Comparison of Isothermal Oxidation Behavior of Co-10Cr-xSi (x=0,5,10) Alloys at 1 073 K
Junhuai Xiang, Ling Wang, Honghua Zhang, Lingyun Bai, Changjun Wan and Ganlan Yang
(Jiangxi Key Laboratory of Surface Engineering, Jiangxi Science and Technology Normal University,Nanchang 330013, China)
Abstract: The oxidation behavior of Co-10Cr-xSi (x=0, 5, 10, nominal composition, at%) alloys in 0.1 MPa pure O2 at 1 073 K was investigated. Co-10Cr presents the worst oxidation resistance with the mass gain of about 7.531 mg/cm2 after 24 h oxidation, while Co-10Cr-10Si presents the best oxidation resistance with the much lower mass gain of about 0.078 mg/cm2. Co-10Cr-10Si is about two magnitudes lower than that of Co-10Cr. The oxidation behavior of Co-10Cr-5Si is intermediate between that of Co-10Cr and Co-10Cr-10Si. The nodular oxides have occupied most of the alloy surface, and their microstructure is similar to Co-10Cr, to some extent. On the contrary, only a fraction of the surface is covered by the Cr2O3 layer, whose microstructure is similar to that of Co-10Cr-10Si.
Key words: cobalt-chromium-silicon; isothermal oxidation; high temperature; microstructure
In gas turbine applications, the operation temperature of engines is being increased to improve engine efficiency and performance; thus, the oxidation resistance of alloys at higher service temperature is becoming an important issue. Cobalt-based superalloys have series of attractive, balanced properties and excellent hot corrosion resistance to degradation in corrosive or oxidizing environments[1-2]. Generally, Co-based superalloys used for high-temperature service rely on the formation of a compact, stable, slow-growing and adherent oxide scale to the base metal, such as Al2O3, SiO2and Cr2O3[3-4]. In addition, SiO2is thermodynamically more stable than Cr2O3, and so the former will consequently tend to form beneath scale interface.
To understand the very complex mechanism of hot corrosion of employed multicomponent superalloys, the oxidation behavior of the fundamental ternary system alloys must first be known. Numerous data concerning the kinetics and mechanism of oxidation of Co-10Cr alloys, as well as the microstructure of scales, have been reported[5-7]. In recent years, a series of Co-based amorphous or nanocrystalline alloys such as CoSi have been studied as better materials for hydrogen storage[8-9]. The oxidation behavior and mechanism of Co-Cr-Al alloys have been studied by Wallwork and Hed[10], and an “oxide map” was drawn for the Co-Cr-Al system at 1 373 K. Jones and Stringer have reported that as little as 0.05 wt% Si is sufficient to change the mode of oxidation of Co-25wt% Cr at temperatures in the range 1 273-1 473 K[11]. Where as there are not enough studies about addition of Si element to Co-10Cr alloys. In particular, a detailed understanding of the variation of kinetics and mechanism of oxidation and microstructure of scales is still insufficient.
The present paper describes the isothermal oxidation behavior of Co-10Cr-0Si, Co-10Cr-5Si and Co-10Cr-10Si alloys at 1 073 K in 0.1 MPa oxygen for up to 24 h. Particular emphasis is placed on the comparison of oxidation rate and microstructure of scales arising from increasing Si content.
1 Experimental Procedures
In the present study, the three Co-based model alloys were prepared by the repeated melting of 99.9% Co, 99.95% Cr and 99.999% Si in a vacuum-induction furnace, and then they were cast into a water-cooled mould. The corresponding actual average composition of each alloy is listed in Tab.1. The ingots were annealed for 24 h at 1 173 K in vacuo (~1.3 Pa) and cut into 10 mm×10 mm×1.2 mm pieces by spark-erosion machining, and then they were polished on 2000# SiC waterproof abrasive papers. All these specimens were washed with distilled water, acetone and ethanol, and then they were dried in warm air.

Tab.1 Actual composition of three alloys
Continuous mass change measurements of the specimens were carried out for 24 h by a Cahn Versatherm TGA system in 0.1 MPa pure O2at 1 073 K. The morphology and composition of the oxide scales were characterized by means of field emission scanning electron microscopy (FESEM) in combination with energy-dispersive X-ray spectroscopy. X-ray diffraction (XRD) was used for oxide phase identification.
2 Results and Discussion
2.1 Isothermal oxidation kinetics
The oxidation kinetics curves and the corresponding parabolic plots for the oxidation of individual specimens of Co-10Cr-0Si, Co-10Cr-5Si and Co-10Cr-10Si alloys at 1 073 K in 0.1 MPa oxygen for up to 24 h are shown in Fig.1. Fig.1c and Fig.1d are the enlarged view of Co-10Cr-10Si alloy due to its very low oxidation rate.
Co-10Cr presents the worst oxidation resistance with a mass gain of about 7.531 mg/cm2after 24 h oxidation, while Co-10Cr-10Si presents the best oxidation resistance with a much lower mass gain of about 0.078 mg/cm2, which is about two magnitudes lower than that of Co-10Cr. In addition, it can be observed that the kinetics curves of all the three alloys approximately followed the parabolic rate law (n=2). This indicates that the process was controlled by diffusion of reactants through the scale. For comparison purposes, approximate parabolic rate constants were obtained for each specimen, and the value was 7.05×10-10g2·cm-4·s-1, 4.67×10-11g2·cm-4·s-1, and 7.42×10-14g2·cm-4·s-2up to 24 h for Co-10Cr-0Si, Co-10Cr-5Si, and Co-10Cr-10Si, respectively. Presumably, the oxidation resistance of Co-10Cr-10Si is far better than that of Co-10Cr due to the addition of 10% Si, which helps to form a continuous protective layer on the surface.

Fig.1 Oxidation kinetics and corresponding parabolic plots of Co-10Cr-xSi(x=0,5,10) alloys after oxidized for 24 h at 1 073 K
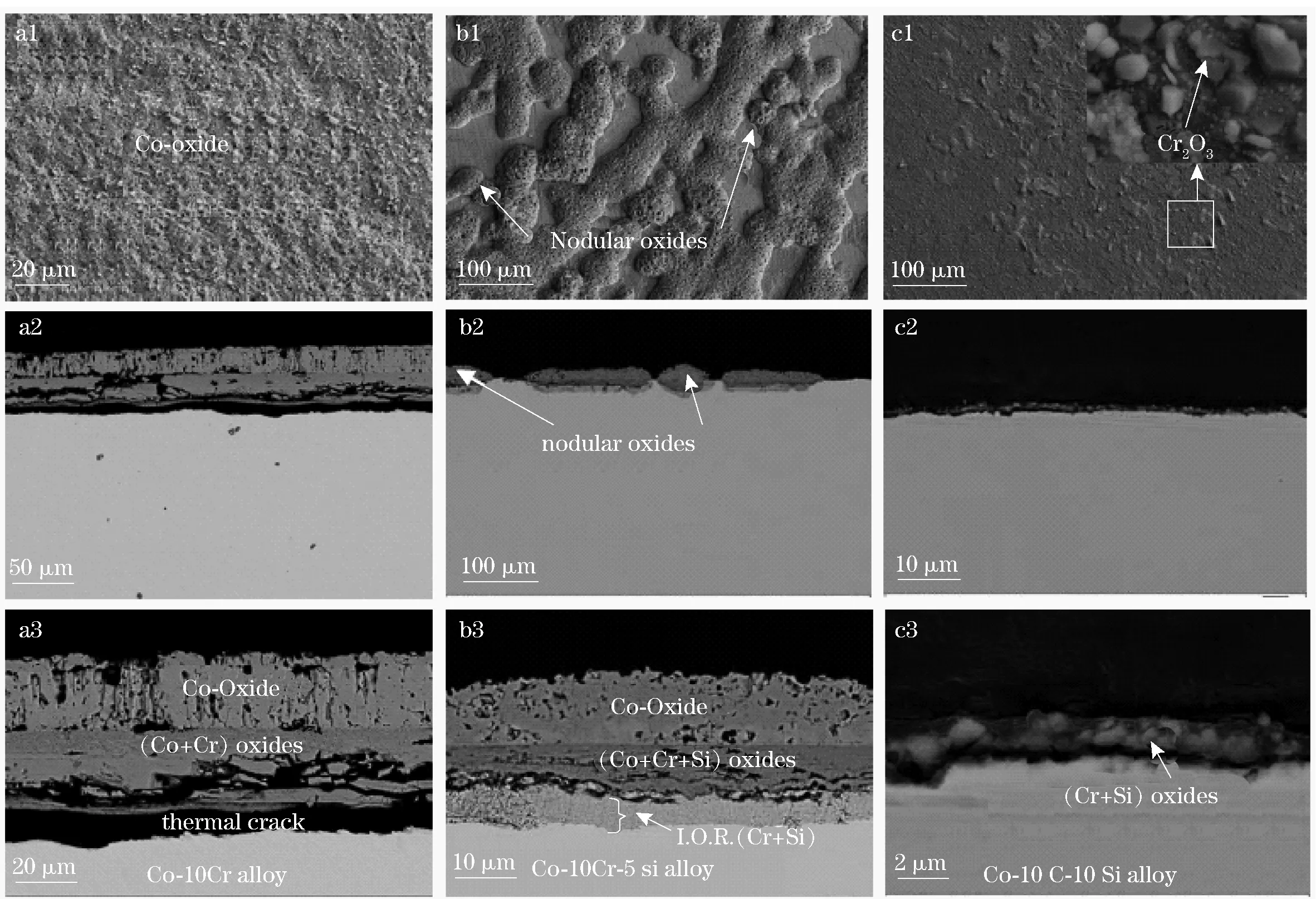
(a1,b1,c1) —surface morphology; (a2,b2,c2) —general views of cross-section; (a3,b3,c3) —expanded views of cross-section; (a1-a3)—Co-10Cr-0Si; (b1-b3)—Co-10Cr-5Si; (c1-c3)—Co-10Cr-10SiFig.2 SEM images of the scales formed on Co-10Cr-xSi (x=0,5,10) oxidized for 24 h at 1 073 K
2.2 Scale morphology and composition
Morphologies of the scales formed on the three alloys at 1 073 K for up to 24 h are shown in Fig.2. The irregular rough surface of Co-10Cr-0Si is formed due to the coalescence of the initial nodular oxides. The thick scales of Co-10Cr-0Si are composed of an outer layer of columnar Co-oxides (CoO+Co3O4), and an inner layer of mixture of oxides of Co and Cr, coupled with some spinel CoCr2O4. There is a wide thermal crack between the scale and the alloy. Furthermore, the internal oxidation region is not found (Fig.3a). However, the addition of 5 at% Si changes the microstructure of the scale a lot. Not all the initial nodular oxides had combined to form continuous thick scales for up to 24 h like Co-10Cr-0Si. Therefore, a fraction of the scales of Co-10Cr-5Si is very thin, while most parts of the scales is very thick with a similar microstructure to Co-10Cr-0Si, to some extent (Fig.3b). The microstructure of the thin part of the scales is like that of Co-10Cr-10Si, which is described below. Compared to the scales of Co-10Cr-0Si, the differences in the thick parts of the scales of Co-10Cr-5Si have four aspects: firstly, the thickness of the scales is much smaller; secondly, the outer layer of Co-oxides is not columnar; thirdly, the inner layer of the mixture of oxides contains SiO2; finally, there is an internal oxidation region of Cr and Si beneath the scale, which is also described elsewhere in our previous published papers[12-13]. Unlike the former two alloys, the scales of Co-10Cr-10Si are very thin with thickness of about only 1.6 μm (Fig.3c). In addition, the scales are mainly composed of fine-grained Cr2O3, coupled with a little SiO2present in the inner part of the scales. As shown in Fig.3, the scales are essentially free from the cobalt element.

(a)—cross-sectional morphology; (b-e)—element distribution mapsFig.3 Cross-sectional morphology and corresponding element distribution maps of oxides formed on Co-10Cr-10Si for 24 h oxidation at 1 073 K
Numerous investigations show that Si is the most reactive element with oxygen to form oxide scales. Its oxides have much more negative formation free energy than that of Co and Cr. Gorr et al. reported that due to the synergistic effect of Si and Cr, the oxidation resistance of the alloys Co-17Re-23Cr-xSi (x=1, 2, 3) is significantly improved[14]. The present study deals with the question of whether the Cr2O3or SiO2scale, which was formed during initial oxidation, can reliably prevent further oxidation of alloys. Thus, in order to understand the behavior of Cr2O3and SiO2scale, the schematic model of scale growth mechanism on three alloys at 1 073 K is presented in Fig. 4.
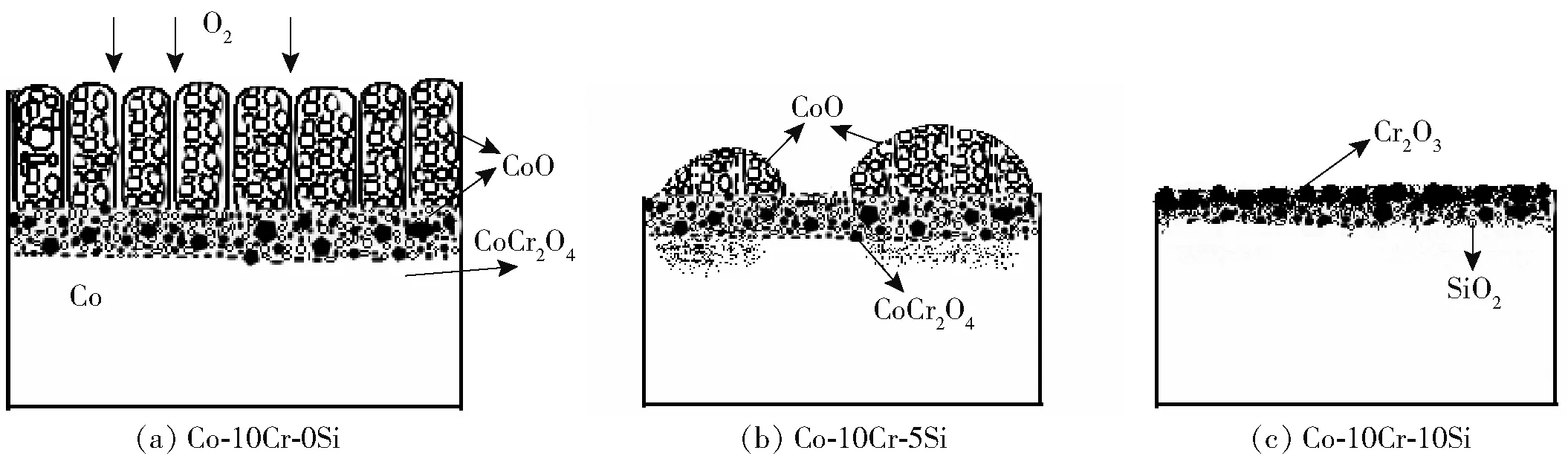
Fig.4 Schematic models of scale growth mechanism on Co-10Cr-xSi (x=0,5,10) alloys at 1 073 K
For the three alloys mentioned above, at the initial stage ofthe oxidation process, oxides of Cr and Si are formed. The oxide scales may be dense and continuous, but after more extended reaction time, such scales usually develop microcracks or microchannels or may be ruptured and spalled[15]. As a consequence, cobalt cations, which diffuse outwards through the Cr2O3oxide, eventually form CoO. Finally, cobalt chromite results from the reaction as
Cr2O3+CoO=CoCr2O4
The Cr2O3scale converts eventually into the spinel CoCr2O4. Numerous investigations on Co-based alloys suggested that the spinel CoCr2O4, as well as CoO, did not provide protective properties at high temperatures. Consequently, oxygen anions and/or molecular O2penetrate through porous CoO and spinel, as well as through the quasi-continuous Cr2O3scale on the metal substrate, and react with chromium forming Cr2O3. Finally, as shown in Fig.4a, oxidation of Co-10Cr produces the outer large columnar Co-oxides and the inner mixture of oxides of Co and Cr coupled with some spinel CoCr2O4. 10 at.% Cr is not sufficient to form a protective Cr2O3layer on the surface of the Co-10Cr alloy. As shown in Fig.4c, once a continuous Cr2O3layer is formed, the oxygen partial pressure governed by the Cr/Cr2O3equilibrium is much lower than that of the Co/CoO equilibrium; therefore, cobalt cannot be oxidized at the oxide/alloy interface. Obviously, the addition of 10 at% Si is able to reduce the critical Cr content needed to form a compact and continuous Cr2O3scale with respect to the binary Co-10Cr alloy. As a result, the oxidation of Co-10Cr-10Si is much more slowly than that of Co-10Cr. The addition of 5 at% Si, however, is not sufficient to help Co-10Cr alloy form a protective Cr2O3scale, although the oxidation resistance has been enhanced to some extent due to the formation of Cr2O3scale on a fraction of the surface of the alloy. The oxidation behavior of Co-10Cr-5Si is intermediate between that of Co-10Cr and Co-10Cr-10Si. As shown in Fig.4b, the nodular oxides have occupied most of the alloy surface and their microstructure is similar to Co-10Cr, to some extent. On the contrary, only a fraction of the surface is covered by the Cr2O3layer, whose microstructure is similar to that of Co-10Cr-10Si.
3 Conclusion
The kinetics curves of all the three alloys approximately followed the parabolic rate law (n=2), and the parabolic rate constant was 7.05×10-10g2·cm-4·s-1, 4.67×10-11g2·cm-4·s-1, and 7.42×10-14g2·cm-4·s-2for up to 24 h for Co-10Cr-0Si, Co-10Cr-5Si and Co-10Cr-10Si alloys, respectively. The addition of 10 at% Si is able to reduce the critical Cr content needed to form a compact and continuous Cr2O3scale with respect to the binary Co-10Cr alloy. As a result, the oxidation of Co-10Cr-10Si is much more slowly than that of Co-10Cr. The addition of 5 at% Si, however, is not sufficient to help Co-10Cr alloy form a protective Cr2O3scale, although the oxidation resistance has been enhanced to some extent due to the formation of Cr2O3scale on a fraction of the surface of the alloy. The oxidation behavior of Co-10Cr-5Si is intermediate between that of Co-10Cr and Co-10Cr-10Si.
 Journal of Beijing Institute of Technology2018年3期
Journal of Beijing Institute of Technology2018年3期
- Journal of Beijing Institute of Technology的其它文章
- Simplified Method for Joint Calibration of 3D Ladar and Monocular Camera
- Force Control of Electro-Hydraulic Servo System Based on Load Velocity Compensation
- Name Relevance and Contact Opportunity-Based Routing Strategy for Mobile Content Sharing
- Identification of Driving Intention Based on EEG Signals
- High-Speed Noise-Based Random Bit Generator by Removing 1/f Noise with Differential Comparison
- Radiometric Calibration Chain Design Based on Uniform Collimated Laser Source
