Synthesis and Structure Determination of Ag-Ni Alloy Nanocluster Ag4Ni2(SPhMe2)8 (SPhMe2 = 2,4-dimethylbenzenethiol)
SUN Guodong, KANG Xi, JIN Shan, LI Xiaowu, HU Daqiao , WANG Shuxin, ZHU Manzhou
Department of Chemistry, Center for Atomic Engineering of Advanced Materials, AnHui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials, Anhui University, Hefei 230601, P. R. China.
Abstract: Atomically precise pieces of metallic matter with nanometer dimensions, which are called nanoclusters, have attracted special research interest as a frontier in nanoscience research. These nanoclusters exhibit unique properties that make them suitable for widespread applications in fields like medical treatments and catalysis. Studies in nanoclusters have been greatly benefited from the use of advanced instrumentation, especially adaptation of mass spectrometry (e.g., matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) and electrospray ionization mass spectrometry (ESI MS)). However, mass spectrometry could not elucidate the bonding between metals and ligands; therefore, single-crystal X-ray diffraction(SC-XRD) analysis has been used. SC-XRD is significant for the development of the nanocluster range in terms of revealing the precise structure of nanoclusters and fully understanding the structure-property relationship. Furthermore,understanding the nature of nanocluster surface has provided possibility to embellish nanocluster surface and to improve their performance. Nowadays, alloy nanoclusters play an important role in catalysis, biology, and materials science.Researchers have synthesized and predicted the alloy structure composed of silver and nickel in ultra-small size(Ag4Ni2(DMSA)4, (DMSA = meso-2,3-dimercaptosuccinic acid)). However, no precise crystal structure has been reported.Herein, we report the crystal structure of the Ag-Ni alloy nanocluster Ag4Ni2(SPhMe2)8. The structure was further confirmed by SC-XRD, X-ray photoelectron spectroscopy (XPS), MALDI-TOF MS, ESI MS and thermo gravimetric analysis (TGA) measurements. The stability experiment suggested that the Ag4Ni2 nanocluster could be stable in ultra-small sizes. This research on Ag-Ni alloy nanoclusters will contribute to the understanding of the alloy in ultra-small sizes. Specifically, based on the structure determination by SC-XRD, the structure of Ag4Ni2(SPhMe2)8 could be divided into three layers: upper and lower layers with Ni(SPhMe2)4 complexes constituting a parallelogram, and the middle layer with four silver atoms constituting a parallelogram like a sandwich. The Ag―Ni, Ag―S and Ni―S bond distances were 0.31–0.32, 0.23–0.24, and 0.22–0.23 nm, respectively. XPS analyses revealed that the Ag/Ni/S atomic ratio was 5.19/2.55/10.28, consistent with the corresponding expected ratio of 4/2/8 in Ag4Ni2(SPhMe2)8. In addition, the Ag 3d3/2 and Ag 3d5/2 binding energy peaks were located at 375.0 and 369.0 eV, respectively, and the Ni 2p1/2 and Ni 2p3/2 are located at 871.50 and 853.90 eV, respectively. Moreover, combined with ESI, the Ag 3d3/2 and Ag 3d5/2 binding energies of Ag4Ni2(SPhMe2)8 were close to the +1 valences, according to previous reports. Meanwhile, the spectra of Ag4Ni2(SPhMe2)8 illustrated that the valence of nickel was +2. Additionally, the MALDI-TOF mass spectrum was in good agreement with the ESI results. Weight loss upon heating was used to confirm the percentage of organic material in nanoclusters (66.31% weight loss was observed in TGA, consistent with the 66.67% loss calculated according to the formula). In the liquid state, the UV-Vis spectra showed no change after exposure to oxygen for a few weeks. Meanwhile,we used UV-Vis spectroscopy at temperatures under 80 °C to test the stability of the Ag4Ni2(SPhMe2)8. The absorption peaks were almost identical with each other, suggesting high stability of the Ag4Ni2(SPhMe2)8. Our study proves that small-sized alloy also has the possibility of diversification, which will play an important role in the synthesis of alloy nanoclusters. Moreover, this research on Ag-Ni alloy nanoclusters will contribute to the understanding of alloys in ultra-small sizes.
Key Words: Nanocluster; Alloy; Crystallography; Surface chemistry; Structure
1 Introduction
Atomically precise alloy nanoclusters (NCs) with the size in the range of 1–3 nm have emerged as a new category of nanomaterials1–5. These NCs have attracted interests across a diverse range of field owing to their synergistic properties such as catalytic as well as optical and electrical properties6–11.During the past decades, series of alloy nanoclusters, including Au-Ag2, Au-Pd12,13, Au-Pt14, Au-Cu15, Ag-Pd16, Ag-Pt17,Ag-Cu18etc., have been synthesized and their structures have been determined. For example, the catalytic activity could be largely enhanced for single Pd or Pt atom doped M1Au24NC(where M = Pd/Pt) compared with its homological Au25nanoclusters13–18. Doping 13 silver atoms into Au25nanocluster leads to the boost of fluorescence quantum yield (400 times)compared with homo-gold Au25rod10. However, as the same group of Pd/Pt, the report on Ni doped silver/gold nanoclusters are rarely reported, which further limits the study of the structure and properties of Ag-Ni alloy nanoclusters. Thus,synthesis and accurately characterized structures of Ni doped silver/gold alloy nanoclusters by X-ray diffraction are highly desired.
Previously, Biltek et al.19have reported the synthesis of Ag4Ni2(DMSA)4(DMSA = meso-2,3-dimercaptosuccinicacid),and proposed the structure by density functional theory (DFT)calculation, which posed the possibility that alloys different from the bulk could be existed in the ultra-small sizes.Although theoretical work has been performed on the structural determination of these alloy nanoclusters, the experimental work is still stagnated and the crystal structure of this corresponding alloy NCs remains a challenge3–10.Consequently, the X-ray crystal structure of bi-metallic NCs(alloying noble metal NCs with Ni) is highly desired because the determination of the corresponding structure can not only fill the blank of alloy NCs structure consisting of the Ag and Ni, but also shed lights on understanding the mechanism of alloys-forming19–23.
Herein, we reported the crystal structure of Ag-Ni alloy nanocluster Ag4Ni2(SPhMe2)8. The structure was further confirmed by single crystal X-ray diffraction (SC-XRD), X-ray photoelectron spectroscopy (XPS), Matrix assisted laser desorption ionization time of flight mass spectrometry(MALDI-TOF-MS), electro spray ionization mass spectrometry(ESI-MS) and thermo gravimetric analysis (TGA)measurements. The stability experiment suggested that the Ag4Ni2 nanocluster could be stable in ultra-small sizes. This scientific research on Ag-Ni alloy nanoclusters will contribute to the understanding of the alloy in ultra-small size.
2 Experimental
2.1 Chemical reagent
Silver (I) nitrate (AgNO3, 99.99%, metals basis), nickel (II)chloride hexahydrate (NiCl2∙6H2O, 99.99%, metals basis),sodium borohydride (NaBH4, 99.99%), 2,4-dimethylthiophenol(C8H10S, 97%), methanol (CH3OH, HPLC, Aldrich), methylene chloride (CH2Cl2, HPLC grade, Aldrich), pure water was purchased from Wahaha Co. Ltd. All reagents were used as received without further purification. All glassware was thoroughly cleaned with aqua regia (V(HCl)/V(HNO3) = 3/1),rinsed with copious pure water, and then dried in an oven prior.
2.2 Synthesis of Ag4Ni2(SPhMe2)8
Briefly, hexahydrate nickel chloride (35.70 mg, 0.15 mmol)and silver nitrate (25.50 mg, 0.15 mmol) were dissolved in 3 mL methanol. The mixture solution was then added to a 50 mL round-bottomed flask and mixed with 15 mL CH2Cl2. The solution turned light green after stirring for 5 min, then 2,4-dimethylbenzenethiol (82.90 μL, 0.60 mmol) was added under fast stirring. After 5 minutes, 57 mg NaBH4(1.50 mmol)dissolved into 3 mL ice cold water were added to the reaction under vigorous stirring. All the experimental procedures were carried out under the condition of ice-bath. According to previous reports19, the reaction continued for 12 h. After the reaction, a black mixture was obtained and was further washed with methanol solvent for at least three times to remove excess thiol. Yellow block crystals were crystallized overnight by mixture of methanol and CH2Cl2.
2.3 Characterization
All UV-Vis absorption spectra of Ag4Ni2(SPhMe2)8dissolved in CH2Cl2were recorded on an Agilent 8453 diode array spectrometer (USA), whose background correction was made using a CH2Cl2blank. Solid samples were first dissolved in CH2Cl2to make a dilute solution, with a subsequent transformation to a 1 cm path length quartz cuvette, followed by spectral measurements. Thermogravimetric analysis (TGA)was carried out on a thermogravimetric analyzer (DTG-60H,Shimadzu Instruments Inc., Japan) with 5 mg of the Ag4Ni2(SPhMe2)8nanocluster in a SiO2pan at a heating rate of 10 K∙min-1from room temperature (~298 K) to 1073 K. X-ray photoelectron spectroscopy (XPS) measurements were performed on a Thermo ESCALAB 250 (USA), configured with a monochromated Al Kα(1486.80 eV) 150 W X-ray source, 0.5 mm circular spot size, a flood gun to counter charging effects, and the analysis chamber base pressure lower than 1 × 10-7Pa, data were collected with Fixed analyzer transmission (FAT) = 20 eV. The data collection for single crystal X-ray diffraction was carried out on a Bruker Smart APEX II CCD diffractometer (Germany) at 296 K, using graphite-monochromatized Mo Kαradiation (λ = 0.071069 nm).Data reductions and absorption corrections were performed using the SAINT and SADABS programs, respectively. The structure was solved by direct methods and refined with full-matrix least squares on F2using the SHELXTL software package. All non-hydrogen atoms were refined anisotropic ally,and all of the hydrogen atoms were set in geometrically calculated positions and refined isotopically using a riding model. All electrospray ionization (ESI) test were recorded on an Thermo Scientific LTQ Orbitrap XL (USA). The sample was directly infused into the chamber at 5 μL∙min-1. To prepare the ESI sample, clusters were dissolved in toluene (1 mg∙mL-1) and then diluted (V(toluene)/V(methanol) = 1/2) by dry methanol containing 5 mmol·L-1CsOAc to ionize the clusters by forming Cs+-cluster adducts. MALDI-TOF MS was recorded on a Bruker Autoflex III smart beam instrument and trans-2-[3-(4-tert-butylphenyl)-2-methyl-2-propenylidene]malononitrile (DCTB) was used as the matrix.
3 Results and discussion
3.1 Atomic structure
Fig. 1 shows the crystal structure of the Ag4Ni2(SPhMe2)8.As shown in Fig. 1, four Ag atoms and two Ni atoms form a distorted octahedral structure, and further capped by thiolates existing at the vertex of Ag-Ni kernel. The structure of Ag4Ni2(SPhMe2)8can also be divided into three layers: upper and lower layers with Ni(SPhMe2)4 complexes constitute a parallelogram, middle layer with four silver atoms constitute a parallelogram like a sandwich. Based on Khanna’s simulation,Ag4Ni2(DMSA)8shows this kind of configuration which maintains the lowest overall energy19. From the perspective of spatial configuration, the atoms of NCs are tight to occupy the smallest space which may lead to a high stability11. The Ag―Ni, Ag―S and Ni―S distances are 0.31–0.32, 0.23–0.24 and 0.22–0.23 nm, respectively21, besides, this cluster is close to 0 valence state on the basis of ESI test (Fig. S1 (Supporting Information)). Another interesting phenomenon is that when we overlook from the plane which consists of a nickel and four sulfur atoms to the whole molecule, we can find four 2,4-dimethylbenzene- thiol ligands arrange anticlockwise (Fig.S2 (Supporting Information)).
3.2 Structure Characterization
As shown in Fig. 2a, the UV-Vis absorption spectrum of Ag4Ni2(SPhMe2)8NC shows several absorption peaks located at 390, 450 and 650 nm, respectively. Additionally, the molecular formula is supported by XPS and TGA.Specifically, the ratio of metal to non-metal in Ag4Ni2(SPhMe2)8is confirmed by TGA (Fig. 2b). Weight loss upon heating is usually used to confirm the percentage of organic material in NCs15,24, As shown in Fig. 2b, 66.31%loss is observed, which is consistent with the calculated loss(66.67%) according to the formula. X-Ray photoelectron spectroscopy (XPS) is used to confirm the composition of the Ag4Ni2(SPhMe2)8NC Fig. 2c,d. The XPS analyses reveals that the Ag/Ni atomic ratio is 5.19/2.55 and the Ag/Ni/S atomic ratio is 5.19/2.55/10.28 respectively, consistent well with the expected ratio of 4/2 and 4/2/8 in Ag4Ni2(SPhMe2)8.In addition, as shown in Fig. 2d, the Ag 3d3/2and Ag 3d5/2binding energy peaks are located at 375.0 and 369.0 eV, and the Ni 2p1/2and Ni 2p3/2are located at 871.50 and 853.90 eV,respectively. Moreover, the Ag 3d3/2and Ag 3d5/2binding energies of Ag4Ni2(SPhMe2)8are close to the +1 valences according to previous reports25, meanwhile, the spectra of Ag4Ni2(SPhMe2)8illustrated that the valence of nickel is+226,27.

Fig. 1 The crystal structure of the Ag4Ni2(SPhMe2)8.Color code: S, yellow; Ni, green; Ag, gray; C and H atoms are shown in wireframe model. Color online.

Fig. 2 Characterization of the Ag4Ni2(SPhMe2)8.(a) UV-Vis spectrum of the Ag4Ni2(SPhMe2)8; (b) TGA of the Ag4Ni2(SPhMe2)8; (c) XPS survey spectrum of purified Ag4Ni2(SPhMe2)8;(d) XPS survey spectrum of Ag 3d and Ni 2p.
To confirm the newly attained Ag4Ni2(SPhMe2)8and probe their charge states, we further performed (ESI and MALDI-TOF) MS analysis. ESI-MS spectrum show both positive mode and negative mode ESI spectrum (Fig. S1A,B)showed no signals. After cesium acetate was added to form Cs+adducts, the peak assigned to Ag4Ni2(SPhMe2)8Cs+(cal.m/z = 1779.78, Fig. S1C) appeared and the experimental and simulated isotope patterns match well. Besides, peak of MALDI-TOF-MS spectrum (Fig. S1D) was assigned to Ag4Ni2(SPhMe2)8(cal. m/z = 1646.88). Taken together, MS indicates that Ag4Ni2(SPhMe2)8is neutral since the number of adducted Cs+ions equals the charge number.
3.3 Thermodynamic stability
Excellent thermal stability of nanoclusters may offer a wide range of applications. In our work, Ag4Ni2(SPhMe2)8showed good stability. For example, the UV-Vis spectra of nanocluster in CH2Cl2solution showed no change after contacting with oxygen for a few weeks. In detail, we conducted a thermal stability test. In this test, we exposed the nanocluster solution in the air for about 30 days and the UV-Vis absorption spectra basically had no change (Fig. S3(Supporting Information)). In addition, the UV-Vis spectra were measured under a series of different time under 80 °C to test the stability of the Ag4Ni2(SPhMe2)8. As shown in Fig.3, the absorption peaks were almost identical which suggested the high stability of the Ag4Ni2(SPhMe2)8.
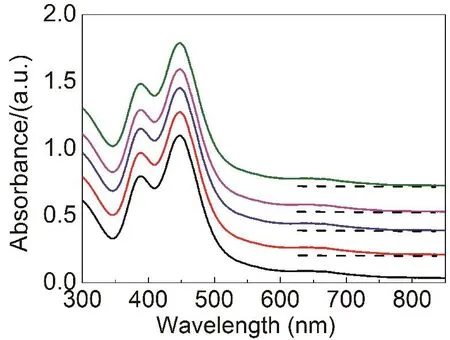
Fig. 3 Time dependent UV-Vis absorption spectra of the Ag4Ni2(SPhMe2)8 under 80 °C.The dotted line represents the reference solvent (CH2Cl2) ultraviolet visible absorption.
4 Conclusions
In summary, we report the synthesis and structure determination of Ag4Ni2(SPhMe2)8. It is an irregular cuboid composed of a plane with four silver atoms, one nickel atoms and four thiol ligands on both sides of the plane of silver atoms. Ag4Ni2(SPhMe2)8was characterized by SC-XRD technique, MS, UV-Vis spectrum, XPS and TGA measurements. Our work prove that the small-sized alloy also has the possibility of diversification, which will play an important role in the synthesis of the alloy NCs.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.
- 物理化学学报的其它文章
- Electronic Stability of Eight-electron Tetrahedral Pd4 Clusters
- Synthesis of High Yield Au21(SR)15 Nanoclusters
- Luminescence Emission of Copper Nanoclusters by Ethanol-induced Aggregation and Aluminum Ion-induced Aggregation
- Construction and NIR Luminescence Properties of Zn-Ln Rectangular Nanoclusters
- Ag7(MBISA)6 Nanoclusters Conjugated with Quinacrine for FRETEnhanced Photodynamic Activity under Visible Light Irradiation
- PPh3: Converts Thiolated Gold Nanoparticles to [Au25(PPh3)10(SR)5Cl2]2+

