Luminescence Emission of Copper Nanoclusters by Ethanol-induced Aggregation and Aluminum Ion-induced Aggregation
GUO Xiaohong, ZHOU Ying, SHI Lihong, ZHANG Yan, ZHANG Caihong, DONG Chuan,ZHANG Guomei , SHUANG Shaomin
School of Chemistry and Chemical Engineering, Institute of Environmental Science, Shanxi University, Taiyuan 030006, P. R. China.
Abstract: Metal nanoclusters (MNCs), as a new type of nano-material,possess excellent properties such as facile synthesis, strong light stability, low toxicity, excellent biocompatibility, and high luminous efficiency.Aggregation-induced emission (AIE), which can enhance the luminescence properties of MNCs, has resulted in MNCs attracting significant attention. In this thesis, L-glutathione (GSH)-protected copper nanoclusters (GS@CuNCs)were prepared by a “one-pot” method in aqueous solution without additional reducing agents. The GS@CuNCs were characterized by UV-Vis absorption spectroscopy and fluorescence spectroscopy. Upon excitation at 370 nm, the fluorescence spectrum of GS@CuNCs displayed the maximum emission peak at 610 nm. The as-prepared CuNCs generate a striking fluorescence intensity via aggregation-induced emission (AIE). The AIE property of GS@CuNCs was examined for the aggregates in different organic solvents, such as ethanol, methanol, and dimethylformamide. Since the aggregation degree was controlled by the content of organic solvent, we further measured the fluorescence intensity of GS@CuNCs in different volume ratios of a water-ethanol mixture solution. The fluorescence intensity of GS@CuNCs exhibited an approximately 30-fold increase at 85% of ethanol content, as compared to that in aqueous solution. A possible mechanism may be that intramolecular motions are restricted in ethanol, resulting in a significant increase of fluorescence intensity. Moreover, only very weak emissions were recorded for the CuNC dispersion in aqueous solution;however, an apparent luminescence enhancement was observed in both luminescence spectra and by naked eyes under UV light, with a gradual increase in the ethanol content in the water-ethanol mixture from 0% to 85%. Additionally, we developed a new selective and sensitive turn-on fluorescent sensor for the detection of trivalent aluminum ions (Al3+)based on cation-induced aggregation methods. Among the 15 types of metal cations studied, only Al3+ visibly increased the fluorescence emission of the GS@CuNCs. These results indicated that the GS@CuNCs were highly selective to Al3+than other metal ions, which may result from the electrostatic and coordination interactions between the trivalent aluminum ions and monovalent carboxylic anions from GSH in the CuNCs. The response of the probe to Al3+ exhibited a good linear range of 2–20 µmol·L-1 and the detection limit was 33 nmol·L-1. Thus, the weak fluorescence intensity of CuNCs was increased markedly by the AIE of Al3+, and could construct an interesting fluorescent platform for sensing aluminum ions. The property of AIE of GS@CuNCs may expand the potential applications of nanocluster materials to biosensors and cell imaging.
Key Words: Fluorescence; Copper nanoclusters; Aggregation-induced emission; Ethanol; Aluminum ion
1 Introduction
The rational design and synthesis of fluorescent chemosensors for the recognition and detection of different metal ions earned great scientific interest due to their importance in environmental, medical, industrial, and agricultural applications1–4. As the third most abundant element in the lithosphere, aluminum has wide spread applications in our daily life, such as automotive, alimentary industries, antacids, automated instrument industries, building materials and so on5,6. Excessive amounts of Al3+inhibits the plant growth7and damages the central nervous system of humans to induce Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis (ALS)8–11. Accordingly, detection of Al3+is crucial to control its impact on the human health and the natural environment. Compared with traditional analytical methods, such as atomic absorption spectrometry12,inductively coupled plasma mass spectroscopy (ICP-MS)13,electrochemiluminscence and electrochemical methods14,15et al., fluorescence sensing approaches have several advantages due to its functional simplicity, excellent sensitivity, cost efficiency, and real-time monitoring16–18. So far, a majority fluorescence chemosensors for the detection of Al3+ions are reported in pure organic or organic-water mixed solutions,which are insufficient water solubility19. In addition, detection of Al3+ions has always been limited due to the lack of spectroscopic characteristics, poor coordination ability and strong hydration ability20. Thus, it is highly desirable to develop a highly selective and sensitive fluorescent probe for the detection of Al3+in aqueous solutions.
Fluorescent metal nanoclusters (NCs) consist of several to tens of metal atoms with properties regulated by their subnanometer dimensions and possess size comparable to the Fermi wavelength of electrons21,22. As one new type of fluorescent material, metal nanoclusters have received much attention for applications in biosensing23,24, catalysis25, and imaging26,27owing to excellent photostability, large Stokes shifts, low toxicity, good water-solubility and their unique size-dependent fluorescence properties28. Prompted by their potential applications, metal NCs have been extensively studied on the synthesis, especially AuNCs and AgNCs29,30. Relative to AuNCs and AgNCs, the synthesis and applications of fluorescent CuNCs have been less performed due to the synthetic difficulty in controlling ultrafine size, the sensibility to oxidation on exposure to air31and their weak photoluminescence intensity. However, metal Cu is the most cost effective and widely used in industries, so the development of biological applications for CuNCs has still attracted sustained research interest. In 2001, aggregation induced emission (AIE), a unique phenomenon that exactly opposite to the aggregation-caused quenching (ACQ) effect, was first presented by Tang’s group32. Instead of emission quenching,AIE-active compounds can emit much enhanced fluorescence in aggregation or solid state, which is because the restriction of the intramolecular rotations prohibits energy dissipation via non-radiative channels33,34. Recently, there have been a few reports concerning of metal nanoclusters via AIE. Xie group35discovered an AIE of Au-thiolate NCs, namely, AuNCs can generate a striking fluorescence enhancement upon solventinduced aggregation. Lu and Zhou group36,37developed cysteine@CuNCs and AuNCs based on the fluorescence enhancement of metal NCs for sensing S2-and Ag+,respectively. However, to the best of our knowledge,Al3+-enhanced fluorescence of metal NCs has not yet been reported, and it is a worthwhile undertaking to explore the photophysical mechanism to induce the aggregation of metal NCs in organic solvent and aqueous solutions.
In this research, the thiolated CuNCs were synthesized facilely using glutathione (GSH) as the reductant and the capping agent, according to the synthetic methods reported by Yang’s group38. The most important finding is an aggregationinduced emission (AIE) of CuNCs. The AIE-active CuNCs emit faint light in aqueous solution, but the compounds emit strong fluorescence in solvent-induced aggregation and cation-induced aggregation (Scheme 1). Besides, we firstly found that Al3+as cation-induced aggregation can dramatically increase the luminescence of AIE-active CuNCs, and the compounds could be used for the detection of Al3+.
2 Exprimental
2.1 Materials and instrumentation
L-Glutathione reduced (GSH, 98%) was obtained from Sigma-Aldrich. Copper sulfate anlydrous (Cu2SO4, > 99%) was provided by Tianjin Chemical Reagent Company (Tianjin,China). Ethanol (C2H5OH, 98%), methanol (CH3OH, ≥ 99.9%),dimethylformamide (DMF, ≥ 99.9%), sodium hydroxide(NaOH, > 96%), aluminum chloride and all other metal salts(analytical reagent grade) were purchased from Beijing Chemical Co. (Beijing, China). All reagents were used without further purification. Ultrapure water (≥ 18.2 MΩ∙cm) from the MilliporeMilli-Q systemwas used in all experiments.
The fluorescence spectra were carried out on F-4500 fluorescence spectrophotometer (Hitachi, Tokyo Japan) with a quartz cell (1 cm × 1 cm). The excitation and emission slits were maintained at 10 nm and 10 nm, respectively. The UV-Vis absorption spectra were recorded on a U-2910 spectrophotometer (Hitachi, Tokyo Japan).
2.2 Synthesisof copper nanocluster (GS@CuNCs)
CuNCs were prepared as follows38. Briefly, 2 mL of 10 mmol∙L-1Cu2SO4aqueous solution was added to 2 mL of 50 mg∙mL-1aqueous solution under vigorous stirring at room temperature, forming white suspension liquid. Then, 200 μL NaOH (1 mol∙L-1) was added dropwise until the turbid liquid turned colorless and the mixture was stirred at 37 °C for 1 h.The color of the solution changed from colorless to light yellow. The as-prepared CuNCs were stored at 4 °C for further use.
2.3 Fluorescence detection of Al3+
The GS@CuNCs solution was diluted 10 times for the fluorescence titration. Different concentrations of Al3+ion were added and mixed thoroughly, and then the fluorescent intensity of the solution was measured. Other cations such as K+, Ca2+,Na+, Mg2+, Fe3+, Zn2+, Cu2+, Hg2+, Ni2+, Pb2+, Co2+, Cd2+, Ag+,Mn2+were tested under the same conditions to evaluate the selectivity of the method.

Scheme 1 Schematic illustration of the synthesis and AIE of CuNCs.
3 Results and discussion
3.1 Aggregation-induced emission of copper nanoclusters
The water-soluble CuNCs were synthesized using a simple one pot procedure while GSH served as both a reducing reagent and a protecting ligand (GS@CuNCs). The as-synthesized CuNCs were characterized successively by fluorescence and absorption spectra. Fig. 1 shows a bright emission at 610 nm(line c) for the GS@CuNCs with an excitation at 370 nm (line b), which indicated the formation of the fluorescent nanoclusters. In its UV-Vis absorption spectrum, no obvious absorption peak could be observed (Fig. 1, line a), indicating the formation of CuNCs instead of large copper nanoparticles due to the characteristic absorption peak at ~500 nm arising from the surface plasmonic resonance of large sized Cu nanoparticles39. The inset photographs of Fig. 1 show that the solution were light yellow under ambient light and exhibited a red luminescence under UV light (365 nm).
CuNCs are prepared via a two-step process40. The first step was the reduction of Cu(II) to Cu(I) by GSH, followed immediately by the coordination of Cu(I) to the thiol group to form an insoluble colloid of Cu(I)-thiolate complexes. The second step, which was initiated by the addition of NaOH, was the dissolution of Cu(I)-thiolate complexes to convert into stable CuNCs41. However, the most important finding is strong luminescence of the complexes upon aggregation-induced emission (AIE).
The aggregation of @CuNCs was induced by two different approaches: solvent-induced aggregation and cation-induced aggregation (Fig. 2). In the first situation, ethanol was used as a poor solvent to destabilize the complexes in water, which the CuNCs are dissolved as isolated species and little restriction is imposed on the intramolecular movements42. In the aggregates,the intramolecular motions are restricted and fluorescence intensity significantly enhanced. As shown in Fig. 2a, there is a striking contrast that the CuNCs upon addition of ethanol (fe=85%) could generate strong luminescence, indicating that the as-synthesized CuNCs exhibited an AIE effect.
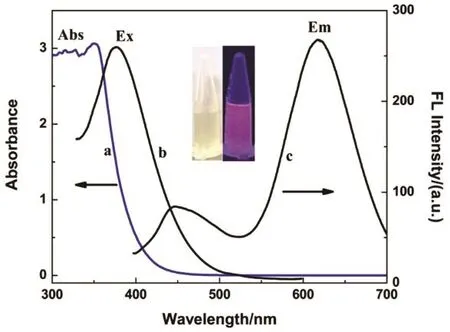
Fig. 1 UV-Vis absorption (a) and fluorescence excitation (b) and emission (c) spectra of the GS@CuNCs.The inset shows photographs of the luminescent CuNCs under visible light (left) and UV light (right)
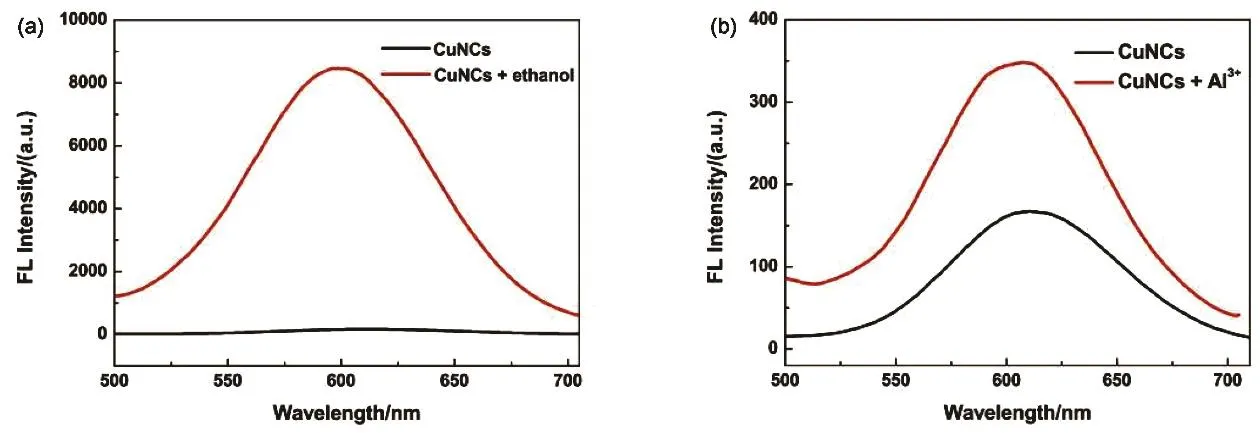
Fig. 2 (a) Fluorescence spectra of the CuNCs (black) and the CuNCs-ethanol (red) (experimental conditions: CuNCs: 0.1 mL, fe = 85% ).(b) Fluorescence spectra of the CuNCs (black) and the CuNCs-Al3+ (red) (experimental conditions: CuNCs: 0.1 mL, [Al3+] = 6 µmol·L-1).
In regard to the cation-induced aggregation method, there is a high affinity between trivalent aluminum ion (Al3+) and the monovalent carboxylic anions from GSH in the CuNCs, by means of electrostatic and coordination interactions43,44.Besides neutralizing the negative charge on the complexes,interaction of Al(Ш)-Al(Ш) also bring the CuNCs closer and facilitated the formation of aurophilic bonds and dense aggregates36. As shown in Fig. 2b, the diluted CuNCs emitted a relatively weak fluorescence; however, the fluorescence intensity of the diluted CuNCs increased markedly in the presence of 6 µmol∙L-1Al3+.
3.2 Ethanol induced luminescence enhancement
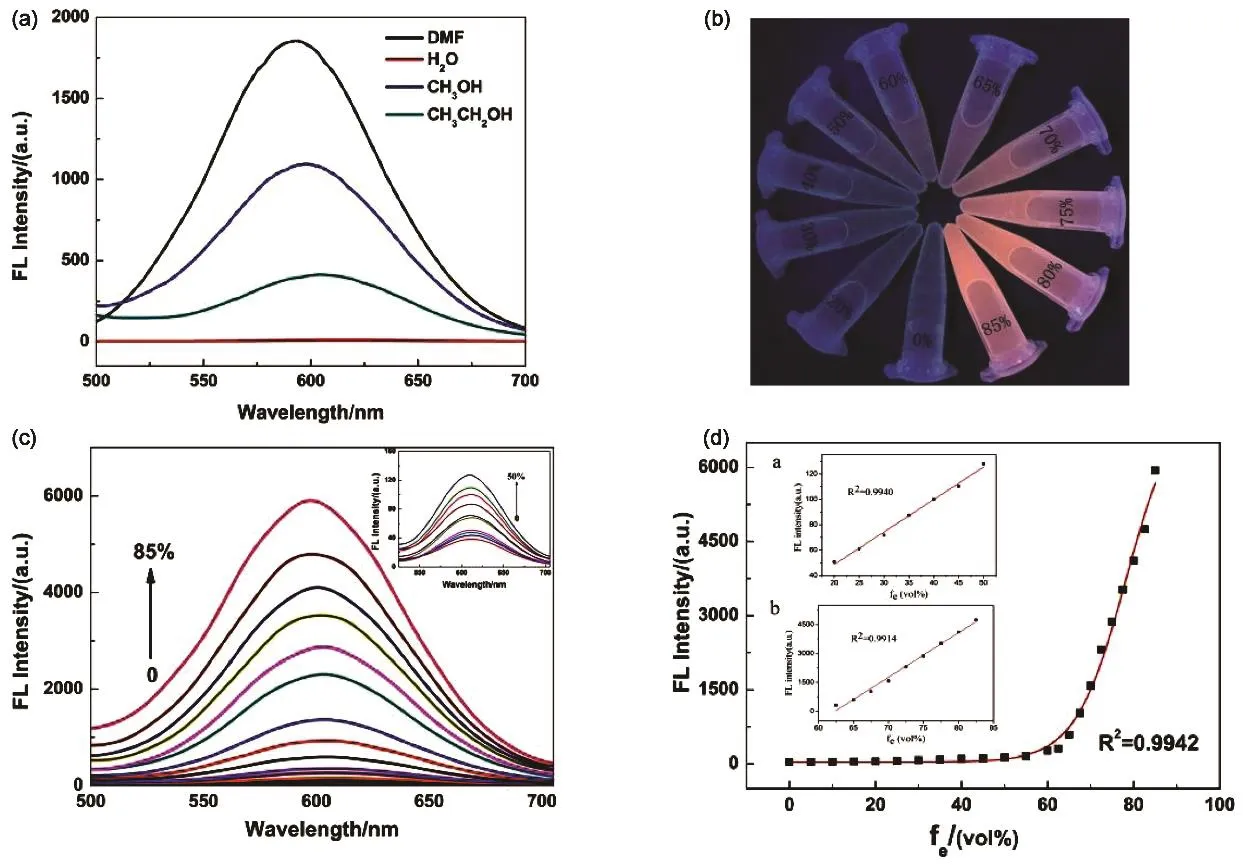
Fig. 3 (a) The AIE effect of the GS@CuNCs in various organic solvents. (b) Digital photos of GS@CuNCs in mixed solvents of ethanol and water with different fe under UV light. (c) Photoemission spectra of GS@CuNCs in mixed solvents with different fe. (Inset) the fe in the range of 0%–50% versus the fluorescence intensity of the GS@CuNCs. (d) The luminescence intensity as a function of ethanol content for water-solubility CuNCs; inset: two linear relationship (a and b) between the fluorescence intensity and different fe.
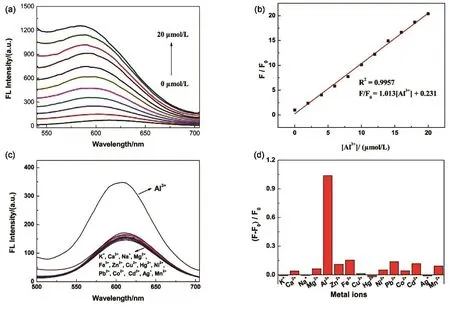
Fig. 4 (a)The fluorescence response of AIE-CuNCs after addition of 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20 µmol·L-1 AlCl3 solution.(b) Plot of the fluorescence change (F/F0) versus the Al3+ concentration. (c) Fluorescence responses of the CuNCs solution to different metal ions. The concentration of Al3+ was 6 μmol·L-1, K+, Ca2+, Na+, Mg2+, Fe3+, Zn2+, Cu2+, Hg2+, Ni2+, Pb2+, Co2+,Cd2+, Ag+, Mn2+ were 1.0 mmol·L-1, (d) Selectivity of the luminescent CuNCs toward Al3+.
The dependence of luminescence properties on the CuNCs was examined for the aggregates from solvent-induced aggregation. Such aggregation-induced emission (AIE)phenomenon has been observed in ethanol, methanol and DMF(Fig. 3a). To get a clear picture about the AIE effect, We chose to use ethanol as the organic solvent in this study due to its low toxicity, low cost, wide application and manage to tune the aggregation states of CuNCs in a mixture of water and ethanol by varying the volume fraction of ethanol, fe= Vethanol/Vethanol+water.As illustrated in Fig. 3b, the CuNCs aggregates generated with increasing fe, and simultaneously emissive light under 365 nm irradiation was gradually intensified. The diluted CuNCs was clear and feeble luminescent until fe was 60%, at which timethe solution turned cloudy with very red emission due to the incipient formation of aggregates. Increasing feto 85%, the solution emitted very strong red luminescence and suggesting the smaller aggregates. Photoemission spectra (Fig. 3c) were also recorded to analyze the luminescence changes due to variations in aggregation degree. The increasing of fe caused an impressive luminescence enhancement of GS@CuNCs in the emission intensity at 610 nm. Among fefrom 0% to 50%,fluorescence intensity was also increasing. A 30-fold enhancement of emission intensity was observed when the fereached 85%. There were two good linear relationships with the in increasing of fe (Fig. 3d). For linear scope (fe) 0%–50% in Fig. 3d Inset a, the regression equation can be expressed as y =2.55x - 2.33 where R2= 0.994. For linear scope (fe) 60%–85%in Fig. 3D Inset b, the regression equation can be expressed as y = 230.93x - 14399.74 where R2= 0.991. From these results,CuNCs in the sensing system can be used to determine water content of ethanol in ethanol. Some inference about the AIE of CuNCs can be made from the above observations, especially the relationship between luminescence intensity and the degree of aggregation.
3.3 Detection of Al3+ based on luminescence enhancement
The capability of the CuNCs for the quantitative detection of Al3+was evaluated. Fig. 4 displays the fluorescence spectra of the CuNCs in the sensitive and selective method to detect various concentrations of Al3+. As provided in Fig. 4a, the fluorescence intensity of GS@CuNCs at 610 nm increases gradually with the addition of 0–20 µmol∙L-1of Al3+, indicating that weak luminescent CuNCs can generate very strong luminescence upon aggregation with addition of Al3+. From Fig. 4b, it can be seen that the developed method exhibited a good behavior for the detection of Al3+in the linear range from 2 µmol∙L-1to 20 µmol∙L-1. The fitting line can be expressed as:F/F0= 1.013[Al3+] + 0.231 (R2= 0.9957), where F0and F represent the fluorescent intensities of CuNCs without and with the addition of Al3+, respectively. The detection limit for Al3+ions was 33 nmol∙L-1on the basis of a signal-to-noise ratio of 3.
In addition, the selectivity of this fluorescent probe was investigated by examining the fluorescence responses of the CuNCs toward Al3+(6 µmol∙L-1) against the other metal ions(K+, Ca2+, Na+, Mg2+, Fe3+, Zn2+, Cu2+, Hg2+, Ni2+, Pb2+, Co2+,Cd2+, Ag+, Mn2+, each 1 mmol∙L-1). As illustrated in Fig. 4c,d,Al3+could apparently enhance the fluorescence intensity of the CuNCs. In contrast, the other metal ions have negligible effects or minor variation for the fluorescence intensity ((F - F0)/F0)of CuNCs. It indicated that the CuNCs probe exhibited high specificity for the detection of Al3+.
4 Conclusions
In conclusion, a red-emitting GS@CuNCs probe has been prepared via a simple and environmentally friendly approach.Two markedly aggregation-induced emission (AIE) approaches of GS@CuNCs were investigated by ethanol-induced aggregation and Al3+-induced aggregation. Moreover, GS@CuNCs were firstly proposed as fluorescence probe for rapid,cheap, selective and sensitive detection of Al3+, based on the aggregation-induced emission of CuNCs.
- 物理化学学报的其它文章
- Electronic Stability of Eight-electron Tetrahedral Pd4 Clusters
- Synthesis of High Yield Au21(SR)15 Nanoclusters
- Construction and NIR Luminescence Properties of Zn-Ln Rectangular Nanoclusters
- Ag7(MBISA)6 Nanoclusters Conjugated with Quinacrine for FRETEnhanced Photodynamic Activity under Visible Light Irradiation
- Synthesis and Structure Determination of Ag-Ni Alloy Nanocluster Ag4Ni2(SPhMe2)8 (SPhMe2 = 2,4-dimethylbenzenethiol)
- PPh3: Converts Thiolated Gold Nanoparticles to [Au25(PPh3)10(SR)5Cl2]2+

