Synthesis of S, N co-doped porous carbons from polybenzoxazine for CO2 capture
JIN Zu-er, WANG Jian-long, ZHAO Ri-jie, GUAN Tao-tao,ZHANG Dong-dong, LI Kai-xi
(1. Key Laboratory of Carbon Materials, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China;2. University of Chinese Academy of Sciences, Beijing 100049, China;3. Shanxi Gangke Carbon Materials Co.Ltd., Taiyuan 030031, China )
Abstract: S, N co-doped porous carbons were synthesized from polybenzoxazine through solidification, carbonization and KOH activation using 4-cyanophenol, thiourea and formaldehyde as the monomers and a triblock copolymer (Pluronic F127) as a soft template. The samples were characterized by FT-IR, SEM, N2 adsorption, elemental analysis and XPS. Results indicate that the activated samples had high surface areas of 1 511.6-2 385.1 m2 g-1 with a large number of micropores and abundant sulfur and nitrogen functionalities. The templated samples had apparently lower contents of sulfur, nitrogen and oxygen than the un-templated ones due to the easy escape of volatile sulfur, nitrogen and oxygen compounds during carbonization and KOH activation. CO2 uptake had contributions from both physical and chemical adsorption and depended on the volume of narrow micropores less than 0.8 nm and the numbers of basic sulfur and nitrogen functional groups. The un-templated sample activated at 600 ℃ had the highest CO2 uptakes of 6.96 and 4.55 mmol g-1 under 1 bar at 0 and 25 ℃, respectively, and was highly selective for CO2/N2 separation and had a high recyclable stability for CO2 capture.
Key words: Polybenzoxazine; Heteroatom; Porous carbons; CO2 adsorption
1 Introduction
Large amounts of CO2emission from the burning fossil fuels have been increasingly recognized as a significant source of global climate deterioration. CO2capture and storage (CCS) using a low cost and energy-efficient method is important for protecting environment and utilizing CO2resources[1,2].There are numerous efforts engaged in CO2capture based on an adsorption method by virtue of its high adsorption efficiency, good recycle performance, and eco-friendly advantages[2]. Various adsorbents, including porous carbon materials, zeolites, porous polymers, and metal-organic frameworks (MOFs) have been widely applied for CO2capture[2-6]. Thereinto, as conventional adsorbents, porous carbons are still in focus in the light of their high surface areas, large pore volumes, tunable pore structures, and good chemical, thermal stabilities[7].
Pore structure and surface chemistry of porous carbons were reported to be the key factors for CO2adsorption[8-12].It has been demonstrated that the micropores smaller than 1 nm (especially smaller than 0.8 nm) are favorable for CO2adsorption, particularly, when adsorption takes place at ambient pressures[9]. A probably explain is that such porespossess an enhanced adsorption potential for CO2, which is similar to the size of the adsorbate molecule (dynamic diameter of CO2is 0.33 nm)[9-11]. On the other side, heteroatom doping is an important way to tailor the surface physical-chemical properties of carbon materials to gain a high CO2adsorption capacity[12-28]. Tremendous researches indicate that an incorporation of basic nitrogen functionalities into the carbon network contributes to a high adsorption capacity of CO2gas, benefiting from the hydrogen bonding and acid-base interactions between acidic CO2molecules and adsorbents[1, 12, 14]. In recent years, the researchers demonstrate that doping sulfur can also significantly improve CO2adsorption capacity of porous carbons[13, 24-28]. Serdych et al found that the sulfur functional groups, such as sulfonic acids, sulfoxides, and sulfones attracted CO2with polar interactions. Moreover, the sulfur incorporated to aromatic rings is also beneficial for CO2adsorption via acid-base interactions in micropores[28]. However, few works pay attention to the interaction of CO2with S, N co-doped activated carbons[29].
Compared with the traditional phenolic resin, polybenzoxazines (PBZs) resin, exhibits many advantages such as wide synthetic material sources, flexible molecular design, good mechanical strength, and thermal stability[18,30]. In general, PBZs are fabricated from phenols, amines, and aldehydes. Recently, nitrogen-doped carbons have been developed using PBZs as the precursors by an in-situ doping method[18,31-35]. Lu and co-workers obtained carbon monoliths from resorcinol, 1,6-diaminohexane, formaldehyde and pluronic F127, which showed an outstanding CO2capture, separation capacity owing to the multiple-length-scale porosity and nitrogen-containing framework[3].They also produced PBZ-based carbon spheres with abundant porosity, for which CO2adsorption capacity could reach 11.03 mmol g-1at -50 ℃ and 1 bar[37]. In our previous work, nitrogen-rich porous carbons derived from PBZ through a soft-templating method, achieved a CO2uptake of 4.02 mmol g-1at 1 bar and 25 ℃[18].In addition, the soft-templating agent F127 created highly interconnected micropores, macropores, and mesopores in porous carbons, which minimized molecular diffusion path. In fact, PBZs as a polymer is suitable for multi-atomic doping by a well-designed monomer at molecular level. To be our knowledge, there are few reports on S, N co-doped porous carbons derived from PBZs for CO2capture.
In present work, PBZ-based S, N co-doped porous carbons (SNPCs) via an in-situ doping method. The benzoxazine monomer was firstly synthesized from 4-cyanophenol, thiourea, formaldehyde and F127 through a solution method, followed by an evaporation-induced self-assembly (EISA) strategy[3,18]. Subsequently, solidification, carbonization, and KOH activation of the polymer were carried out consecutively. Finally, a series of SNPCs with developed porosities and tunable heteroatom amount were prepared. The influence of pore structure and sulfur, nitrogen functionalities in the SNPCs on CO2capture performance were investigated through FT-IR, SEM, N2adsorption, XPS and elemental analysis.
2 Experimental
2.1 Materials
4-Cyanophenol (99%) and thiourea (99%) were purchased from J&K China. Formaldehyde (37 wt% in water), absolute ethanol, hydrochloric acid (36 wt%-38 wt%), triethanolamine solution (0.5 mol L-1) and KOH were obtained from Tianjin Tianli Chemical Corp. All chemicals were utilized without any further purification.
2.2 Preparation of SNPCs based on PBZs
The synthesis routine of SNPCs is similar to our previous research[18].In a typical run, thiourea (5.1 g) and formaldehyde (10 mL) were added in a 250 mL three-necked round bottom flask equipped with a magnetic stirrer, a thermometer and a reflux condenser. Then, the triethanolamine solution (0.5 mol L-1) was used to adjust the pH value of the solution to 8-9. The mixture was gradually heated to 70 ℃ for 15 min and then cooled to room temperature, which was denoted as the solution A. F127 (2.1 g) and 4-cyanophenol (7.95 g) were dissolved in absolute ethanol (45 mL) and stirred for 10 min at 40 ℃, which was denoted as the solution B. The solution B was added into the solution A, and the mixture was heated under reflux at 100 ℃ for 6 h. After cooled to room temperature, the reaction mixture was poured into dishes to evaporate ethanol at room temperature for 24 h.
As-obtained PBZ was heated stepwise in an oven at 120, 150, 180, 220 and 250 ℃ for 4 h. The cured PBZ was further carbonized under nitrogen atmosphere by heating at 600 ℃ for 5 h with a heating rate of 1 ℃ min-1. The carbonized PBZ was denoted as SNPC-F127-c. Subsequently, the carbonized PBZ was mixed with an aqueous KOH solution at a weight ratio of KOH to the carbonized PBZ of 2∶1. The activation was carried out at 600, 700, 800 ℃ for 1 h in a tube furnace under flowing nitrogen with a ramp rate of 3 ℃ min-1to obtain three SNPCs, which were denoted as SNPC-F127-1, SNPC-F127-2, and SNPC-F127-3, respectively. For comparison, the sample named SNPC-c was prepared without adding any soft-templating agent F127 with the same conditions as SNPC-1. The SNPC-1 (3.0 g) was mixed with concentrated HCl (36 wt%-38 wt%, 30 mL) and stirred adequately for 24 h at room temperature. The treated SNPC-1 was denoted as SNPC-1-HCl. SNPC-1-HCl was washed with abundant water until the pH of the filtrate was neutral, and dried at 110 ℃ overnight.
2.3 Characterization
Fourier transform infrared (FT-IR) spectroscopy was performed on a Bruker Vertex70 spectrometer over the wavenumber range of 4 000-400 cm-1. Surface morphology of SNPCs was detected by a scanning electron microscope (SEM, JSM-7500F). The pore structure was obtained from N2adsorption at -196 ℃ by a Micromeritics ASAP 2020 sorption analyzer. Before measurement, the samples were treated under vacuum at 200 ℃ for 6 h. The specific surface area (SBET) was calculated from adsorption data based on the Brunauer-Emmett-Teller method in the relative pressure (p/p0) range of 0.04-0.20, whereas the total pore volume (Vtotal) was calculated from the adsorbed amount at a relative pressure of 0.99. The micropore surface area (Smicro) and micropore volume (Vmicro) were calculated according to the t-plot method. The pore size distribution (PSD) was calculated by the density functional theory (DFT) model assuming split-shaped pores. Elemental analysis was obtained on an Elementar Vario Macro EL Cube microanalyzer. X-ray photoelectron spectroscopy (XPS) was carried out using an XIS Ultra DLD spectrometer with an exciting source of MgKα(1 486.6 eV).
2.4 Evaluation of CO2 adsorption performance
IGA-002 gravimetric adsorption instrument (Hiden Isochema, Warrington, UK) was used to detect CO2isotherms at a pressure range of 0-1 bar. Before the adsorption experiments, all the samples were degassed under high vacuum at 230 ℃ for 2 h and then cooled to 25 ℃ or 0 ℃. N2or CO2was introduced to reach adsorption equilibrium at the setting pressure.
3 Results and discussion
3.1 Structure and composition of SNPCs based on PBZ

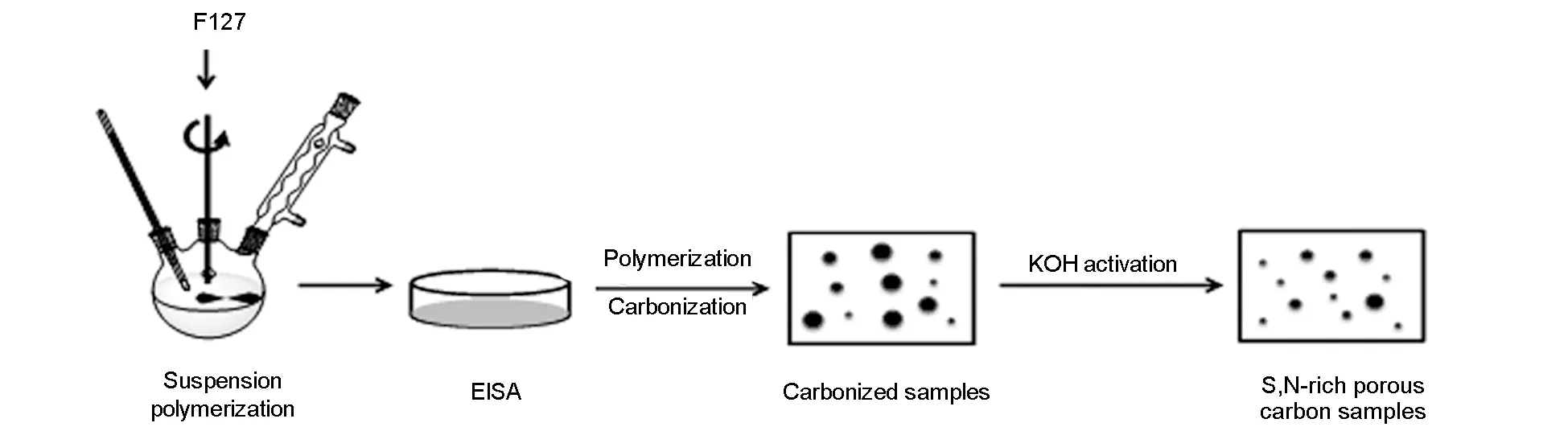
Fig. 1 Schematic diagram of preparation of SNPCs.
Fig. 2 FT-IR spectra of the benzoxazines and the SNPCs.
SEM images of the SNPCs are shown in Fig. S1. No obvious voids or pores were observed on the surface of SNPCs except SNPC-F127-3. With an increase of activation temperature, SNPC-F127-1 and SNPC-F127-2 still have smooth surface without voids or pores. The smooth surface morphology is different from our previous research, and the reason may be due to the different dosages of F127[38]. Remarkably, SNPC-F127-3 shows some shallow cavities with sizes of over several hundreds of nanometers, which may come from an over activation at high temperature.
In order to further study the influence of F127 on SNPCs, pore structure of samples was characterized. N2adsorption-desorption isotherms and pore-size distributions (PSD) are presented in Fig. 3. The porosity parameters are summarized in Table 1. N2adsorption-desorptionisotherms of SNPC-c with a hysteresis loop is of type IV according to the IUPAC classification, indicating the presence of mesopores, as shown in Fig. 3. Noticeably, the PSD of SNPC-c in Fig. 3 also demonstrates the presence of mesopores in the range of 2-2.8 nm. Compared with SNPC-c, SNPC-F127-c is dominated by micropores of size 0.4-1.5 nm. The result is in good accordance with its PSD curves shown in Fig. 3. As we all know, KOH is usually used as a chemical activating agent to prepare microporous carbon materials. In order to obtain abundant micropores, few F127 ( molar ratio of F127 and 4-cyanophenol is 1∶400 ) is used for pore-forming in this work[38,40]. After activation with KOH, the samples exhibit type I isotherm with significant nitrogen uptake at relative pressuresp/p0lower than 0.1, demonstrating the presence of large amounts of micropores. SNPC-F127-1 activated at 600 ℃, has a high BET surface area of 1 512 m2g-1with a pore volume of 0.71 cm3g-1. The changes are ascribed to KOH activation that opens some closed pores, excavates some new narrow micropores, and widens some pre-existent pores[41]. With increasing of activation temperature,SBETandVtotalof samples are obviously enhanced. Under the conditions of high temperature, the severe activation not only causes a destruction of microporous structure such as the breakage of pore walls between adjacent micropores, but also has an influence on impurity atoms. It can be seen that activated samples are still composed of abundant micropores, especially large amounts of micropores at around 0.8 nm (Fig. 3), which are very beneficial for CO2capture. The microporous feature of SNPCs is in contrast to previous reports that the soft-templating agent F127 can generate mesopores by decomposition at high temperature[18,38,42]. A probably reason is that the dosages of F127 in preparing SNPCs are lower than the values in the literature.
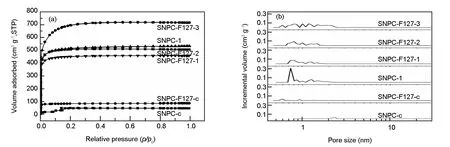
Fig. 3 (a)N2 adsorption-desorption isotherms and (b)DFT pore size distributions of SNPCs.
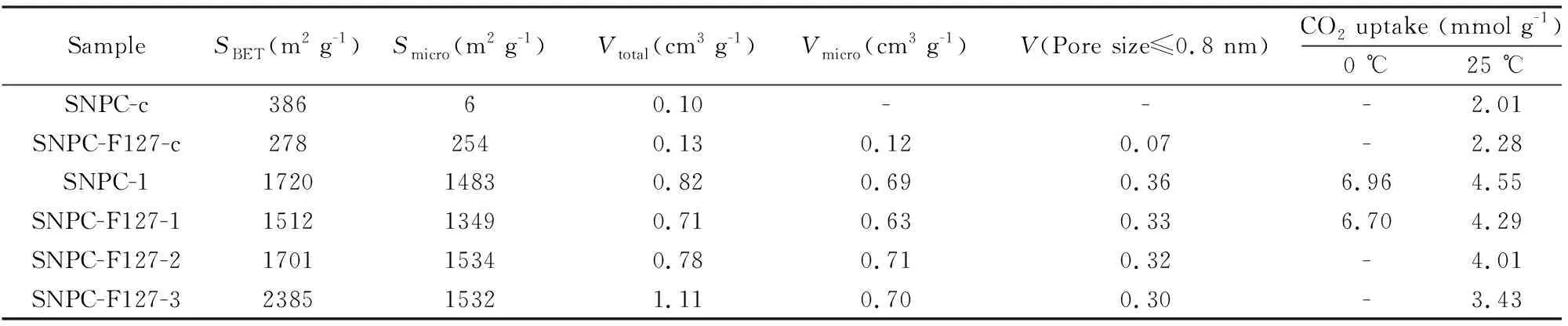
SampleSBET(m2 g-1)Smicro(m2 g-1)Vtotal(cm3 g-1)Vmicro(cm3 g-1)V(Pore size≤0.8 nm)CO2 uptake (mmol g-1)0 ℃25 ℃SNPC-c38660.10---2.01SNPC-F127-c2782540.130.120.07-2.28SNPC-1172014830.820.690.366.964.55SNPC-F127-1151213490.710.630.336.704.29SNPC-F127-2170115340.780.710.32-4.01SNPC-F127-3238515321.110.700.30-3.43
The chemical compositions of the SNPCs were further determined by elemental analysis (Table 2). It can be seen that the nitrogen content of the carbonized samples SNPC-c and SNPC-F127-c can reach 11.35 wt% and 7.82 wt% respectively, and decreases with activation temperature for activated samples. The similar trend can be found in sulfur content of obtained samples Significantly, the nitrogen, sulfur content of SNPC-c is higher than that of SNPC-F127-c. It is probably due to easier decomposition of S/N species in the carbon framework with F127 during thermal treatment[18].However, with increasing activation temperature, the loss of S/N content in SNPC-F127 samples are more serious. It is mainly due to the introduction of the copolymer F127 in the sample, whose decomposition provides porous channel for escaping of volatile nitrogen and sulfur compounds.
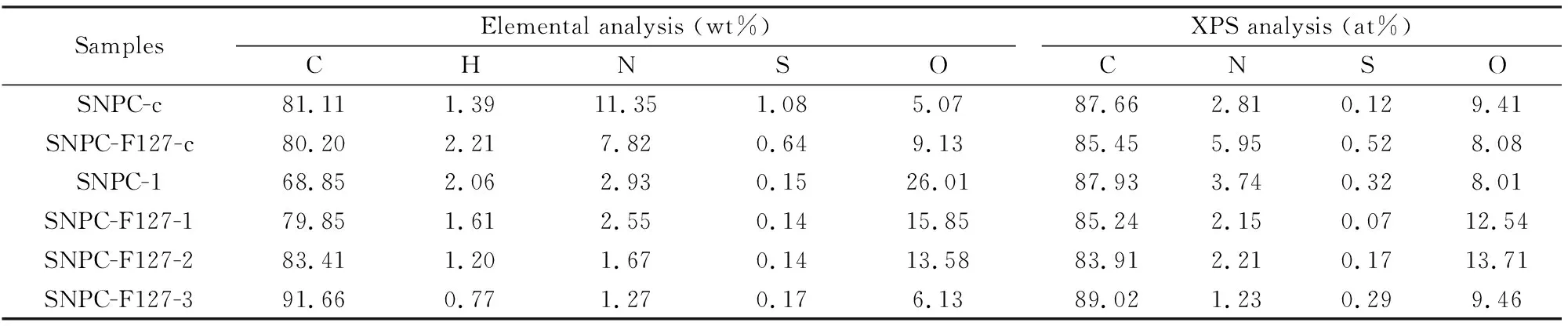
Table 2 Elemental and XPS analysis of the SNPCs.
Elemental types and functional groups on the surface of SNPCs are further investigated by XPS, as shown in Fig. S2, Fig. 4, and Tables 2, 3. It can be seen that O, N and S functional groups in the surface of SNPC samples and S/N content of SNPC-F127-c are more than that of SNPC-c. After KOH activation, S/N content of SNPC-F127-1-3 decreased with increasing activation temperature. XPS spectra of the N1s is fitted into four peaks at 398.5, 399.8, 401.0, and 403.0 eV for SNPC samples (Fig. 4), which correspond to the pyridine-N (N-6), pyrrole-N or pyridone-N (N-5), quaternary-N (N-Q), and pyridine-N-oxide (N-X), respectively[38,43].The contents of N-6 and N-5 gradually reduce, but more N-X are formed and content of N-Q is almost unaltered with increasing activation temperature (Fig. 4, Table 3). It implies that N-6 and N-5 are partially converted into N-X, and N-Q is the most stable one in the nitrogen species[18]. It can be found that the introduction of F127 for SNPC-F127-c can accelerate the conversion of N-5 into N-6, which is verified by the increased content of N-6. It is probably due to easier decomposition of N species in the carbon framework of SNPC-F127-c[18].

Fig. 4 (a) N1s and (b) S2p XPS spectra of the SNPCs.
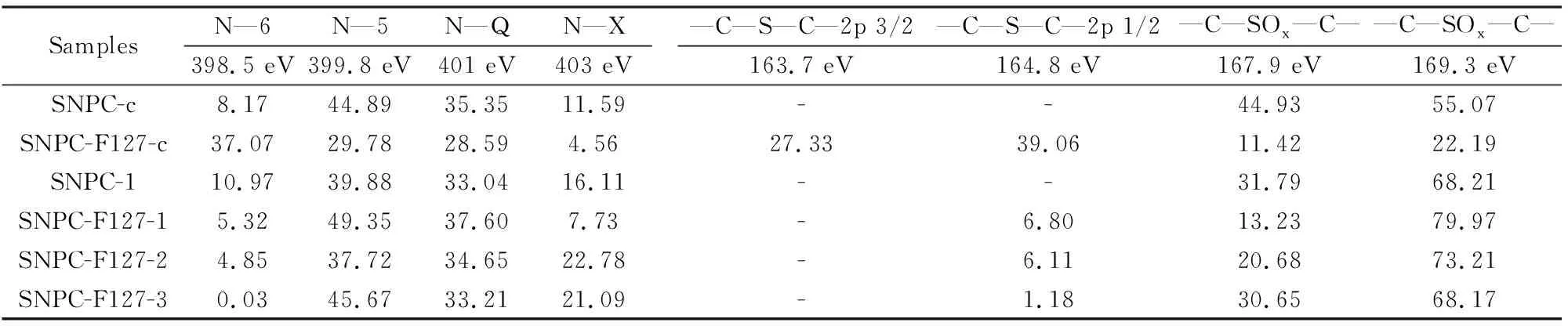
SamplesN—6N—5N—QN—X398.5 eV399.8 eV401 eV403 eV—C—S—C—2p 3/2—C—S—C—2p 1/2—C—SOx—C——C—SOx—C—163.7 eV164.8 eV167.9 eV169.3 eVSNPC-c8.1744.8935.3511.59--44.9355.07SNPC-F127-c37.0729.7828.594.5627.3339.0611.4222.19SNPC-110.9739.8833.0416.11--31.7968.21SNPC-F127-15.3249.3537.607.73-6.8013.2379.97SNPC-F127-24.8537.7234.6522.78-6.1120.6873.21SNPC-F127-30.0345.6733.2121.09-1.1830.6568.17
3.2 CO2 capture performance
SNPCs were utilized as adsorbents for CO2capture at 25 ℃ or 0 ℃ and 1 bar. CO2adsorption isotherms of SNPCs are shown in Fig. 5. The corresponding uptake at 1 bar is presented in Table 1. SNPC-F127-c, which has a low surface area (278 m2g-1) and low pore volume (0.13 cm3g-1), exhibits a CO2uptake of 2.28 mmol g-1. Carbonized sample without F127 also shows a high CO2adsorption capacity. SNPC-c has almost no microporous but displays a CO2adsorption capacity of 2.01 mmol g-1at 25 ℃, 1 bar. Previous research demonstrates that N-5, N-6, and oxidized S, which exhibit stronger Lewis basicity than other types of nitrogen functionalities, generally have a great contribution to CO2capture[38,45,49]. So, the high capacity of carbonized samples is due to the existence of the heteroatom functional groups in their surface, which can promote the adsorption capacity of acidic CO2gas. Compared with the carbonized sample SNPC-F127-c, activated sample SNPC-F127-1 displays a high CO2uptake (4.29 mmol g-1at 25 ℃ and 6.70 mmol g-1at 0 ℃). Especially, SNPC-1 has the maximum values of 4.55 mmol g-1at 25 ℃ and 6.96 mmol g-1at 0 ℃, which are higher than those of various heteroatom doping porous carbons materials reported previously[1,3,9,11,12,14,17,18,29,45]. Such improved CO2uptake may be due to the well-developed micropore structure by KOH activation. Furthermore, CO2uptakes for SNPC-F127-2 and SNPC-F127-3 decrease with the increase of activation temperature. Although SNPC-F127-2 and SNPC-F127-3 have high surface areas (1 700.6, 2 385.1 m2g-1) and pore volumes (0.78, 1.11 cm3g-1), theV(Pore size≤0.8nm)of SNPC-F127-2 and SNPC-F127-3 were reduced at high activation temperatures (shown in Table 1), leading to reduced CO2uptakes. As we know, the kinetic diameter of a single CO2molecule is 0.33 nm, and the micropore only with a narrow pore size of 1 nm, which have a high adsorption potential, is effective for CO2gas physical adsorption at atmospheric or low pressure[36]. SNPC-1 has the highestV(Pore size≤0.8nm)(Table 1) of all the samples, which is one of the reasons for its highest CO2uptake values.
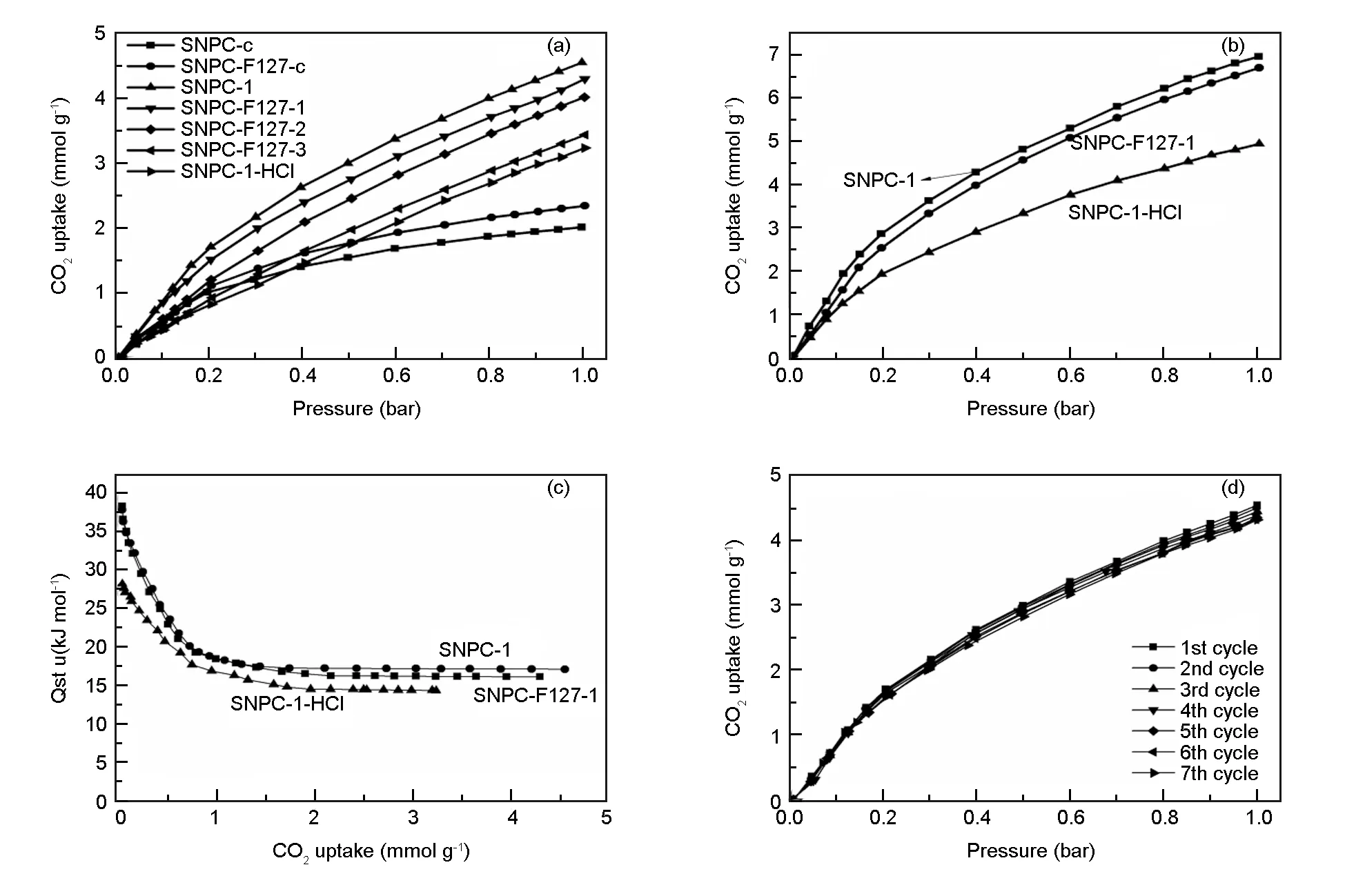
Fig. 5 CO2 adsorption isotherms of the SNPCs at (a) 25 ℃, (b) 0 ℃, (c) isosteric heat of CO2adsorption and (d) CO2 multi-cycle adsorption isotherms.
Besides pore structure, the surface functionalities on porous carbons also have an influence on the CO2capture at atmospheric pressure. Based on the acid-base interactions between basic N-containing groups and acidic CO2, increasing the content of pyridine N, pyrrole-like N groups can effectively enhance their CO2uptakes[38]. It has been found that oxidized S can act as an anchor for CO2capture and enhance CO2adsorptive capacity of porous carbons owing to its high polarity and basicity[24-28]. In this work, the contents of sulfur and nitrogen on the surface of activated samples decrease with increasing activation temperature as shown in Table 2. The loss of oxidized-S,N-6,and N-5 functionalities in the surface of carbon framework leads to a weaken affinity with CO2molecule, resulting in a low CO2adsorption capacity[38,45,50]. In order to investigate the effect of heteroatomic groups on CO2capture, the groups on the surface of SNPC-1 was neutralized by the treatment with concentrated HCl. The treated sample was denoted as SNPC-1-HCl. The narrowing of pores of SNPC-1-HCl, has been observed (Table S2) such asSBETandVtotal. Moreover, XPS and elemental analysis reveal the existence of Cl in the SNPC-1-HCl ( Tables S3, S4 and Fig. S4, S5 ). It implies that Cl ions might be adsorbed into the narrow pores and chemically bonded to the surface functional groups[18]. In general, concentrated HCl treatment has effects on the surface functionality content of SNPC-1. N1s signal reflects the decrease of N-6 content and the increase of content N-Q after neutralization (Table S4). At the same time, S2p signal also changes. Exactly, —C—S—C— appears and the content of —C—SOX—C— reduces after HCl treatment. After HCl treatment, the content of micropores (pore diameter ≤0.8 nm) decreases by 22%, the contents of S and N functional groups on surface decrease by 77% and 34% (Tables S2, S3), respectively. CO2adsorption isotherms of SNPC-1-HCl were detected at 0 and 25 ℃. As shown in Fig. 5a and b, CO2uptakes of SNPC-1-HCl can reach 4.93 and 3.23 mmol g-1at 0 and 25 ℃, respectively, which are smaller than those of SNPC-1 (6.96 and 4.55 mmol g-1). The result demonstrates that the S/N functionalities in surface of carbon materials contribute to CO2uptake. The result is similar to the work of Lu et al, who revealed that the heteroatom of adsorbents was a booster for CO2capture at low pressures, while the porosity played an essential role in achieving high CO2adsorption capacity[37].
It is clear that CO2uptake of samples reduces with increasing adsorption temperature from Fig. 5a, b. The result indicates that adsorption is an exothermic process[51]. The isosteric heat of CO2adsorption (Qst) was calculated by Clausius-Clapeyron equation based on CO2adsorption isotherms at 0 and 25 ℃, as shown in Fig. 5c[9,52].Qstof SNPC-F127-1 decreases and levels off in the range of 38.3-16.1 kJ mol-1with the CO2uptakes from 0.03 to 4.29 mmol g-1, andQstof SNPC-1 is from 37.8 to 17.1 kJ mol-1with CO2uptakes from 0.03 to 4.55 mmol g-1. In the initial stage of CO2adsorption,Qstreaches a maximum, but with increasing of the surface coverage of CO2molecules,Qstvalues decrease and level off obviously. As for SNPC-1-HCl,Qstvaries from 14.3 to 28.2 kJ mol-1with CO2capacities from 0.03 to 3.23 mmol g-1.Qstof physical adsorption for capture CO2gas by porous carbon materials under atmospheric pressure and normal temperature usually lies in the range from 16 to 25 kJ mol-1[53]. This data suggests that the adsorptive nature of CO2by SNPC-1-HCl is physical adsorption, which mainly depends on its micropore structure[18]. In addition, compared with SNPC-1, a lowQstvalue for SNPC-1-HCl can be attributed to the loss of basic groups for SNPC-1-HCl after neutralization treatment. Moreover, CO2adsorption of SNPC-F127-1 and SNPC-1 may combine both physical adsorption and chemical adsorption. Such highQstcan be attributed to not only the porous configuration, but also the strong interaction between sulfur, nitrogen functionalities and CO2molecules.

Fig. 6 (a) CO2 /N2 adsorption isotherms of SNPC-F127-1 and SNPC-1 at 25 ℃ and (b) CO2/N2 selectivity versus CO2 molar fraction at an overall pressure of 1 bar.
As an ideal CO2adsorbent, the CO2/N2selectivity of samples also needs to be investigated. N2adsorption capacity of SNPC-F127-1 and SNPC-1 was detected under the same experimental conditions and the results are shown in Fig. 6a, which show that their N2uptakes are 0.51 and 0.49 mmol g-1, respectively, which are much lower than their CO2uptake. According to the ideal solution selectivity theory (IAST), the adsorption selectivity (Sads) for the binary mixtures of CO2and N2can be calculated by determining the pure component isotherm of binary mixtures at the same conditions (Fig. 6b). The isotherms of pure CO2and N2are fitted with Langmiur model andR-squares are 0.999 7 (CO2), 0.996 6 (N2) and 0.998 5 (CO2), 0.999 6 (N2) for SNPC-F127-1 and SNPC-1, respectively. It can be seen that CO2/N2selectivity decreases with the increase of CO2molar fraction. The CO2/N2selectivity of SNPC-1 is higher than SNPC-F127-1 due to its unique micropore structure (V(Pore size≤0.8 nm)) and the higher oxidized-S contents. Reutilization is another property of adsorbent. After CO2adsorption, the used SNPC-1 was easily regenerated by heating under vacuum to a temperature of 230 ℃ and then retested for CO2uptake. Cyclic adsorption/desorption of CO2for SNPC-1 shows no significant alteration after 7 cycles (Fig. 5d), which indicates a high recyclable stability for CO2capture because of the well-developed micropores, appropriate pore size distribution, and abundant S/N functional groups.
4 Conclusions
A series of novel S, N co-doped porous carbons from polybenzoxazine resins were prepared by in-situ doping, carbonization and KOH activation. The activated samples exhibit abundant micropores (Pore size≤0.8 nm) and rich S(—C—SOX—C—)/N(N—6, N—5) functional groups, which have a significant influence on CO2adsorption properties. The CO2adsorption capacity for SNPC-1 can reach 4.55 and 6.96 mmol g-1at 1 bar, 25 and 0 ℃, respectively. CO2adsorption includes physical and chemical adsorption and is attributed by a synergistic effect of the pore structure and the heteroatomic functionalities. SNPC-1 can be recycled seven times without significant alternation. Such polybenzoxazine-based S, N co-doped porous carbons will show excellent prospect in the field of CO2adsorptive application.