泰地罗新注射液在猪体内的药动学及生物利用度研究
闫超群,黎健业,张申,谢顺,胡浪,顾欣,曹莹,黄士新,黄显会
泰地罗新注射液在猪体内的药动学及生物利用度研究
闫超群1,黎健业1,张申1,谢顺1,胡浪1,顾欣2,曹莹2,黄士新2,黄显会1
(1华南农业大学兽医学院/广东省兽药研制与安全评价重点实验室,广州 510642;2上海市动物疫病预防控制中心,上海 201103)
【目的】以健康猪为研究对象,开展泰地罗新注射液在猪体内的药动学特征及生物利用度的研究,从而为泰地罗新的临床应用提供科学依据。【方法】选取20头健康猪,随机分为2组,按4 mg·kg-1进行单次静注、肌注泰地罗新注射液,于注射后5 min、10 min、15 min、0.5 h、1 h、2 h、4 h、8 h、12 h、1 d、2 d、3 d、4 d、5 d、6 d、7 d、8 d、9 d、10 d、11 d、12 d、13 d、14 d、15 d进行前腔静脉采血,采用Phenomenex Luna C18(150 mm×2 mm,5 μm)。以乙腈-0.1%甲酸水溶液为流动相,梯度洗脱程序洗脱,流速0.25 mL·min-1,柱温为30℃,进样量 5.0 μL。样品自然解冻,准确吸取0.5 mL血浆于5 mL离心管内,加入2 mL乙腈,涡旋混匀,震荡10 min,8 000 r/min离心10 min,取上清液35℃下氮气吹干,1 mL复溶液复溶,过0.22 μm微孔滤膜,LC-MS/MS检测分析。泰地罗新在4—1 000 ng·mL-1的浓度范围内呈现良好的线性关系,相关系数为0.994—0.998,检测限为2 ng·mL-1,定量限为4 ng·mL-1,该方法的相对回收率为87.91%—104.93%,呈良好的线性关系,相关系数为0.994—0.998。批内变异系数为2.12%—12.09%,批间变异系数3.92%—10.65%。该方法灵敏度高,操作简便,可以用于泰地罗新在猪体内的药代动力学研究。泰地罗新采用电喷雾离子源(ESI)离子化;正离子模式扫描;多反应监测模式(MRM),电喷雾电压(IS)为5500V;雾化气压力(GS1)为50psi;辅助气流速(GS2)为50 L·min-1,气帘气压力(CUR)为25 psi;离子源温度(TEM)为550 ℃;碰撞室压力(CAD)为6 psi。m/z→734.6/98.1和m/z→734.6/174.4。HPLC-MS/MS法检测猪血浆中泰地罗新的浓度。采用药动学软件 Winnonlin5.2.1的非房室模型分析方法,计算有关药物动力学参数。【结果】猪静脉注射给药后,AUClast和AUCinf(pred)分别为(18030.30±7560.75) h·ng·mL-1和(18795.31±7455.23) h·ng·mL-1。t1/2为(99.42±22.25) h,MRT为(81.71±12.15) h。肌肉注射给药后,Cmax为(886.00±155.63) ng·mL-1,Tmax为(0.51±0.30)h,AUClast和AUCinf(pred)分别为(19702.05±6442.36)h·ng·mL-1和(20840.08±6849.76) h·ng·mL-1。t1/2为(100.83±20.23) h,MRT为(81.80±9.44) h。绝对生物利用度为109.27%。【结论】泰地罗新肌注后在猪体内具有吸收迅速,分布广泛,达峰迅速,消除较慢,生物利用度高等特点,可在兽医临床上安全使用。
泰地罗新;药动学;生物利用度;猪
0 引言
【研究意义】引起猪呼吸道疾病(SRD,swine respiratory disease)病原体多样,具有高的发病率和死亡率,每年给全球带来巨大的经济损失[1]。常见的临床致病菌主要有猪胸膜肺炎放线杆菌()、多杀性巴氏杆菌()、支气管败血波氏杆菌()以及副猪嗜血杆菌()等[2-6]。大环内酯类药物是临床上常用的治疗SRD感染的药物。其与原核生物50S核糖体亚基中的23S rRNA结合,通过阻断肽链的延长,从而抑制细菌蛋白质合成[7]。大环内酯类治疗效果通常呈时间依赖性[8],但泰地罗新呈浓度依赖性[9]。大环内酯类药物一般是抑制微生物的生长,但对某些病原体可以起到杀菌的作用。替米考星、泰乐菌素等大环内酯类抗生素因为具有较差的细胞外膜穿透力,因此很少用来治疗具有细胞壁的阴性菌感染[10]。泰地罗新(tildipirosin)是新型动物专用的16元大环内酯类抗生素,是半合成泰乐菌素的衍生物,其分子式为C14H71N3O8,分子量为734.02D,微溶于水中,易溶于有机溶剂(如甲醇、丙酮等)。化学式见图1。较其他大环内酯类抗生素,泰地罗新独特的化学结构是在C20和C30的位点有两个哌啶取代基,在大环内酯环的C5位置上有一个碱性碳霉糖,由于三个氮原子可以质子化,因此泰地罗新为有机碱,且脂溶性高,易穿过细胞壁[11]。【前人研究进展】泰地罗新最早由Intervet公司生产,CAS号为:328898-40-4。泰地罗新为单次给药,牛的给药方式为皮下注射(规格为18%),猪的给药方式为肌肉注射(规格为4%),给药剂量均为4 mg·kg-1[8]。2011年欧盟兽用药品委员会(CVMP)准许了Intervet公司以泰地罗新为主要成分的无菌注射液(商品名为Zuprevo)的市场许可申请,随后美国也相继批准上市[12]。在国内已有某些厂家自主研制泰地罗新注射液,而国产泰地罗新注射液在动物体内的药动学尚未研究。【本研究切入点】本研究探讨了健康猪静注、肌注泰地罗新注射液的药动力学过程,并阐明其药动学特征和肌注给药的生物利用度,旨在为国产泰地罗新在国内临床上的使用制定合理的给药方案,并提供理论依据。【拟解决的关键问题】泰乐菌素和替米考星等大环内酯类药物在临床上均具有良好的治疗效果,但一般需要多次重复给药才能发挥较好的药效,大多还以拌料或饮水方式给药[13]。但是从长远来看药物大剂量、长期重复使用[14],加速了临床耐药菌的出现[15-16]。而新型大环内酯类抗生素泰地罗新,具有给药后分布广泛,半衰期长等特点,以单剂量注射给药即可有效治疗猪SRD、牛的呼吸道疾病(BRD,bovine respiratory disease),除此之外泰地罗新对猪布氏杆菌病()也有好的治疗效果[17]。BARTRAM等对比了泰拉霉素与泰地罗新在感染牛支原体的临床治疗效果上,结果表明,泰拉霉素的治疗效果优于泰地罗新[18]。本研究确定了猪血浆中泰地罗新检测方法的建立及确证,以及泰地罗新在猪体内药代动力学规律的认识,为泰地罗新注射液临床合理给药方案的制定提供参考。

图1 泰地罗新化学结构式
1 材料与方法
1.1 试验动物
20头健康的杜洛克、长白、约克夏杂交猪,雌、雄各半,8周龄左右,体重20 kg左右。按常规饲养,自由饮水和采食,饲料为全价日粮,不含抗菌药物。临床观察两周表现健康。20头猪随机分为2组,每组10头,雌、雄各半,分别静脉注射、肌肉注射给药进行药动学试验,给药剂量均为4 mg·kg-1体重。试验前16 h起及给药后4 h期间禁食,仅自由饮水。
1.2 药品和试剂
受试药物:泰地罗新注射液,含量4%(100 mL:4 g),批号:20150401。泰地罗新标准品,批号:20150209,含量:99.26%,均由上海同仁药业有限公司提供。
1.3 仪器设备
美国Agilent公司1200型高效液相色谱仪(配四元泵、脱气泵、自动进样器、柱温箱等),美国应用生物系统公司API 4000 电喷雾—串联四级杆质谱仪(配Analyst1.5 软件),美国BECKMAN COULTER公司Avanti J-26 高速冷冻离心机,上海青浦沪西仪器厂XW—80A 旋涡混合仪。美国Beckman-Coulter公司Avanti J-26 XP型高速冷冻离心机。Milli-Q公司Milli-Q去离子水发生器。德国eppendorf公司Research型可调微量移液器。瑞士Mettler公司生产AT-261型电子分析天平。
1.4 分组、给药及采样
猪只仰卧保定,从前腔静脉采血,给药前所有试验动物采集一次空白血。静脉(耳缘静脉)、肌肉注射给药后分别于5 min、10 min、15 min、0.5 h、1 h、2 h、4 h、8 h、12 h、1 d、2 d、3 d、4 d、5 d、6 d、7 d、8 d、9 d、10 d、11 d、12 d、13 d、14 d、15 d采集血样。每次采血5 mL左右,置于含肝素的离心管中,离心分离血浆,-80℃冰箱保存,待测。
1.5 血浆中泰地罗新含量测定
采用Phenomenex Luna C18(150 mm×2 mm,5 μm)。流动相为0.1%的甲酸水和乙腈;梯度洗脱程序洗脱,流速为0.25 mL·min-1;柱温为30℃;进样量为5.0 μL。
泰地罗新采用电喷雾离子源(ESI)离子化;正离子模式扫描;多反应监测模式(MRM),电喷雾电压(IS)为5 500 V;雾化气压力(GS1)为50 psi;辅助气流速(GS2)为50 L·min-1,气帘气压力(CUR)为25 psi;离子源温度(TEM)为550℃;碰撞室压力(CAD)为6 psi。泰地罗新用于定量和定性的离子分别为:m/z→734.6/98.1和m/z→734.6/174.4。
在上述条件下,泰地罗新的检测限和定量限分别为2 g·mL-1和4 ng·mL-1。血浆中的泰地罗新在4— 1 000 ng·mL-1的范围内,泰地罗新的相对回收率为87.91%—104.93%,呈良好的线性关系,相关系数为0.994—0.998。批内变异系数为2.12%—12.09%,批间变异系数为3.92%—10.65%。
1.6 样品处理
血浆样品自然解冻,混匀。准确吸取0.5 mL血浆于5 mL离心管内,加入2 mL乙腈,涡旋混匀,震荡10 min,8 000 r/min离心10 min,取上清液35℃下氮气吹干,1 mL复溶液复溶,过0.22 μm微孔滤膜,LC-MS/MS检测分析。
1.7 数据分析处理
采用美国Pharsight公司药动学软件Winnonlin 5.2.1的非房室模型来处理药动数据,计算出每头试验猪的关键药动学参数(F、AUC、Tmax、Cmax等),然后计算平均值()及标准差(S.D.),同时以血药浓度平均值对时间作药-时曲线图(图2)。
2 结果
2.1 静注泰地罗新在猪体内的药动学特征
本试验得到的数据经Winnonlin5.2.1软件采用非房室模型进行分析。静脉注射结果表明,按4 mg·kg-1的剂量给猪静脉注射后,泰地罗新在猪体内获得的血药浓度—时间数据符合非房室模型。泰地罗新给药后,消除半衰期t1/2为(99.42±22.25)h,药时曲线面积AUClast为(18030.30±7560.75)h·ng·mL-1。消除半衰期t1/2为(99.42±22.25)h。MRT为(81.71±12.15)h。说明静脉注射给药后分布广泛,表观分布容积大,半衰期长,消除缓慢(表1,2)。
2.2 肌注泰地罗新在猪体内的药动学特征
本试验得到的数据经Winnonlin5.2.1软件采用非房室模型进行分析。泰地罗新按4 mg·kg-1的剂量肌肉注射后,泰地罗新在猪体内获得的血药浓度-时间数据符合非房室模型。肌注泰地罗新后,达峰时间tmax为(0.51±0.30)h,峰浓度Cmax为(886.00±155.63)ng·mL-1,消除半衰期t1/2为(100.83±20.23)h,药时曲线下面积AUClast为(19702.05 ±6442.36)h·ng·mL-1。MRT为(81.80±9.44)h。泰地罗新注射液的绝对生物利用度为109.27%,说明肌注给药吸收完全。
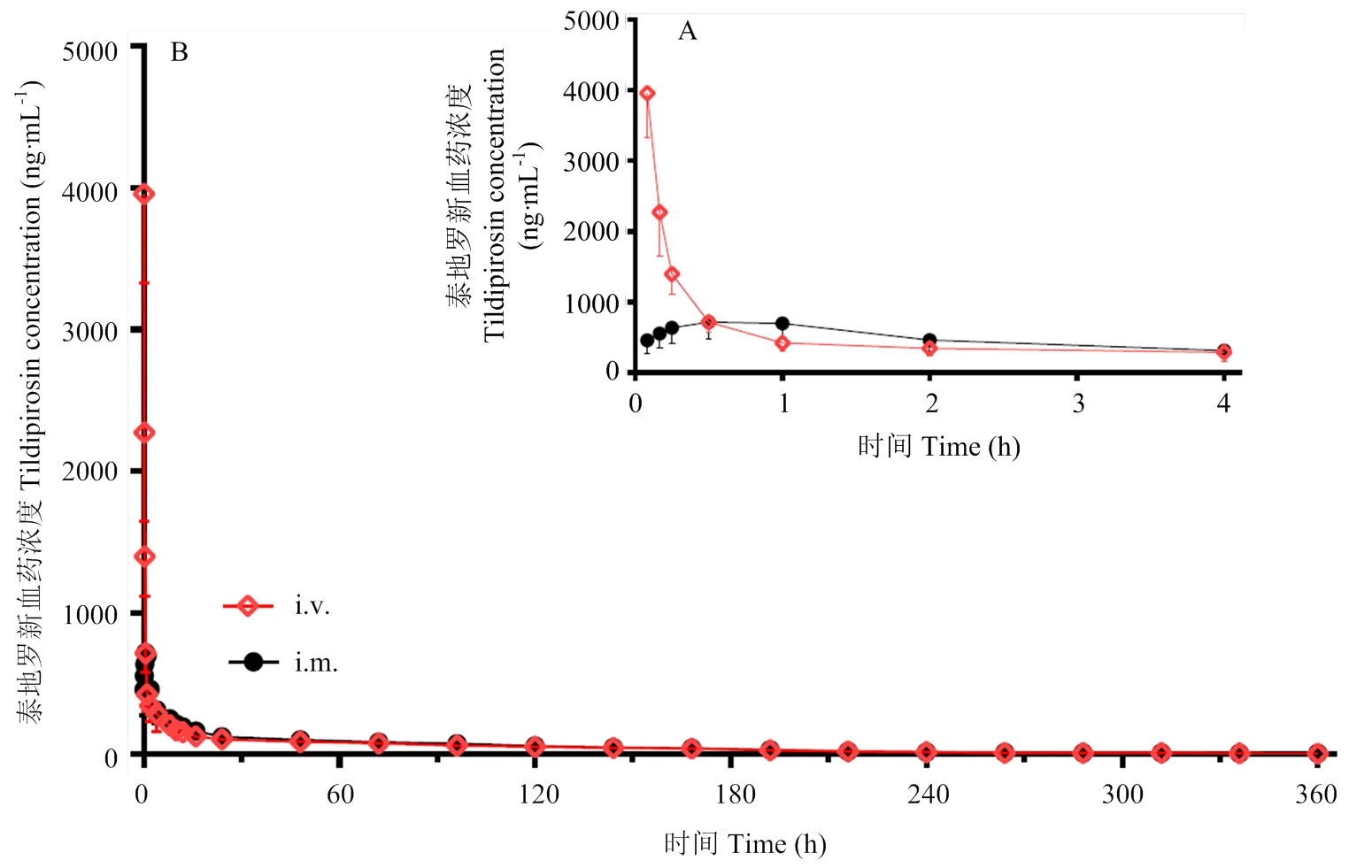
A:0-4 h,B:0-360 h
3 讨论
3.1 安全性
据EMA报道,大鼠按2 000 mg·kg-1bw口服泰地罗新,表现出皮肤褶皱、镇静、身体躬起、闭目、失衡、流涕等症状。剖检无其他病理变化。小鼠按6.25、12.5、25 和50 mg·kg-1bw静脉注射后,高于12.5 mg·kg-1bw剂量组全部死亡,且剂量越大死亡越快,并表现出失衡、呼吸急促、不安、抽搐、痉挛等症状。小鼠静脉注射泰地罗新的LD50(半数致死量)的范围为6.25<LD50<12.5 mg·kg-1bw[19]。与替米考星一样,泰地罗新有一定的心脏毒性,犬按20 mg·kg-1bw,肌肉一次注射,给药后,犬血压有轻微且明显的下降。在靶动物猪的安全性试验中。猪按4、8、12、20 mg·kg-1bw的剂量进行肌肉注射给药,每个注射位点的给药体积不超过5 mL。在所有进行多倍推荐剂量注射的试验组中,猪会短暂地表现出精神萎靡。3倍推荐剂量组和5倍推荐剂量组的实验动物会出现短暂的震颤症状,5倍剂量组甚至出现休克症状。猪肌肉注射5倍推荐剂量给药后,注射部位出现不适,肿胀。白细胞、嗜中性粒细胞、单核细胞以及肌酸磷酸激酶增加[20]。除了注射部位,试验动物的其他部位并没有发现明显的病理变化[21]。本试验过程中肌肉注射和静脉注射,给药剂量均为4 mg·kg-1bw,给药后临床无不良反应。
表1 猪肌肉、静注泰地罗新注射液后平均血药浓度(4 mg·kg-1b.w.)(±SD,n=10)
Table 1 Mean ± SD plasma concentrations in pigs administered a single intramuscular(i.m.) or intravenous (i.v.) injection of tildipiroson at 4 mg·kg-1body weight, respectively (ng·mL-1) (±SD, n=10)

表2 猪静脉、肌肉注射泰地罗新注射液(4 mg·kg-1bw)后的药代动力学参数(注射液规格为:4%)
Table 2 Plasma pharmacokinetics parameters after single intramuscular (i.m.) or intravenous (i.v.) administration of 40 mg·mL-1tildipirosin solution for injection at 4 mg·kg-1body weight

3.2 药动学
据EMA报道,猪按4 mg·kg-1bw肌肉注射泰地罗新注射液后,tmax小于0.5 h,14 d后血浆药物浓度低于定量限(10 ng·mL-1),t1/2大于4 d,AUClast为11 012 h·ng·mL-1,MRTlast大于3.5 d[20-21]。Rose等亦报道了泰地罗新肌肉注射给药后(4 mg·kg-1),tmax为(0.38±0.14)h,Cmax为(895±453)ng·mL-1,t1/2为(106±15)h,AUClast为(11012±2989) h·ng·mL-1[11]。廖远军报道泰地罗新肌肉注射后(4 mg·kg-1),tmax为(0.39±0.18)h,Cmax为(0.97±0.39)μg·mL-1,t1/2为(124.5±42.1)h,AUClast为(15.75±5.19)h·μg·mL-1[22]。这与本研究得到的泰地罗新药动学参数接近。AUC有较大的差异,这可能是样品的检测限和纳入试验动物的品种等不同引起的差异。说明该制剂的生物利用度比已报道的更高,达到109.27%。已报道泰地罗新在不同动物的药动学参数见表3。
表3 泰地罗新在不同动物体内经肌肉、皮下、静脉注射后的主要药代动力学参数(±SD)
Table 3 Summary of the mean (±SD) plasma pharmacokinetic parameters in different animals administered a single intramuscular (i.m.), subcutaneous(s.c.) or intravenous (i.v.) injection of tildipirosin

Cmax:血药峰浓度;Tmax:药物达到峰浓度的时间;AUClast:0 h到最后一个采样点药时曲线下面积;AUCinf (predicted):0 h到无穷大药时曲线下面积;T1/2:体内药物下降一半所需要的时间;MRTlast:0 h到最后一个采样点平均滞留时间
Cmax, maximum observed concentration in plasma; Tmax, time to reach maximum observed concentration in plasma; AUClast, area under concentration-vs.-time curve from time 0 to the last sampling time associated with a quantifiable drug concentration; AUCinf (predicted), area under concentration vs.time curve from time zero extrapolated to infinity (predicted); T1/2, terminal half-life; MRTlast, mean residence time from the time of dosing to the time of last measurable concentration
3.3 药效学
泰地罗新的抗微生物活性谱包括:胸膜肺炎放线杆菌(),多杀巴氏杆菌(),支气管败血波氏杆菌()和副猪嗜血杆菌(),它们是最常与猪呼吸道疾病(SRD,swine respiratory disease)相关的细菌病原体。在体外,泰地罗新的作用是抑制多杀巴斯德氏菌和支气管败血波氏杆菌,对胸膜肺炎放线杆菌和副猪嗜血杆菌是杀菌的。EMA报道的泰地罗新对常见致病菌见表4。本试验给药后血浆药物浓度Cmax为(886.00±155.63)ng·mL-1,值得一提的是,大环内酯类抗生素的在组织中的分布是远高于血浆[25- 26],尤其是在吞噬细胞(如多核白细胞和巨噬细胞)周围[27-29]。ROSE等报道给药后的泰地罗新被迅速吸收和广泛分布到呼吸道感染部位。在肺中,泰地罗新在给药后第24 h达到峰值,为(4.25±0.72)μg·kg-1,而同时间的血浆浓度仅为(0.051±0.005)μg·mL-1,肺/血浆的比率为83。之后缓慢下降,在给药后第5天比率达到最高的148。直到给药后17 d,依然可以检测到肺部药物浓度,其在肺部的消除半衰期约为7 d。同样考察了支气管液中泰地罗新的药物含量,给药后5 d,支气管液中泰地罗新含量高达(14.31±3.67)μg·g-1,支气管液/血浆比率为681[11]。还有研究表明,猪流行性胸膜肺炎放线杆菌(APP)可以长期在扁桃体中成为这种微生物的无症状携带者。给药后,扁桃体中泰地罗新的最大浓度在给药后1 d观察到,一直持续到第15天。最后,扁桃体AUC/血浆AUC为97.9,和扁桃体t1/2(h)明显高于血浆。抗菌治疗已被用于根除扁桃体中的APP[30]。
表4 泰地罗新对常见致病菌MIC
Table 4 Tildipirosin against common pathogens MIC (μg·mL-1)

4 结论
本试验通过静脉注射和肌肉注射给药研究了国产泰地罗新注射液在猪体内的药动学,试验结果表明国产泰地罗新注射液在健康猪体内具有吸收迅速,半衰期长,生物利用度高等特点,兽医临床上可安全使用。本试验首次科学的评价了国产泰地罗新注射液在猪体内的药动学特征,为其新兽药审批及在临床上合理使用提供了试验依据。对兽医临床上治疗由胸膜肺炎放线杆菌、支原体、巴氏杆菌、副猪嗜血杆菌等引起的疾病具有重大意义。
[1] STARK K D C. Epidemiological investigation of the influence of environmental risk factors on respiratory diseases in swine: A literature review.2000, 159(1): 37-56.
[2] OPRIESSNIG T, GIMENEZ-LIROLA L G, HALBUR P G. Polymicrobial respiratory disease in pigs., 2011, 12(2): 133-148.
[3] AHMAD T A, RAMMAH S S, SHEWEITA S A, HAROUN M, EI-SAYED L H. Development of immunization trials against., 2014, 32(8): 909-917.
[4] GOTTSCHALK M. The challenge of detecting herds sub-clinically infected with.2015, 206 (1): 30-38.
[5] MACEDO N, ROVIRA A, TORREMORELL M.: infection, immunity and enrofloxacin., 2015, 46(1): 1-6.
[6] PENG Z, WANG H, LIANG W, CHEN Y, TANG X, CHEN H C, WU B. A capsule/lipopolysaccharide/MLST genotype D/L6/ST11 ofis likely to be strongly associated with swine respiratory disease in China.2018, 200(1): 107-118.
[7] SCHLUENZEN F, ZARIVACH R, HARMS J, BASHAN A, TOCILJ A, ALBRECHT R, YONATH A, FRANCESCHI F. Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria.(London), 2001, 413(6858): 814-821.
[8] Lees P C D A. Drug selection and optimization of dosage schedules to minimize antimicrobial resistance.. Washington, DC: ASM Press, 2006: 49-60.
[9] LEI Z, LIU Q, YANG B. The pharmacokinetic-pharmacodynamic modeling and cut-off values of tildipirosin against, 2018, 9(2): 1673-1690.
[10] FITTIPALDI N, KLOPFENSTEIN C, GOTTSCHALK M, BROES A, PARADIS M A, DICK C P. Assessment of the efficacy of tilmicosin phosphate to eliminatefrom carrier pigs., 2005, 69(2): 146-150.
[11] ROSE M, MENGE M, BOHLAND C, ZSCHIESCHE E, WILHELM C, KILP S, METZ W, ALLAN M, RÖPKE R, NÜRNBERGER M. Pharmacokinetics of tildipirosin in porcine plasma, lung tissue, and bronchial fluid and effects of test conditions onin vitro activity against reference strains and field isolates of., 2013, 36(2): 140-153.
[12] Fda. RE: NADA 141-334, Zuprevo ™ (tildipirosi. n) promotional labeling and advertisements[Z]. 2014.
[13] WATTS J L, SWEENEY M T. Antimicrobial resistance in bovine respiratory disease pathogens: Measures, trends, and impact on efficacy.2010, 26(1): 79.
[14] PYÖRÄLÄ S, BAPTISTE K E, CATRY B. Macrolides and lincosamides in cattle and pigs: Use and development of antimicrobial resistance., 2014, 200(2): 230-239.
[15] GAUTIERBOUCHARDON A V, FERRÉ S, LE G D. Overall decrease in the susceptibility of mycoplasma bovis to antimicrobials over the past 30 years in france., 2014, 8: 1-9.
[16] OLSEN A S, WARRASS R, DOUTHWAITE S. Macrolide resistance conferred by rRNA mutations in field isolates ofand.2015, 70(2): 420.
[17] DIESTE-PÉREZ L, FRAILE L, DE MIGUEL M J, BARBERÁN M, BLASCO J M. Studies on a suitable antibiotic therapy for treating swine brucellosis.2015, 38: 357-364.
[18] BARTRAM D J, MOYAERT H, VANIMISETTI B H. Comparative efficacy of tulathromycin and tildipirosin for the treatment of experimental Mycoplasma bovis infection in calves., 2016, 2(3): 170-178.
[19] Ema. European public MRL assessment report (EMA/CVMP/709377/ 2009)[Z]. 2010.
[20] Ema. European public MRL assessment report (EMA/CVMP/684147/ 2011)[Z]. 2014.
[21] Ema. Committee for Medicinal Products for Veterinary Use(EMA/ CVMP/709377/2009)[Z]. 2010.
[22] 廖远军. 泰地罗新注射液在猪体内的药代动力学研究[D]. 长春: 吉林大学, 2015.
LIAO Y J. Studies on pharmacokinetics of tildipirosin injection in pigs[D]. Changchun: Jilin University, 2015. (in Chinese)
[23] WANG J, ZHAO T, SUN X, CAO X. Pharmacokinetics of tildipirosin in beagle dogs.2018, 41(1): e49-e52.
[24] MENGE M, ROSE M, BOHLAND C, ZSCHIESCHE E, KILP S, METZ W, ALLAN M, RÖPKE R, NÜRNBERGER M. Pharmacokinetics of tildipirosin in bovine plasma, lung tissue, and bronchial fluid (from live, nonanesthetized cattle)., 2012, 35(6): 550-559.
[25] ANADON A, REEVE-JOHNSON L. Macrolide antibiotics, drug interactions and microsomal enzymes: Implications for veterinary medicine., 1999, 66(3): 197-203.
[26] NIGHTINGALE C H. Pharmacokinetics and pharmacodynamics of newer macrolides., 1997, 16(4): 438-443.
[27] COX S R, MCLAUGHLIN C, FIELDER A E. Rapid and prolonged distribution of tulathromycin into lung homogenate and pulmonary epithelial lining fluid of holstein calves following a single subcutaneous administration of 2. 5 mg/kg body weight., 2010, 8(3): 129-137.
[28] GIGUERE S, HUANG R, MALINSKI T J. Disposition of gamithromycin in plasma, pulmonary epithelial lining fluid, bronchoalveolar cells, and lung tissue in cattle., 2011, 72(3): 326-330.
[29] VENNER M, PETERS J, HOEHENSTEIGER N. Concentration of the macrolide antibiotic tulathromycin in broncho-alveolar cells is influenced by comedication of rifampicin in foals., 2010, 381(2): 161-169.
[30] TORRES F, SANTAMARIA R, JIMENEZ M, MENJÓN R, IBANEZ A. Pharmacokinetics of tildipirosin in pig tonsils., 2016, 39(2): 199-201.
(责任编辑 林鉴非)
Pharmacokinetics and Bioavailability of Tildipirosin Solution in Pigs
YAN ChaoQun1, LI JianYe1, ZHANG Shen1, XIE Shun1, HU Lang1, GU Xin2, CAO Ying2, HUANG ShiXin2, HUANG XianHui1
(1College of Veterinary Medicine, South China Agricultural University/Guangdong Provincial Key Laboratory of Veterinary Pharmaceutics Development and Safety Evaluation, Guangzhou 510642;2Shanghai Animal Disease Control Center, Shanghai 201103)
【Objective】The aim of this study was to investigate the pharmacokinetic properties and bioavailability of tildipirosin in pigs after an intravenous or intramuscular administration of 4 mg·kg-1body weight. 【Method】Twenty healthy pigs were selected and randomly divided into 2 groups and received 4 mg·kg-1of tildipirosin injection by either intravenous or intramuscular administration. The blood was collected at 5 min, 10 min, 15 min, 0.5 h, 1 h, 2 h, 4 h, 8 h, 12 h, 1 d, 2 d, 3 d, 4 d, 5 d, 6 d, 7 d, 8 d, 9 d, 10 d, 11 d, 12 d, 13 d, 14 d and 15 d after administration. Phenomenex Luna C18 (150 mm×2 mm, 5 μm) was used. Acetonitrile-0.1% formic acid aqueous solution was used as the mobile phase. The gradient elution procedure was used. The flow rate was 0.25 mL·min-1, the column temperature was 30℃, and the injection volume was 5.0 μL. The sample was thawed naturally, 0.5 mL of plasma was accurately pipetted into a 5 mL centrifuge tube, 2 mL of acetonitrile was added, vortexed and shaken for 10 min, centrifuged at 8 000 r/min for 10 min. And then the supernatant was dried with nitrogen at 35℃ and reconstituted in 1 mL of solution. 0.22 μm microporous membrane, LC-MS/MS detection analysis. Tildipirosin showed a good linearity in the concentration range of 4-1000 ng·mL-1, with a correlation coefficient of 0.994-0.998, a detection limit of 2 ng·mL-1, and a limit of quantification of 4 ng·mL-1. The relative recovery rate of this method was 87.91%-104.93%, which showed a good linear relationship with the correlation coefficient of 0.994-0.998. The coefficient of intra-assay coefficient of variation was 2.12%-12.09%, and the inter-assay coefficient of variation was 3.92%-10.65%. The experimental method had high sensitivity and simple operation, and could be used for the pharmacokinetic study of tildipirosin in pigs. MS conditions of detection method: ESI ion source, positive ion scan, ion spray voltage: 5 500 V, TEM: 550℃, the pressure of CUR: 25 psi, the pressure of GS2 50 L/min, the pressure of collision CAD: 6 psi. the quantitative ion is: tildipirosin m/z→735.1/98.0. Blood samples were collected and detected by high-performance liquid chromatography (HPLC) with tandem mass spectrometry (LC-MS/MS). Pharmacokinetic parameters were estimated using the WinNonlin5.2.1 software package and SPSS 16.0 analysis of the time and concentration data.【Result】After intravenous injection, AUClastand AUCinf (pred)were (18030.30 ±7560.75) h·ng·mL-1and (18795.31±7455.23) h·ng·mL-1, respectively;t1/2was (99.42±22.25) h, and MRT was (81.71±12.15) h. After intramuscular injection, Cmaxwas (886.00±155.63) ng·mL-1; Tmaxwas (0.51 ± 0.30) h. AUClastand AUCinf (pred)were (19702.05±6442.36) h·ng·mL-1and (20840.08±6849.76) h·ng·mL-1, respectively; t1/2was (100.83±20.23) h and MRT was (81.80±9.44) h; the absolute bioavailability was 109.27%. 【Conclusion】After intramuscular injection, the tildipirosin was absorbed quickly in pigs, distributed widely, peaked rapidly, and eliminated slowly.
tildipirosin; pharmacokinetic; bioavailability; pig
2018-01-24;
2018-05-23
“十三五”国家重点研发计划项目(2016YFD0501306)
闫超群,E-mail:1368268753@qq.com。 通信作者黄显会,Tel:020-87344801;E-mail:xhhuang@scau.edu.cn
10.3864/j.issn.0578-1752.2018.19.017
