Synthesis of High Yield Au21(SR)15 Nanoclusters
REN Xiuqing , LIN Xinzhang , FU Xuemei , LIU Chao , YAN Jinghui , HUANG Jiahui
1 Gold Catalysis Research Center, State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, Liaoning Province, P. R. China.
2 School of Chemistry & Environmental Engineering, Changchun University of Science and Technology, Changchun 130022, P. R.China.
3 University of Chinese Academy of Sciences, Beijing 100049, P. R. China.
Abstract: In recent years, Au nanoclusters have attracted much attention as new nanomaterials, which contain several to two hundred Au atoms and are protected by ligands. The structures and properties of Au nanoclusters are usually sensitive to the particle size due to quantum confinement effect. Au nanoclusters have been applied to different fields, such as optical properties,catalysis, and biology. There are two common methods for the synthesis of atomically precise Au nanoclusters: “size focusing” and “ligand exchange”.Although a series of Au nanocluster have been obtained via “size focusing” and“ligand exchange”, obtaining high yield of such Au nanoclusters is a challenge.Au21(S-Adam)15 was previously synthesized via etching Au18 nanoclusters with excess thiols, and its crystal structure was determined by X-ray diffraction crystallography; however, the yield of Au nanoclusters was low. In this study, we prepared Au21(S-Adam)15 in high yield via conversion of Au23(S-Adam)16 to Au21(S-Adam)15. Firstly, Au23(S-Adam)16 nanoclusters were synthesized using adamantanethiols(HS-Adam) as the protecting ligand and HAuCl4 as the gold resource in ethyl acetate solvent. Au23(S-Adam)16 were further etched with excess thiols at room temperature. After reacting for 30 min, highly pure Au21(S-Adam)15, with high yield of ~20% based on HAuCl4 precursor, were successfully prepared. Au23(S-Adam)16 and Au21(S-Adam)15 were characterized by electrospray ionization (ESI), UV-Vis absorption spectroscopy, matrix-assisted laser desorption ionization (MALDI) mass spectrometry, and thermogravimetric analysis (TGA). ESI-MS and UV-Vis spectra confirm the high purity of the Au23(S-Adam)16. After conversion, UV-Vis spectra show the absorption peaks of Au21(S-Adam)15 at 700, 540, 435 and 380 nm. The MALDI-MS of Au21(S-Adam)15 shows several peaks at 6502, 6471, 6106, 5411, and 5048, assigned to Au21(S-Adam)14S, Au21(S-Adam)14, Au20(S-Adam)13, Au19(S-Adam)10, and Au18(S-Adam)9, respectively. The fragments of Au nanoclusters were produced by the strong laser intensity, which easily removes carbon tails from HS-Adam.Thermogravimetric analysis (TGA) was also performed to check the purity of Au21(S-Adam)15 nanoclusters. The TGA curve shows a weight loss of 42% (expected value, 38%). UV-Vis absorption spectroscopy was performed to track the conversion of Au23(S-Adam)16 to Au21(S-Adam)15. It was found that Au23(S-Adam)16 can convert to Au21(S-Adam)15 with a conversion efficiency of up to 97%, using excess thiols at room temperature within 30 min. In general, we successfully synthesized highly pure Au21(S-Adam)15 nanoclusters, with high yield of ~20% based on HAuCl4, by etching Au23(S-Adam)16 with excess thiols at room temperature.
Key Words: Gold nanocluster; Au21(S-Adam)15; High-yield
1 Introduction
Gold nanoclusters with size smaller than 2 nm as new nanomaterials have received wide interest in nanoscience and nanotechnology1–5. Gold nanoclusters are usually composed of exact Au atom number ranging from several to a few hundred atoms and exact protecting ligands. Gold nanoclusters are very different from plasma gold nanoparticles (NPs), usually exhibit very remarkable quantum confinement effect6and are very sensitive to their sizes2. In past decade, a number of gold nanoclusters with precise atom number have been achieved,and the crystal structures have already been determined as well,such as Au257,8, Au389,10, Au6011and Au13012. Due to the quantum effect, gold nanoclusters possess unique chemical and physical properties13,14, which endow gold nanoclusters with the great application potential in many fields, such as optic7,15,biology16, catalysis17–24and magnetism25,26.
There are two main methods to synthesize gold nanoclusters:“size focusing”27and “ligand exchange”28–30. In term of “size focusing” process, the important point is to obtain proper size distribution of polydispersed gold nanoclusters. During this process, the polydispersed gold nanoclusters were further etched under harsh environment (high temperature or excess thiols). Finally, the unstable gold nanoclusters convert to stable ones. On the other hand, “ligand exchange” means the protecting ligands on gold nanoclusters were replaced with other ligands. New methodology has been developed to control the size and structure of nanoclusters, which is usually hard to achieve via simple one-step method. Liu et al. prepared high-yield Au99(SPh)42via ligand exchange of polydispersed gold nanoclusters31. This method also provides idea to synthesize new size gold nanoclusters via conversion from one stable gold nanoclusters to another stable one. Murry et al.synthesized Au75(SR)40via replacing PPh3with thiols32. In recent, Zeng et al. obtained a series of gold nanoclusters via“ligand exchange-induced transformation”33.
In previous work, Au21(S-Adam)15(HS-Adam:1-adamantane-thiolate) was synthesized by “ligand exchangeinduced transformation”, that is, Au18(SR)15reacted with excess HS-Adam at high temperature34. In this work, we report the synthesis of highly pure Au21(S-Adam)15with high yield via etching Au23(S-Adam)16with excess thiols at room temperature. The high yield of Au21(S-Adam)15was greatly improved and reached up to 20% (based on HAuCl4precursor).
2 Experimental
2.1 Chemicals
All the chemicals were used without further purification.Tetrachloroauric(III) acid (HAuCl4∙4H2O, 99.99%, Acros),tetrabutylammonium bromide (TOAB, 99%, TIC),adamantanethiols (HS-Adam, 95%, Sinopharm), sodium borohydride (NaBH4, 99.99%, Sinopharm), methanol (99%,Sinopharm), ethyl acetate (99%, Sinopharm), acetone (99%,Sinopharm), dichloromethane (CH2Cl2,99%, Sinopharm).Nanopure water (resistivity 18.2 MΩ∙cm) was purified with a Barnstead NANOpure DiwaterTM system. All glassware was thoroughly cleaned with aqua regia (V(HCl) : V(HNO3) = 3 :1), rinsed with copious nanopure water, and then dried in an oven prior to use.
2.2 Synthesis of Au23(S-Adam)16 nanoclusters
61.8 mg HAuCl4∙4H2O (0.15 mmol) was dissolved in 15 mL ethyl acetate. Then, 95.2 mg TOAB was added into ethyl acetate and stirred for 30 min. 126 mg HS-Adam was further added to react with Au(III) to Au(I)-SR. At last, 57 mg NaBH4dissolved in 4 mL cold water (4 °C) was used to reduce the Au(I)-SR to Au(0). The reaction lasts 7 days. After reaction, the product was washed with methanol for four times. Then, the crude product was extracted with acetone for twice.
2.3 Transformation of Au23(S-Adam)16 to Au21(S-Adam)15
5.0 mg Au23(S-Adam)16was dissolved in 10 mL CH2Cl2.Then, 50 mg HS-Adam was added to CH2Cl2. After reaction at room temperature for 60 min, the color of solution turned from purple into brown, which meant Au23(S-Adam)16converted to Au21(S-Adam)15.
2.4 Characterization
UV-Vis spectra of Au nanoclusters (dissolved in CH2Cl2)were performed on a UV-8000s spectrophotometer at room temperature. Matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) was carried out on a PerSeptive Biosystems Voyager DE super-STR time-of-light (TOF) mass spectrometer, and trans-2-[3-(4-tert-butylphenyl)-2-methyl-2-propenylidene]malononitrile (DCTB) was used as the matrix35.Typically, 0.1 mg matrix and 10 mL analytic stock solution were mixed in 10 mL CH2Cl2. Electro spray ionization mass spectrometry (ESI-MS) were recorded on a Bruker microTOFQ system in positive ion mode. The product was dissolved in mixture of methanol and CH2Cl2(Volume ratio of 5 : 7) via adding cesium acetate into sample. Thermo- gravimetric analysis was carried out on a TG/DTA 6300 analyzer (Seiko Instruments Inc.).
3 Result and discussion
Au21(S-Adam)15nanoclusters were synthesized via etching Au23(S-Adam)16with excess thiols at room temperature. In Fig.1a, the UV-Vis absorption spectrum of Au23(S-Adam)16shows one strong optical peak at ~580 nm and a weak shoulder peak at ~450 nm, consistent with previous report36In order to further confirm the exact molecular mass of this nanocluster,ESI-MS was used to characterize its mass via adding cesium acetate (CsOAc) into it. ESI-MS spectrum in Fig. 1b shows four m/z peaks 7206.4, 6778.1, 6645.4 and 3323.1, which are attributed to Au23(S-Adam)16, Au21(S-Adam)15Cs, Au21(SAdam)15and [Au21(S-Adam)15H]2+. Basis on UV-Vis absorption and ESI-MS, it can be confirmed that Au21(SAdam)15is a fragment of Au23(S-Adam)16by losing Au2SR.
In previous report, Au21(S-Adam)15was prepared via etching Au18in excess HS-Adam at high temperature34. To realize the conversion of Au23(S-Adam)16to Au21(S-Adam)15, excess HS-Adam was used to react with Au23(S-Adam)16at the room temperature. In this process, it was found that Au23(S-Adam)16was not stable under harsh condition (excess thiols). As shown in Fig. 2, after reaction for 15 min, the peak at 580 nm decreased and one weak peak at 700 nm appeared. After reaction for 25 min, the peak at 580 nm obviously weakened and the peak at 700 nm gradually strengthened, meanwhile,new peaks at 540, 435 and 380 nm appeared, indicating Au23(S-Adam)16almost turned into Au21(S-Adam)15.Furthermore, after reaction for 30 min, the purple solution of gold nanoclusters totally changed to brown, and UV-Vis spectrum shows multiple absorption bands at 700, 540, 435 and 380 nm, respectively, indicating the conversion process of Au23(S-Adam)16to Au21(S-Adam)15completed. The conversion efficiency of Au23(S-Adam)16to Au21(S-Adam)15reached up to 97% (Au atom basis), and the yield of Au21(SR)15reached up to~20% (based on HAuCl4precursor). However, it has been reported that the conversion efficiency of Au18to Au21is 20%(Au atom basis)34.
To obtain highly pure Au21(S-Adam)15, the crude product was purified by washing with methanol and extracted with CH2Cl2. The purified Au21(S-Adam)15shows multiple absorption bands in UV-Vis spectrum at 700, 540, 435 and 380 nm, respectively (Fig. 3a). The MALDI-MS of purified Au21(S-Adam)15in Fig. 3b shows several peaks at 6502, 6471,6106, 5411 and 5048, which are assigned to Au21(S-Adam)14S,Au21(S-Adam)14, Au20(S-Adam)13, Au19(S-Adam)10and Au18(S-Adam)9, respectively. However, it is hard to obtain the complete molecular mass of Au21(S-Adam)15via MALDI-MS spectrometry, because thiol of HS-Adam is easy to loss carbon tail under strong laser environment.
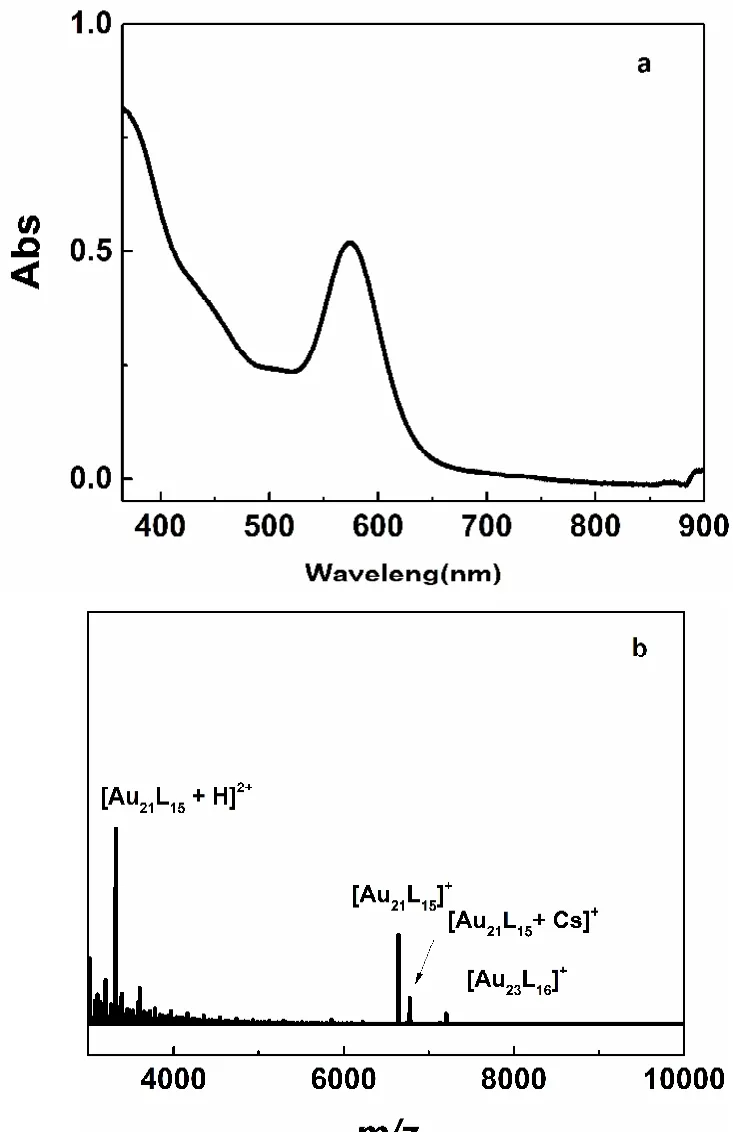
Fig. 1 UV-Vis absorption (a) and ESI-MS spectra (b) of[Au23(S-Adam)16]+ nanoclusters.
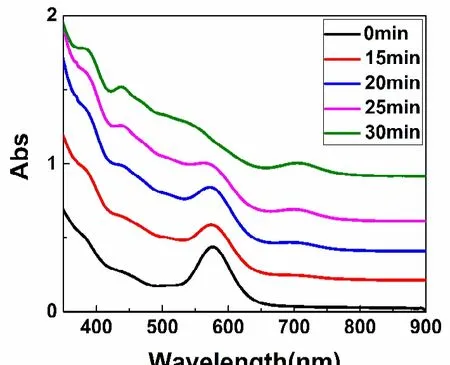
Fig. 2 Time-dependment UV-Vis spectra of the conversion process of Au23(S-Adam)16 to Au21(S-Adam)15.
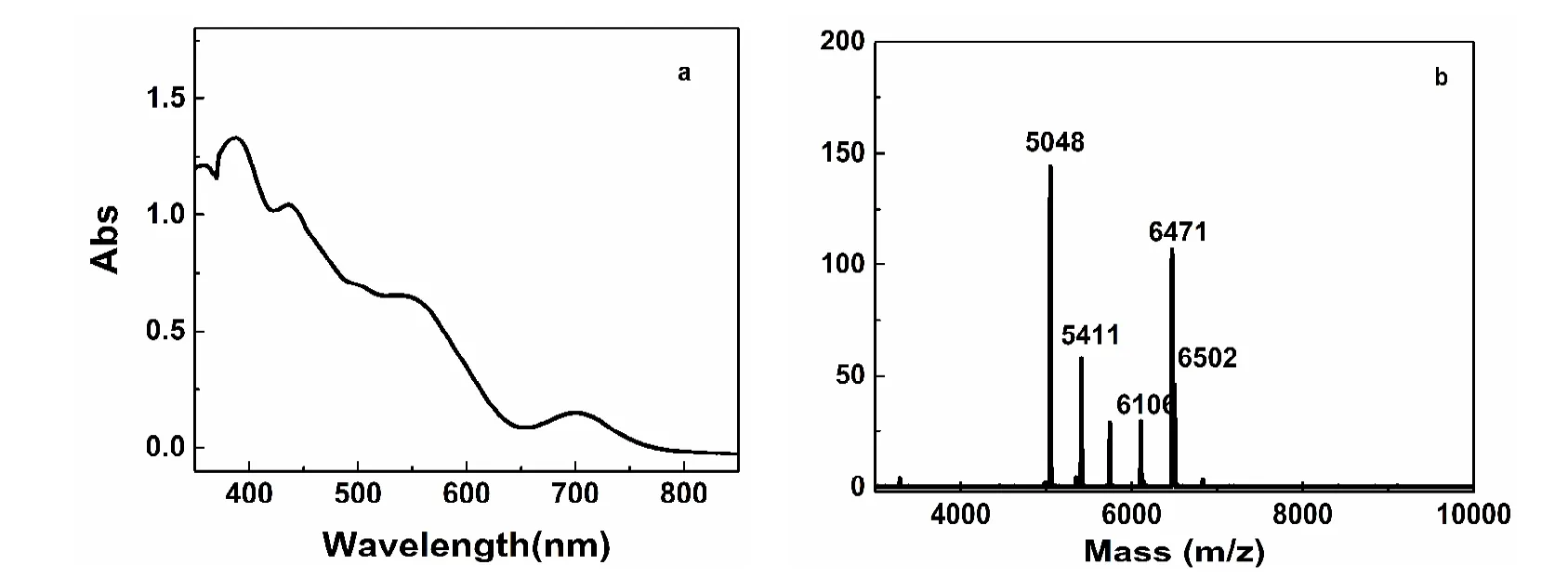
Fig. 3 UV-Vis absorption (a) and MALDI-MS spectra (b) of purified [Au21(S-Adam)15] nanoclusters.
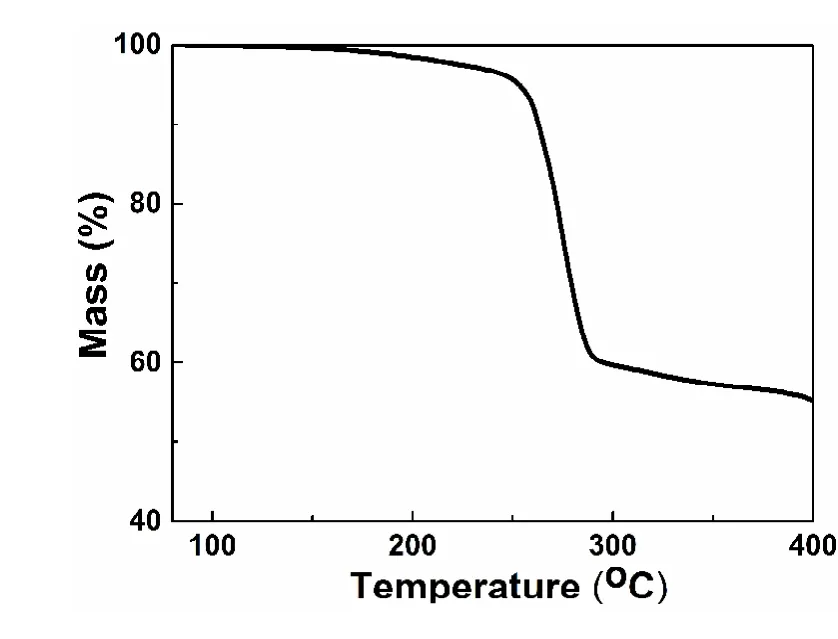
Fig. 4 Thermogravimetric analysis of purified Au21(S-Adam)15 nanoclusters.
Furthermore, we performed thermogravimetric analysis(TGA) to check the purity of Au21(S-Adam)15nanoclusters.TGA curve in Fig. 4 shows a weight loss of 42%,corresponding to the loss of the surface thiolate ligands,consistent with the expected value (38%) according to the formula of Au21(S-Adam)15and the previous work34.
In summary, we successfully synthesized highly pure Au21(S-Adam)15nanoclusters with high yield of ~20% based on HAuCl4via converting Au23(S-Adam)16with excess thiols at room temperature. The optical absorption and molecular mass of Au21(S-Adam)15were checked with UV-Vis spectroscopy and matrix-assisted laser desorption ionization mass spectroscopy. Thermogravimetric analysis were further carried out to confirm the purity of Au21(S-Adam)15.
Acknowledgment: Thanks Prof. Jianping Xie and Dr.Qiaofeng Yao for ESI-MS characterization.
- 物理化学学报的其它文章
- Ag7(MBISA)6 Nanoclusters Conjugated with Quinacrine for FRETEnhanced Photodynamic Activity under Visible Light Irradiation
- Thiolate-Protected Hollow Gold Nanospheres
- Controlled Synthesis of Au36(SR)24 (SR = SPh, SC6H4CH3, SCH(CH3)Ph,and SC10H7) Nanoclusters
- PPh3: Converts Thiolated Gold Nanoparticles to [Au25(PPh3)10(SR)5Cl2]2+
- Synthesis and Structure Determination of Ag-Ni Alloy Nanocluster Ag4Ni2(SPhMe2)8 (SPhMe2 = 2,4-dimethylbenzenethiol)
- Luminescence Emission of Copper Nanoclusters by Ethanol-induced Aggregation and Aluminum Ion-induced Aggregation

