage of antioxidant activity from three different tests. Ascorbic acid was used for standard curve and antioxidant activity was calculated by following equation.
Inhibition % = (Ac-As/Ac) ×100
Where, Acis the absorbance of the control reaction (containing all reagents except the test sample), and Asis the absorbance of the extracts/reference. Experiments were run in triplicate and the results were conveyed as average values with standard error of the mean(SEM).
2.7. Cholinesterase activity
Inhibition of AChE and BChE was determined by Ellman’s method[20,21] with slight modifications[22,23]. To the reaction mixture 0.12 U AChE enzyme and plant extract 100 µL (1 mg/mL) was added along with 640 µL Tris-HCl buffer 50 mM pH 8 into the 1 mL quartz cuvette with path length of 1 mm. This reaction mixture was incubated at room temperature for 5 min. A total of 100 µL DTNB (7.5 mM)and 100 µL acetylcholinesterase (AChE) inhibitor (1 mM) were added and the reading was measured at 405 nm in spectrophotometer. The reaction was monitored for 104 s in the interval of 13 s. The velocities of the reactions were measured. Enzyme activity was calculated as the percentage of the velocities compared to that of the assay using buffer without any inhibitor. Inhibitory activity was calculated from 100 subtracted by the percentage of enzyme activity and every experiment was done in triplicate.
2.8. Anti-amyloidogenic activity
2.8.1. Preparation of amyloid beta 42 (Aβ42) peptide solution
Aβ42lyophilized powder formerly stored at -20 ℃ was dissolved in chilled HFIP and vortexed gently for 10 min. Then, the dissolved Aβ42was allowed to form pellet at 25 ℃ and distributed in different vials to get final concentration of 1 µg/µL. The formed pellet of Aβ42vials was defrosted at -20 ℃ for future use. The required working vial was thawed for 30 min by keeping at room temperature. The 50µL of DMSO was added into the pellet, vortexed to mix thoroughly followed by adding 50 µL of sterile double distilled water to obtain final concentration of 1 µg/µL of 221.5 µM[24].
2.8.2. Inhibition of oligomerization, fibrillation and defibrillation of Aβ42
Inhibition of Aβ42oligomerization among all four extracts,each one (i.e. 50 µg/mL) was incubated with 25 µM of Aβ42after dissolving in 0.4 mM DMSO with ratio of 1:2 in 50 mM Tris-HCl buffer (pH-7.4) incubated at 37 ℃ for 120 h[25]. Time course Aβ42fibril formation inhibition by CCMeOH-kinetic study or time lapse study was carried out with 1:1 ratio concentrations of peptide and extracts. Aliquots were collected at different hours 0, 12, 24, 48, 72 and 120 h[26]. For inhibition of fibrillization of Aβ42by CCMeOH,i.e. formation of mature fibril, its inhibition by extract was performed by incubating CCMeOH with peptide in 1:1 (50 µM: 50 µg) ratio for 9 d at 37 ℃ and control was devoid of extract. There were four groups in ThT assay including CCAI9d (CC is CCMeOH, AI is along with incubation that refers to CCMeOH incubated with Aβ42for 9 d),CCBI9d (CC is CCMeOH, BI is before incubation Aβ42allowed to form fibrils for 120 h, then treated with extract and further incubated for a total of 9 d), ConAβ429d (control for fibrillization and defibrilization), and ConAβ42120h (Aβ42incubated for 5 days i.e. 120 h). For defibrillation of Aβ42fibrils by CCMeOH, defibrillation study was done by allowing the 50 µM peptide to form fibrils till 120 h and later 50 µg extract was added, mixed and incubated for 9 d. The control was devoid of extract so for fibrillization and defibrilization control was same. Even 120 h data was considered to compare the increase in fluroscence intensity and formation of mature fibrils[27].For all experimernt control Aβ42was incubated without the extracts,and reaction mixture remained same. Assays were carried out in triplicate.
2.8.3. ThT fluroscence assay
The formation of Aβ42fibril and its inhibition by the extracts were observed by ThT dye. The binding efficacy of benzothiazole dye to the beta sheet structure of amyloid fibril enhances the fluorescence.The 25 µL (1 mM) of ThT in 50 mM glycine-NaOH (pH 9) was added to peptide solution and incubated for 30 min at 25 ℃. Later,using glycine buffer, final volume made up to 1 mL in 1 mm quartz cuvette to the incubated sample, fluorescence was measured at Ex450nmand Em482nmby adjusting the slit width to 5 nm and 10 nm,respectively, in spectroflurophotometer (RF-5301PC Shimadzu[28,29].
2.9. Transmission electron microscopy (TEM) study
The 10 µL of incubated aliquots were mounted on 400-mesh carbon coated copper grids, after 2-3 min excess was wicked off from edges of grid using ash less filter paper. Sample was then stained by 5 µL of 1% uranyl acetate (w/v) and excess wicked off. Uranyl acetate dissolved in sterile water was mixed thoroughly and filtered using 0.2 µm syringe filter to avoid unsolubilized uranyl acetate. The sample was allowed to be dried and observed under microscope at 120 kV Technai G2 BiotwinFei[30].
2.10. Statistic analysis
The results in spectroscopic studies were represented as triplicate mean±SEM of each sample and graph was plotted in Origin 8.Statistical analysis was carried out using GraphPad Prism 6.01. Oneway ANOVA (analysis of variance) followed by Dunnett’s multiple comparison tests was carried out and P value less than 0.05 was regarded as statistically significant.
3. Results
3.1. Extraction yields, total phenolic and flavonoids content,and HPLC fingerprint profiling
Among all extracts, from non-polar to polar solvent, CCMeOH extract showed 6.423% w/w dry weight of total C. crista extract.Total content of polyphenols was measured equivalent to mg of gallic acid per gram of dry extract. The phenolic content of CCMeOH was 182.25 mg GAE/g dry extract. Subsequently, the quantification of flavonoid content was expressed with respect to quercetin equivalents.The yield of flavanoid content was 441 mg QE/g of dry extract in CCMeOH which showed the presence of the highest amount of polyphenols in C. crista followed by CCPE which contained 412 mg QE/g of dry extract (Table 1).
The presence of highest amount of total polyphenolic content in CCMeOH was further subjected to HPLC profiling. A total of 14 standards were compared as shown in Figure 1, in CCMeOH extract there existed sinapic acid (215.28 µg/mL), rutin hydrate (499.31µg/mL), myricitin (317.49 µg/mL) and quercetin (119.20 µg/mL)as in Table 2 along with other polyphenols, which yielded less as compared to above mentioned contents.
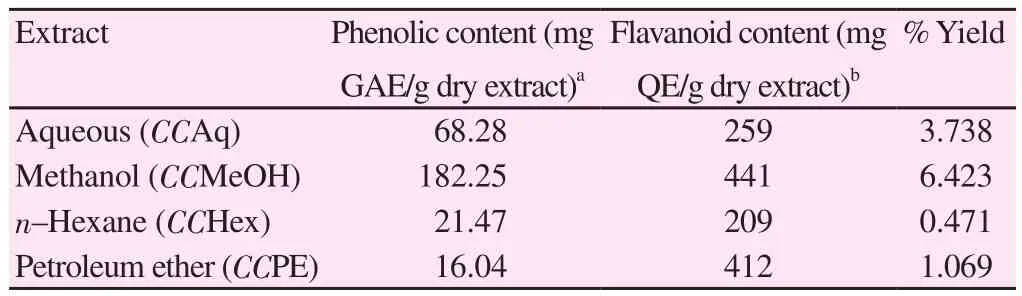
Table 1Total phenolic and flavonoid contents (mg/g) of the C. crista extracts.
3.2. Antioxidant activity
Maximum scavenging of synthetic DPPH radical activity was exhibited by CCMeOH with 93.01% at 400 µg/mL concentration.The highest activity of CCAq activity was 55.74% at 400 µg/mL.CCHex and CCPE extracts did not show any scavenging activity(Table 3).
3.3. Cholinesterase inhibition activity
Cholinesterase activity of both enzymes AChE and BChE of CCMeOH found to be 51.19% and 63.86% respectively, and was compared with standard galantamine (96.68% and 83.68%) at 100µg/mL. The other extracts n-hexane, petroleum ether and aqueous extracts did not show the AChE and BChE inhibition activities.
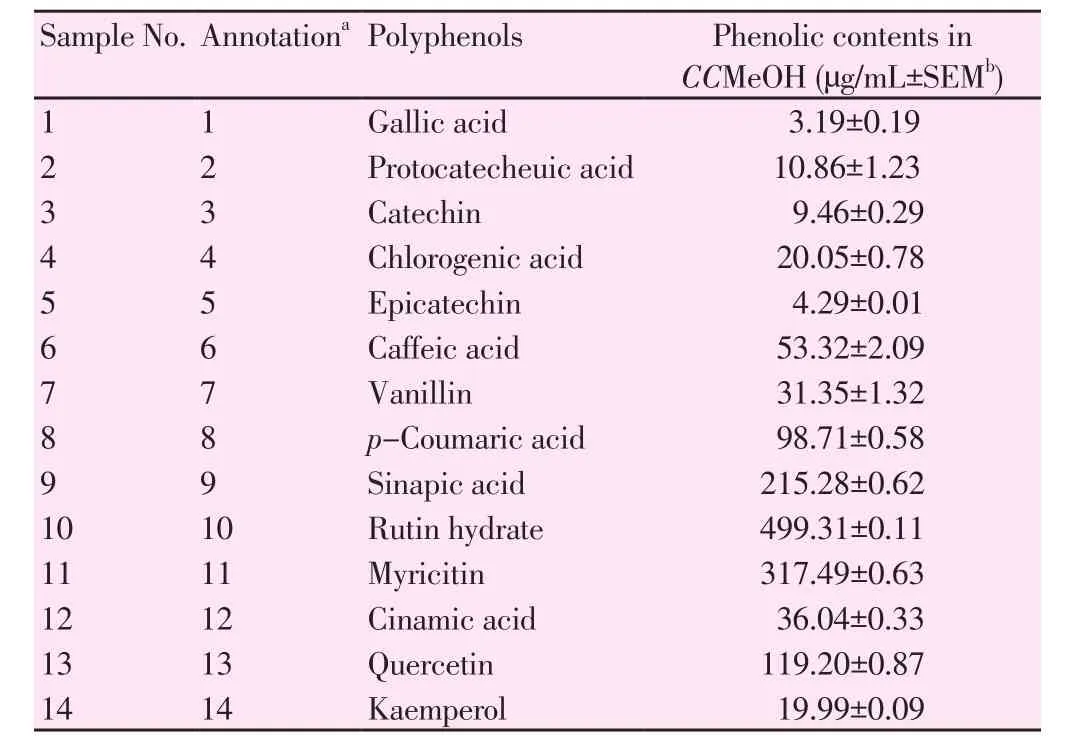
Table 2HPLC fingerprinting of the methanol extract of C. crista.

Table 3DPPH radical scavenging activity of C. crista extract.
3.4. Anti-amyloidogenic studies: ThT fluorescence and TEM analysis
Assessment of anti-amyloidogenic property was carried out by treating C. crista extracts with Aβ42to check Aβ42oligomerization.The CCMeOH extract was treated with Aβ42to analyse the inhibition activity kinetics of fibrillar structure, fibrillation, and defibrillation activity and control was taken without treating Aβ42with C. crista extract.
3.4.1. C. crista extracts in inhibiting Aβ42 aggregation
All the four extracts (50 µg) were treated with Aβ42(25 µM) at 37 ℃for 120 h by ThT assay displayed promising inhibition activity as shown in Figure 2a among which, CCMeOH showed significant (P<0.000 1) inhibition activity. Corresponding TEM electrogram of CCMeOH treated with Aβ42was presented at 500 and 200 nm bar scale while control Aβ42showed the aggregation of Aβ42at 500 nm and 100 nm bar scale in Figure 2b. Comparing with control, electrogram showed a completely disorientation of the structural deformation and diminishing of secondary structure of Aβ42.
3.4.2. Aβ42 aggregation kinetic study with CCMeOH inhibition

Figure 1. Representative HPLC profiling chromatograms of mixed standards and methanolic extract of C. crista traced at 270 nm.
The Aβ42peptide aggregation kinetics study was conducted by following series of stages - misfold, oligomers (dimmers, trimmers etc) in lag phase and protofibril formation at growth phase, later leading to amyloid fibrils representing sigmoid growth curve of insoluble fibrillar toxic species. As shown in Figure 3a ThT assay,the activity of CCMeOH in inhibiting Aβ42aggregation process was significantly evident. The CCMeOH was screened for time lapse studies, where a 25 µg/mL of the extract was incubated with 25 µM of Aβ42with time intervals 0, 12, 24, 48, 72 and 120 h. As time increased, there was an increase in the fluorescence intensity in control Aβ42as that of CCMeOH treated with Aβ42. This specifies the significant decrease in reduction of amyloid aggregation by CCMeOH. These results are also supported by electrogram in TEM analysis which was taken at 24, 48, 72 and 120 h in both control(Aβ42) and CCMeOH with Aβ42at 500 nm and 200 nm scale. In the Figure 3b, at 24 h (3b1A and 3b2A) of incubation there was globular structure formation but in CCMeOH treatment (3b3A and 3b4A) there was a drastic reduction in structural arrangement. At 48 h control (3b1B and 3b2B), a structural network appeared between globular structures but in CCMeOH treatment (3b3B and 3b4B),there was an absence of these structure. Observing control (3b1C and 3b2C) and the CCMeOH treatment (3b3C and 3b4C) at 72 h,complete structural demodulation was found. At 120 h (3b1D and 3b2D), there was fibrilar structure and in CCMeOH treatment (3b3D and 3b4D), a significant reduction of structural morphology was observed. This specifies the effective CCMeOH role in reduction of amyloid aggregation.
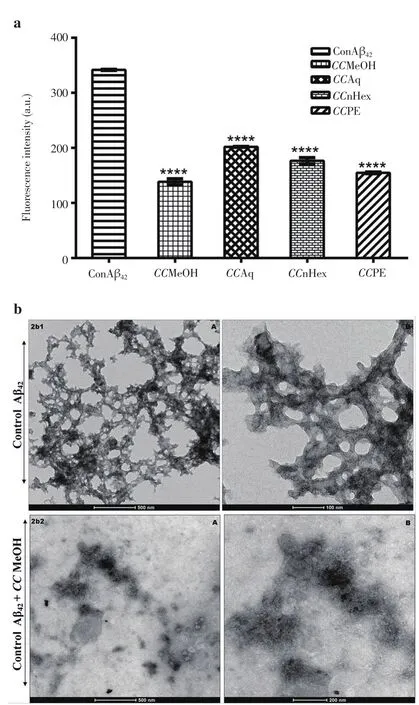
Figure 2. Propensity of C. crista extracts in inhibiting Aβ42 aggregation.
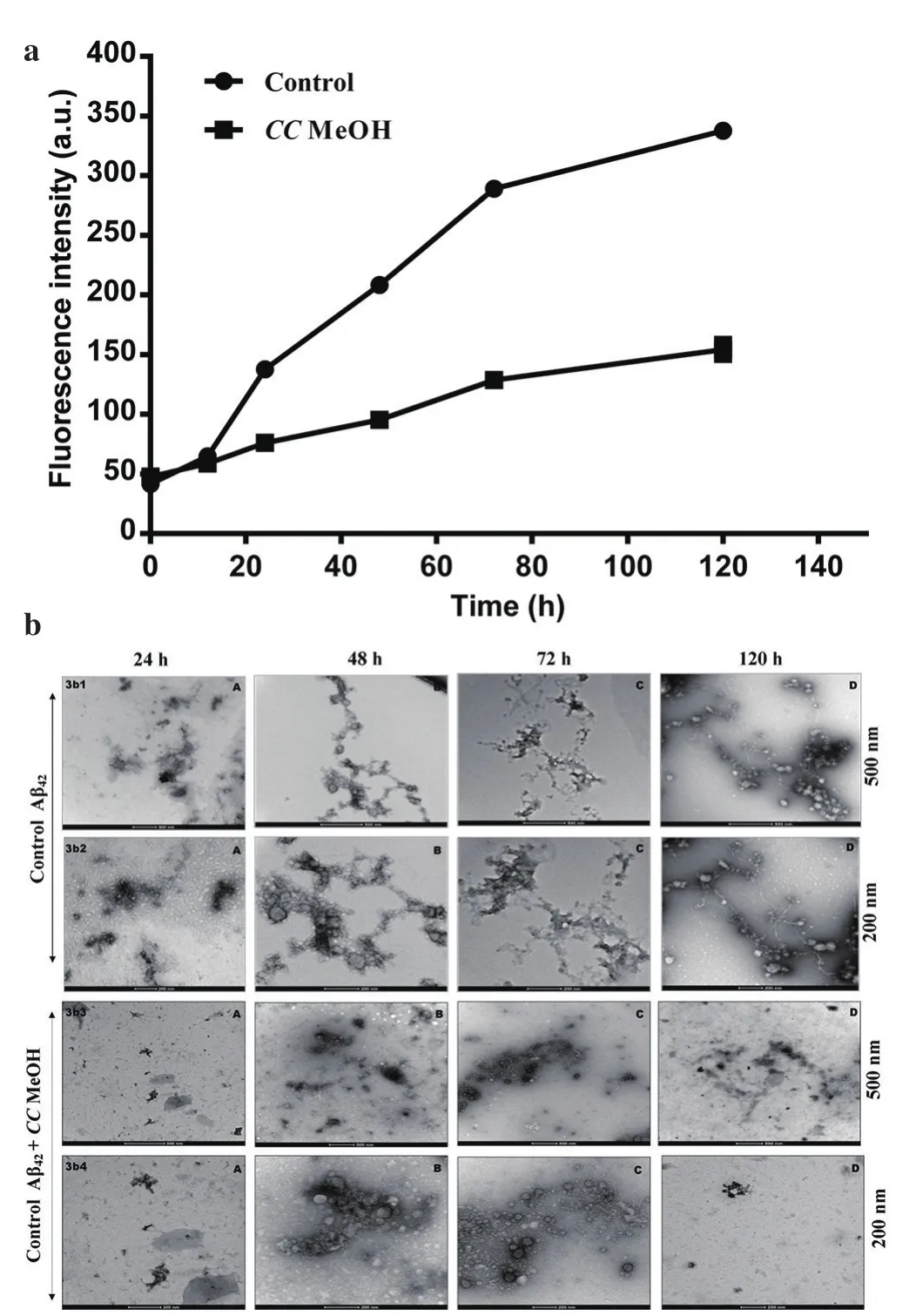
Figure 3. Aβ42 aggregation kinetic study with CCMeOH inhibition.
3.4.3. Inhibition of Aβ42 aggregation to form mature fibrils and defibrillation
Amyloid aggregation studies were carried out for 9 days (9 d) to see activity of CCMeOH on mature fibrils and study was performed at 50 µM:50 µg of Aβ42and CCMeOH co-incubated at 37 ℃. The ThT assay was shown in Figure 4a. ConAβ429d in which Aβ42was allowed to aggregate has shown increased fluorescence intensity compared to that of ConAβ42120h. We have compared 9d with 120h in order to know the increase in fluroscence which specifies the growth of higher structural formation of Aβ42aggregation. There is significant decrease in quenching of fluorescence and is evident in the decrease of fluorescence intensity. In TEM analysis, matured fibrillar structure formation was observed in control Aβ42in Figure 4b (4b1A, 4b1B1 and 4b1B2). The CCMeOH treated with Aβ42was shown in Figure 4b (4b2 A and B) both at 500 nm and 200 nm scale.A drastic decrease in structure, only in globular like structure, was observed which was evident from the electrogram. These studies give insights into the possible better activity of CCMeOH extract in inhibiting the amyloid aggregation.
In Figure 4a, there was significant decrease in quenching of fluorescence as compared with control ConAβ429d. TEM electrogram supported the result as in Figure 4c (4c2 A and B) there was a drastic change in structure of amyloid fibril. These observations essentially proved the effectiveness of the extract even in destabilizing the matured fibrils.

Figure 4. Inhibition of Aβ42 aggregation to form mature fibrils and defibrillation.
3.5. TLC, LC-MS and GC-MS analysis of CCMeOH
The TLC of CCMeOH extract showed different bands (Figure 5),which shows the presence of various phytochemical presence. For further study of these phytochemials, we performed GC-MS and LCMS. GC-MS analysis of CCMeOH extract was carried out. Peaks obtained were shown in chromatogram (Figure 6) and a total of 26 compounds were identified with their peak number, retention time(RT), area under peak (%) and height of the peak (%) as represented in the Table 4. Most of the identified compounds have medicinal properties. For LC-MS study, the column chromatography was carried out with 60-120 mesh silica. Total of seventeen fractions were collected, among which only sixteenth and seventeenth were brown compared to rest that was yellow or green in color, and were eliminated and subjected to LC-MS along with whole CCMeOH extract. The whole extract of CCMeOH shown in the MS spectra(Figure 7Aa and 7Ab) demonstrated the various peaks represented the presence of phytochemicals that was considered with retention time 3.124 (7Aa) and 5.839 (7Ab) of LC chromatogram. The column fraction of CCMeOH was brown color fraction as in Figure 7B and the MS spectra showed retention time 3.124 (7Ba) and 3.517 (7Bb)of LC chromatogram. Both the spectra mass was compared with help of MSLAMPS database. In Table 5, we could be able to identify thirteen polyketides and one prenol lipids as in Figure 7A showing the MS spectra of RT time 3.124 (7Aa) and 5.839 (7Ab) of LC chromatogram for CCMeOH whole extract. Both the spectra mass was also compared with help of MSLAMPS database. As in Table 6,we could be able to identify nine polyketides for CCMeOH column fraction from MS chromatogram 7Ba and 7Bb. Some of the mass peak in Figure 7B with RT 3.124 (7Ba) MS spectra of CCMeOH column fraction was as same as in CCMeOH whole extract in Figure 7Aa with RT 3.124 (7Aa) which was excluded. Both the whole fraction and column fraction molecules compared with mass number represented indicate the presence of flavanoids like kaempferol,myricitin, catechin, quercetin, chrysosplenol etc. Spectral studies give an insight into the presence of active ingredients present in the extracts which could be isolated, and the studies in detail show the plausible mechanism that may play a vital role in inhibition of pathogenesis of AD.

Figure 5. Thin layer chromatographic analysis of CCMeOH extract.A) Long UV, B) Short UV, C) Visible light.
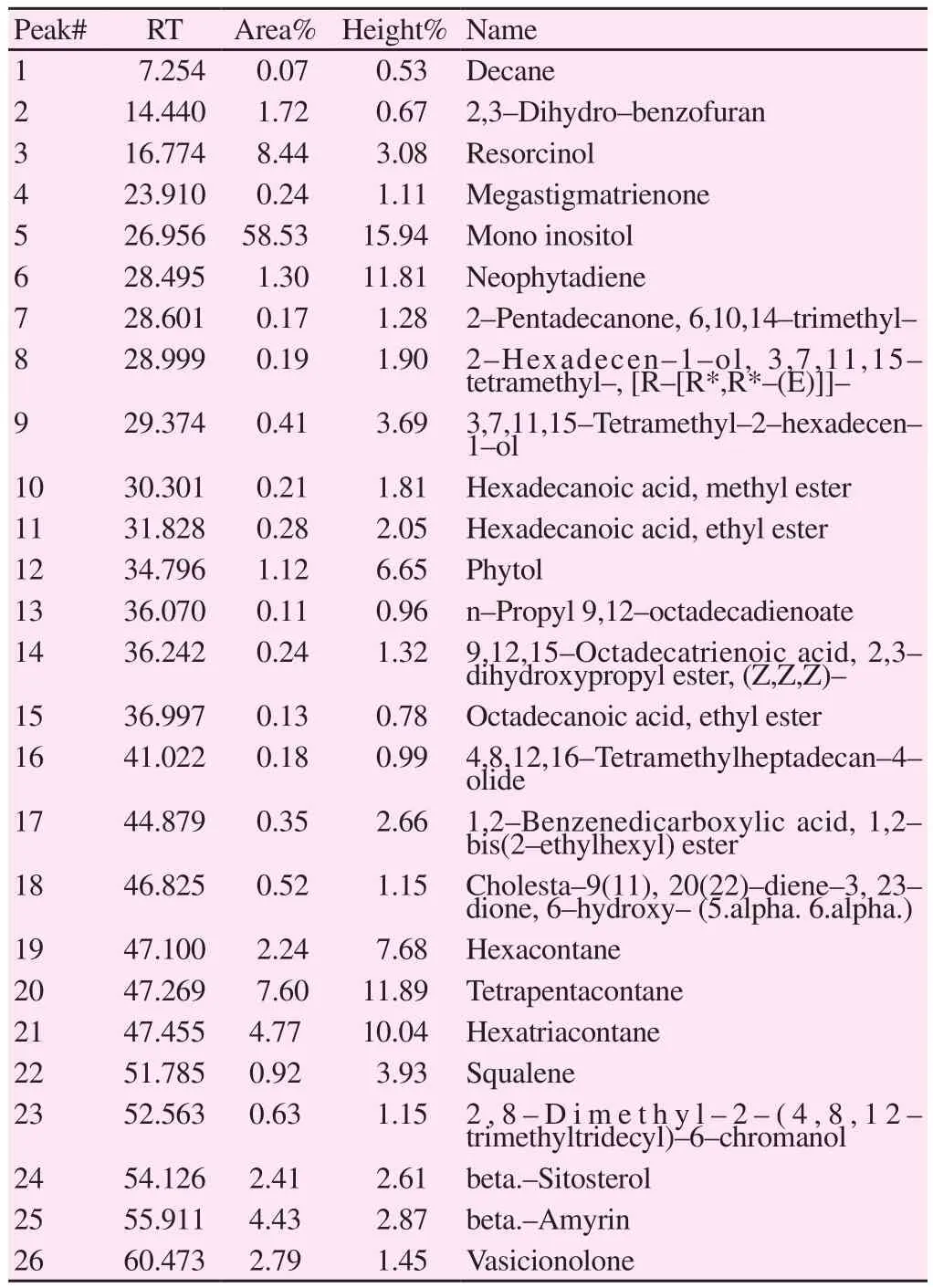
Table 4Chemical ingredients of methanolic leaf extract of C. crista by GC-MS.
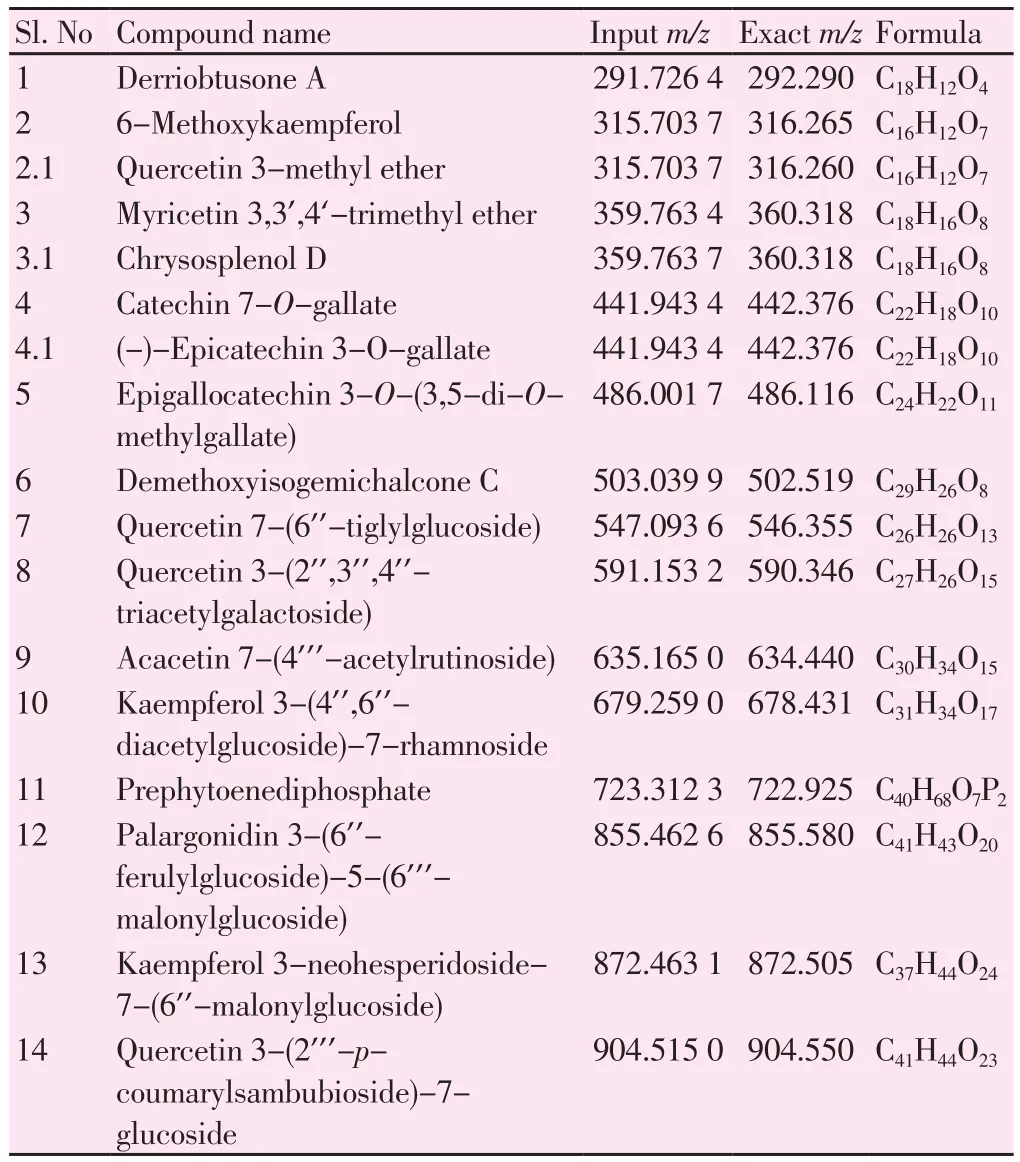
Table 5Chemical ingredient of methanolic leaf extract of C. crista by LC-MS.

Table 6Chemical ingredient of methanolic leaf extract fraction of C. crista by LC-MS.
4. Discussion
C. crista is a common climber with bipinnate, oblong, obtuse, and mucronate leaves. In ayurveda, it consumed as astringent with a bitter taste[9]. Advances in studies on leaves of C. crista have highlighted many activities as discussed above. Our version of current study gives an insight into the fractional study of leaves extracts from non-polar to polar solvent,which can guide for the better active fraction or active moieties for AD at in vivo condition. Plants produce various secondary metabolites such as alkaloids, terpenoids sterols, saponins, polyphenols, anthraquinones,glycosides etc. We have quantified the presence of phenolic and flavonoid contents among the four different extracts of C. crista. The potential CCMeOH with the highest yield could explain better activity against the oxidative stress and the cholinergic inhibition. The HPLC fingerprinting also showed the presence of gallic acid, protocatechuic acid, catechin,chlorogenic acid, epicatechin, caffeic acid, vanillin, p-coumaric acid,sinapic acid, rutin hydrate, myricitin, cinnamic acid, quercetin, and kaempferol.
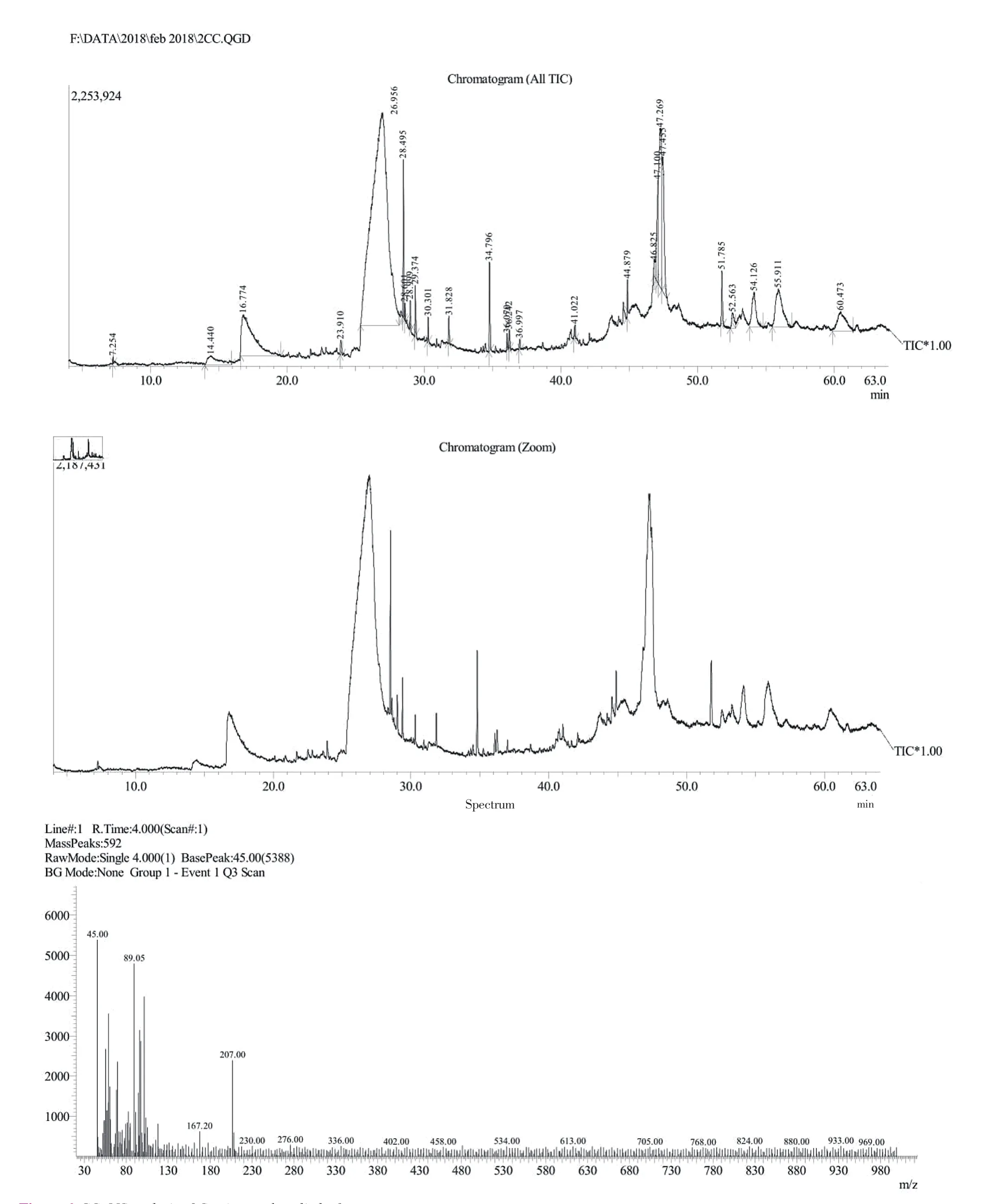
Figure 6. GC-MS analysis of C. crista methanolic leaf extract.

Figure 7. LC-MS analysis of C. crista.
Mandal et al.,[31] have studied the potential activity of CCMeOH for various other reactive oxygen species such as hydroxyl, superoxide, nitric oxide, singlet oxygen and hypochlorous acid with scavenging activity with IC50values being 0.44 µg/mL, 24.9 µg/mL, 33.72 µg/mL, 61.13µg/mL and 170.51 µg/mL, respectively. The CCMeOH also showed iron chelating activity of 279.85 µg/mL. These oxidative activities of CCMeOH pour light on combating the oxidative stress. Oxidative stress caused by Aβ42is by intercalation of peptide aggregates into lipid bilayer thus resulting in oxidative alteration of proteins and lipid peroxidation[32,33]. Also, the cell model studies have paved evidence of increase in levels of hydrogen peroxide and lipid peroxides upon treatment of Aβ to the cells[34]. AChE is a membrane bound enzyme that has a major role in cognition and the evidences show the role of AChE in amyloidosis, where AChE can colocalize with amyloid aggregates that are present in pre-amyloid diffuse deposits, mature senile plaques, and cerebral blood vessels[35-37]. Interaction of AChE that occurs between the growing Aβ fibrils formed complex thus triggering the assembly of Aβ peptides[38]. Suppressing the action of AChE is an essential for reducing the pathogenesis. To suppress AD pathology polyphenols have major role in combating the disease and show potential activities against AChE enzyme. Quercetin, rutin,quercetin 3-glucuronide, kaempferol, myricitin, epigallocatechein,epicatechein, catechin etc have shown the AChE and BChE inhibition activity[39]. Scientific studies have untangled the reason behind the process of amyloidosis. Beta-sheet rich senile plaques structures are the major hallmark in AD. For in vivo quantification of these amyloid beta fibrils ThT assay is used, where the fluorescent dye has higher affinity towards fibrillar beta-sheet rich structures inducing red shift in the excitation spectrum, thus increasing the quantum yield which helps the study condition in the formation of Aβ fibrillar structure and its inhibition at in vivo condition[40-42]. The reason behind the formation of beta structure of peptides is due to interaction between planar aromatic rings of amino acids forming π-π interactions or π-stacking which plays major role in supramolecular structure like senile plaques. Parallel displaced, T shaped, parallel staggered, or herringbone are the four higher order possible clusters formed by aromatic rings[43]. Formation of secondary structure is crucial and the elucidation of structural morphology of peptides to senile plaques and its inhibition is an essential approach towards the therapeutic approach. Natural drug therapy has its wider application and positive merits over the synthetic therapeutic treatment. Plants are rich source of polyphenols and various metabolites, over the years scientific evidences have supported the elucidation and provided proof for the active moieties that have been used to treat various diseases. In kinetic study of Aβ42aggregation myricitin has more significant role in intercalating the conformational transition from monomer to oligomer than defibrillation of aggregation cascade[44]. Kaempferol-3-O-rhamnoside has shown effective activity against neurotoxicity of SH-SY5Y induced by Aβ42and also anti-amyloidogenic property by inhibiting fibrillogenesis and secondary structural transformation of the peptide[45]. Studies on major polyphenols like epicatechin,epigallocatechin, along with the 3-O-gallate esters of epicatechin gallate and epigallocatechin gallate which contained flavan-3-ol unit of catechins in the structure system showed key interaction between Aβ42, PrP106-126, and ataxin-3 oligomers inhibition and also presence of gallate has enhanced the activity[46]. All the above detailed studies give an insight towards the potential mechanism of action of structural based interaction in inhibiting the oligomers, protofibrils or mature fibrils. Over the years, plants have gained attraction for finding the drug like candidature for treating the AD. With growing statistics of dementia, it gives the call towards searching or developing the proper therapeutic approach in need to treat the disease. Over the decade, there has been only treatment of mild and moderate condition of AD. Drugs available for symptomatic cure are not sufficient to combating the diseases that is imposing on the society. Natural source could yield the better option for its nutraceutical property. Plants like C. crista can be a better option to search the active moieties for multi-targeting drug approach as it exhibits aggregation inhibitory property in addition to cholinergic activity. Various studies show the presence of polyphenols, terpenoids and other metabolites which are potential source of medicinal value. Previous studies have shown whole aqueous extract of C. crista leaves as anti-amyloidogenic potential.Accordingly, we have studied from non-polar to polar solvent extract in which the CCMeOH extract has shown better antioxidant,anticholinergic and excellent anti-amyloidogenic property that gives insights to exploring the novel drug-like candidature for AD therapeutics. Thus, C. crista could be a potential source of moieties that could possibly have aggregation inhibitory property in addition to the cholinergic activity for treating AD. All in all, the natural products that are rich in polyphenols, and possess multiple functions,might be preferable choices for the cure of AD. The further studies will be involved to know the better mechanistic approach of C. crista extracts towards the treatment of AD.
Conflict of interest statement
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgements
Authors acknowledge Jain University, Bangalore for the financial support.
 Asian Pacific Journal of Tropical Biomedicine2018年10期
Asian Pacific Journal of Tropical Biomedicine2018年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Beneficial effects of anthocyanins against diabetes mellitus associated consequences-A mini review
- Does prospective permutation scan statistics work well with cutaneous leishmaniais as a high-frequency or malaria as a low-frequency infection in Fars province, Iran?
- Effect of extracts from Stachys sieboldii Miq. on cellular reactive oxygen species and glutathione production and genomic DNA oxidation
- Eicosane, pentadecane and palmitic acid: The effects in in vitro wound healing studies
- Endophytic actinobacteria of medicinal plant Aloe vera: Isolation, antimicrobial,antioxidant, cytotoxicity assays and taxonomic study
