PPh3: Converts Thiolated Gold Nanoparticles to [Au25(PPh3)10(SR)5Cl2]2+
ZHU Min , LI Manbo , YAO Chuanhao , XIA Nan , ZHAO Yan ,2, YAN Nan , LIAO Lingwen ,WU Zhikun ,*
1 Key Laboratory of Materials Physics, Anhui Key Laboratory of Nanomaterials and Nanotechnology, CAS Center for Excellence in Nanoscience, Institute of Solid State Physics, Chinese Academy of Sciences, Hefei 230031, P. R. China.
2 University of Science and Technology of China, Hefei 230026, P. R. China.
Abstract: Research on gold nanoclusters is at the frontier of nanoscience and nanotechnology. The introduction of the first phosphineprotected gold nanocluster, Au11(PPh3)7(SCN)3 (where PPh3 stands for triphenylphosphine and Ph stands for benzene), can be dated back to 1969. As research in the field progressed, many structures of phosphineprotected nanoclusters such as Au5, Au8, Au13, and Au39 were reported.However, the stability of these phosphine-protected nanoclusters was not satisfactory, which handicapped their research and application. In an attempt to find alternatives for phosphine-protected nanoclusters,thiolated gold nanoclusters have attracted extensive attention in recent years. So far, there has been great progress primarily owing to the development of wet-chemical synthesis techniques,among which the utilization of ligand-exchange has been proved to be very effective to synthesize thiolated gold nanoclusters. It can be easily understood that phosphine in gold nanoclusters can be exchanged with thiolate because the latter has stronger affinity for gold. However, we recently found that the reverse ligand-exchange, i.e., the exchange of thiolate with phosphine, can also take place. Some questions have naturally arisen: Is the reverse ligand-exchange only applicable to superatomic [Au25(SR)18]- (SR: thiolate) nanoclusters? Can it occur in other thiolated gold nanoclusters? If so, is this reverse ligand-exchange also dependent on the starting nanoclusters? These intriguing issues have inspired us to conduct this work.
Key Words: Thiolated gold nanoparticles; PPh3; Universal converter; Luminescence
1 Introduction
The study of gold nanoclusters (ultrasmall nanoparticles)protected by phosphine has long history1–10, much longer than that of the thiolated ones. The first phosphine-protected gold nanocluster can be dated back to 1969, when McPartlin et al.1reported the Au11(PPh3)7(SCN)3nanocluster. As research in the field progressed, many structures of phosphine-protected nanoclusters such as Au52, Au73, Au84, Au111,5,6, Au137,8and Au399were unravelled. However, the stability of these phosphine-protected nanoclusters was not satisfactory. For this reason, people had to move their sights to the more stable species,thiolated gold nanoclusters, thus booming the related research11–47.So far, great progress has been achieved primarily owing to the development of wet-chemistry synthesis techniques, among which, ligand-exchange, developed by Schmid, Hutchison,Tsukuda, Jin, et al. has been proved to be very effective to synthesize thiolated gold nanoclusters25,48–57. It can be easily understood that phosphine in gold nanoclusters can be exchanged with thiolate because the latter has stronger affinity for gold. However, we recently found that the reverse ligandexchange, i.e., thiolate was exchanged by phosphine, can also take place58,59. Questions naturally arising are: Is the reverse ligand-exchange only applicable to superatomic Au25? Can it occur in other thiolated gold nanoclusters? If so, is this reverse ligand exchange also dependent on the starting nanoclusters25,43,57,60? These intriguing issues inspired us to make an investigation on the reactions of PPh3 with some other thiolated gold nanoclusters. Surprisingly, the experimental results show that thiolated gold nanoclusters (nanoparticles) with different compositions, structures, sizes and protecting thiolates can be uniformly transformed to [Au11(PPh3)8Cl2]+(Au11for short), and finally to [Au25(PPh3)10(SR)5Cl2]2+(SR: thiolate)under the action of PPh353, while [Ag25(SPhMe2)18]-(Ag25 for short)61, and PVP(citrate)-protected gold nanoparticles can’t be transformed to [Au25(PPh3)10(SR)5Cl2]2+(or [Ag25(PPh3)10(SR)5Cl2]2+) under the same conditions, indicating the unique chemistry of thiolated gold nanoparticles. Employing this special chemistry, we synthesized seven [Au25(PPh3)10(SR)5Cl2]2+species with various ligands, and investigated the ligand influence on the luminescence properties of [Au25(PPh3)10(SR)5Cl2]2+.
2 Experimental and section
2.1 Chemicals
Tetraoctylammonium bromide (TOABr, 98%), 2-phenylethanethiol (PhC2H4SH, ≥ 99%), 4-(tert-butyl) benzene-1-thiol (t-Bu-PhSH, 99.0%), cyclohexanethiol (C6H11SH,99.0%), 1-hexanethiol (C6H14S, ≥ 98%), 1-dodecanethiol(C12H26S, ≥ 98%), benzyl mercaptane (PhCH2SH, 99.0%), 4-tert-butylbenzylmethanethiol (t-Bu-PhCH2SH, 99.0%) were purchased from Sigma-Aldrich. Sodium borohydride (NaBH4, ≥96%), dichloromethane (CH2Cl2, ≥ 99.5%), and methanol(CH3OH, ≥ 99.5%) were purchased from Sinopharm Chemical Reagent Co. Ltd.
All chemicals were used as received. The water used in all experiments was ultrapure (resistivity 18.2 MΩ∙cm), produced with a Milli-Q NANO pure water system.
2.2 Materials
[Au23(SC6H11)16]-(Au23for short)62, Au24(SC2H4Ph)20(Au24for short)60, Au36(TBBT)28(Au36for short)57, Au38(SC2H4Ph)24(Au38for short)40, 3 nm Au nanoparticles38, PVP/citrate protected Au nanoparticles63,64and Ag2561were synthesized following the previous methods.
2.3 Measurements
All UV-Vis-NIR absorption spectra were recorded using a UV-2550 spectrophotometer (Shimadzu, Japan) at room temperature. Electrospray ionization mass spectra (ESI-MS)were acquired on a Waters Q-TOF mass spectrometer equipped with a Z-spray source. The sample was dissolved in toluene (~1 mg∙mL-1) and diluted 1 : 1 in dry ethanol (5 mmol∙L-1CsOAc).The sample was directly infused at 5 μL∙min-1. The source temperature was fixed at 70 °C. The spray voltage was set at 2.20 kV and the cone voltage at 60 V. Fluorescence spectra were recorded on a Fluoromax-4 spectrofluorometer (HORIBA JobinYvon), and the excitation wavelength was kept at 514 nm with slit of 10 nm.
2.4 Isolation and purification of[Au15(PPh3)7(SC6H11)7]+
Au23was dissolved in CH2Cl2and 20 equivalents of PPh3were added to the solution in a dropwise fashion. The reaction mixture was stirred at room temperature for 30 min. After the reaction was completed, the reaction mixture was concentrated by vaporating solvent under a reduced pressure.[Au15(PPh3)7(SC6H11)7]+(Au15for short) was purified by fractional precipitation with petroleum ether as the precipitator.
2.5 Isolation and purification of [Au11(PPh3)8Cl]2+and [Au(PPh3)2]+
Au23(Au24and Au36) was dissolved in CH2Cl2and 20 equivalents of PPh3were added to the solution in a dropwise fashion. The reaction mixture was stirred at room temperature for 5 h. After the reaction was completed, the reaction mixture was concentrated by evaporating solvent under a reduced pressure. Au11and [Au(PPh3)2]+were purified by column chromatography on silica gel (methanol/dichloromethane = 1/20,V/V).
2.6 Isolation and purification of[Au25(PPh3)10(SR)5Cl2]2+
Au23(Au24and Au36) was dissolved in CH2Cl2and 20 equivalents of PPh3were added to the solution in a dropwise fashion. The reaction mixture was stirred at room temperature for 24 h. After the reaction was completed, the reaction mixture was concentrated by evaporating solvent under a reduced pressure. [Au25(PPh3)10(SR)5Cl2]2+was purified by column chromatography on silica gel (methanol/ dichloromethane =1/20, V/V).
2.7 Synthesis of Aux(SR)y mixture nanocluster
HAuCl4∙4H2O (0.42 mmol, dissolved in 2 mL of water) was mixed with TOAB (0.48 mmol, dissolved in 10 mL of dichloromethane), the solution was vigorously stirred to facilitate phase transfer of the Au(III) salt into the organic phase.After 15 min., the aqueous layer was removed and 2-phenylethanethiol (6.0 equivs. relative to gold) was added. After 1 h, 5 mL of aqueous NaBH4(10 equivs. relative to gold atom)was rapidly added to the solution. The reaction was allowed to proceed under constant stirring for 2 h. The CH2Cl2 phase was then removed via rotary evaporation and washed with methanol.
3 Results and discussion
Au23is the second case that is negatively charged as Au25, and its molecular composition differs from Au25only by an Au2(SR)2unit (without considering the ligand difference), but the structure and protecting thiolate are utterly different from those of Au2562,so Au23was first chosen as the starting nanocluster. In a typical transformation test (details are provided in the experimental section), Au23was dissolved in dichloromethane (DCM), after which a freshly prepared dichloromethane solution of PPh3(20 equivs. per mole of Au nanoclusters) was added dropwise. The reaction mixture was stirred at room temperature and monitored by UV-Vis-NIR spectrometry, which reveals three main reaction stages similar to those in the reaction of Au25with PPh3(Fig. 1a).In the first stage, the characteristic peak of Au23at 450 nm blueshifted to 430 nm, and the characteristic peak of Au23at 570 nm blue-shifted to 550 nm, with a new absorption band centered at 690 nm appeared in the UV-Vis-NIR spectrum. In the second stage, the peak at 430 nm blue-shifted to 415 nm, and the peak at 550 nm red-shifted to 580 nm, with the peak at 690 nm disappeared and a new absorption band centered at 380 nm appeared in the UV-Vis-NIR spectrum in the meantime. Thirdly,two new peaks at 450 and 700 nm were observed with the previous peaks at 415 and 380 nm unchanged. The UV-Vis-NIR spectra of the products at stage 2 and 3 are analogous to those of the reaction between Au25and PPh358, respectively, indicating that Au11and biicosahedral [Au25(PPh3)10(SR)5Cl2]2+rod might also be formed in the reaction of Au23 with PPh3. Electrospray ionization mass spectrometry (ESI-MS), a well-known technology for the formula determination of metal nanoclusters,indeed confirm this, see Fig. 2.

Fig. 1 UV-Vis-NIR spectrometry monitoring the reaction between Au23 (a), Au24 (b), Au36 (c) and PPh3; UV-Vis-NIR spectrum of PPh3 (d) (solvent: CH2Cl2).

Fig. 2 The UV-Vis-NIR and the corresponding ESI-MS spectra of stage 1 (a, b), stage 2 (c, d) and stage 3 (e, f) for the reaction between Au23 with PPh3.Inset: The comparison of simulated and experimentally obtained isotopic patterns.
Since the ligand-exchange reaction is closely related to the cluster size25,57and might be influenced by the protecting ligand25,43,57,60, a larger nanocluster with different protecting thiolate Au3657was then chosen to react with PPh3. After the addition of 20 equivs. of PPh3, the characteristic peaks of Au36disappeared and new absorption bands centered at 415 nm and 380 nm appeared in the UV-Vis-NIR spectra, indicating the formation of Au11. A few hours later, the characteristic peaks of Au11disappeared and new absorption peaks at 341, 392, 426, 460 and 694 nm appeared, indicating that Au11was transformed to[Au25(PPh3)10(TBBT)5Cl2]2+, which was identified by ESI-MS.Interestingly, Au25, Au23 and Au36 are all uniformly transformed to [Au25(PPh3)10(SR)5Cl2]2+through the same intermediate Au11.Due to the fact that the kernels in Au25, Au23and Au36are all bigger than Au11(Au13for Au2535,36, Au15for Au2362, and Au23for Au3657), we speculate that the nanoclusters with kernels smaller than Au11may not be tranformed to Au11, and finally to[Au25(PPh3)10(SR)5Cl2]2+after etched by PPh3. To test this, Au24 was chosen as the starting nanocluster since it has a Au8kernel60.However, the reaction process of Au24with PPh3is similar to the case of Au36with PPh3: Au11was first generated and then[Au25(PPh3)10(SR)5Cl2]2+formed, which was identified by the UV-Vis-NIR and ESI-MS spectrometry (see Figs. 1b and 3a–d).Further experiments demonstrate that even Au38 and the polydisperse gold nanocluster protected by 2-phenylethanethiolatecan be transformed to [Au25(PPh3)10(SR)5Cl2]2+, see Figs. S1b and 4b. The fact that nanoclusters with kernels smaller than Au11can be transformed to Au11and[Au25(PPh3)10(SR)5Cl2]2+indicates that the peeling process found in the case of Au25 may not be applicable to these cases.To address this, we conducted more investigations on the intermediates of the above mentioned reactions.

Fig. 3 UV-Vis-NIR and corresponding ESI-MS spectra of the reaction between Au24 (a–d), Au36 (e–h) and PPh3.Inset: The comparison of simulated and experimentally obtained isotopic patterns.

Fig. 4 UV-Vis-NIR spectra of the reaction between ~3 nm thiolated Au nanoparticles (a), polydispersed Aux(SR)y (b) and PPh3.
In the reaction of Au23and PPh3, we obtained three different products by controlling the reaction time. The first one (denoted as S1) exhibiting similar absorption as that in stage 1 (see above)was isolated when Au23and PPh3were reacted for 30 min, the second one (denoted as S2) showing a similar UV-Vis-NIR spectrum as that in stage 2 was obtained after 5 h’s reaction, and the third product (denoted as S3) with similar absorption as that in stage 3 was isolated after reaction of 24 h. Two distinct peaks centered at M/Z 4335 and 5598 are shown in ESI-MS spectrum of S1, which are assigned to [Au11(PPh3)8Cl2]2+and[Au15(PPh3)7(SC6H11)2]+, respectively, and the isotopic patterns are in good agreement with the simulated ones, see Fig. 2b,confirming these assignments. Moreover, S2 and S3 were identified to be [Au11(PPh3)8Cl2]2+(Fig.2d) and [Au25(PPh3)10(SC6H11)5Cl2]2+(Fig. 2f) by ESI-MS, respectively. It’s known that Au23has a Au15core62, thus the identification of Au15(PPh3)7(SC6H11)7+indicates that the peeling of staples may also occur to the etching of Au23by PPh3. However, in the cases of Au36and Au24, Au11was formed immediately after the addition of PPh3 monitored by UV-Vis-NIR, indicating that the staples peeling is very fast or not applicable to the two cases. Of note, in all of these reactions,we isolated and identified three small nanoclusters [Au2(PPh3)2(SR)]+, [Au3(PPh3)2(SR)2]+and[Au(PPh3)2]+(See Fig.5a), among which, [Au3(PPh3)2(SR)2]+is newly found, while [Au2(PPh3)2(SR)]+65and [Au(PPh3)2]+58have been previously reported.
Why can thiolated gold nanoclusters with different compositions, structures, sizes and protecting ligands be transformed into [Au25(PPh3)10(SR)5Cl2]2+(see Fig. 6)? The reason could be assigned to the exceptional stability of[Au25(PPh3)10(SR)5Cl2]2+under the investigated conditions. We exclude the quantumn size effect reason by revealling that ~3 nm thiolated gold nanoparticles can also be transformed to[Au25(PPh3)10(SR)5Cl2]2+, while gold nanoparticles protected by polyvinylpyrrolidone (PVP)/citrate or Ag25(SPhMe2)-18(for their synthesis and characterization, see experimental section)cannot be transformed to [Au25(PPh3)10(SR)5Cl2]2+(or[Ag25(PPh3)10(SR)5Cl2]2+) under the similar conditions (Fig. S2).Especially, Ag25 shares the similar structure with Au2561,however, there are no change in the UV-Vis-NIR spectrum of Ag25upon the addition of PPh3(Fig. 5b) even if the reaction time is extended to 48 h, indicating that Ag25is inert to PPh3. These experiment results indicate the unique reactivity of thiolated gold nanoparticles with PPh3, in other words, PPh3acts as a universal converter for thiolated gold nanoparticles.

Fig. 5 ESI-MS spectrum of three small byproducts [Au(PPh3)2]+,[Au2(PPh3)2(SR)]+ and [Au3(PPh3)2(SR)2]+(a), UV-Vis-NIR spectral evolution for the reaction between Ag25 and PPh3(b).
One utility of this kind of uniform transformation is that it provides ideal opportunities to investigate ligands influence on the properties of gold nanoclusters and screen ligands for special applications. For example, we synthesized seven[Au25(PPh3)10(SR)5Cl2]2+species with different thiolates(including S-c-C6H11, SC6H13, SC12H25, SC2H4Ph, SCH2Ph,SCH2Ph-t-Bu and SPh-t-Bu) (see Fig. 7a) by using this reverse exchange method, investigated their luminescence properties,and found that their luminescence quantum yields follow the order of [Au25(PPh3)10(SCH2Ph-t-Bu)5Cl2]2+(1.32 × 10-4) >[Au25(PPh3)10(SCH2Ph)5Cl2]2+(8.23 × 10-5) > [Au25(PPh3)10(SC2H4Ph)5Cl2]2+(5.35 × 10-6) > [Au25(PPh3)10(SC12H25)5Cl2]2+(5.02 × 10-6) > [Au25(PPh3)10(SPh-t-Bu)5Cl2]2+(3.97 × 10-6) >[Au25(PPh3)10(SC6H13)5Cl2]2+(3.73 × 10-6) > [Au25(PPh3)10(S-c-C6H11)5Cl2]2+(1.53 × 10-6). (See Fig. 7b). Basing on the QY comparison, we might conclude that SCH2Ph-t-Bu is the best ligand, while S-c-C6H11is the worse ligand for the luminescence triggering of gold nanoparticles in these investigated ligands. It is known that the surface ligands greatly influence the luminescence properties of metal nanoclusters, and one effecting factor is the electron donability of the ligands18. However, it is worth noting that the fluorescence mechanism is complex,and there are some other influencing factors on basis of our previous work18,43,60,66–68. Such diversity in surface ligand is not found in other nanoclusters, for example, although several Au25(SR)-18(SRH: thiols, including 1-hexanethiol, 1-octanethiol, 1-dodecanethiol, 2-phenylethanethiol, glutathione, 2-naphthalenethiol) nanoclusters have been reported32,69, the TBBT-protected Au25nanocluster is not obtained yet until now due to steric hindrance, which limits the systematic investigation on ligand effect.
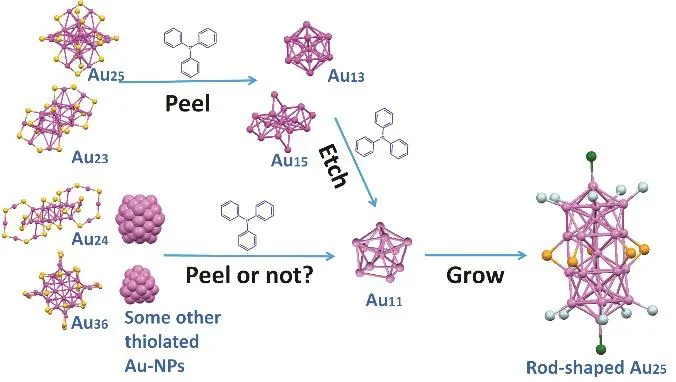
Fig. 6 Mechanism illustration of the etching process. Purple,Au atoms; yellow, S atoms; blue, P atoms; green, Cl atoms.Note: The structures used here are all from the solid single crystals,might different from those in liquid phases.
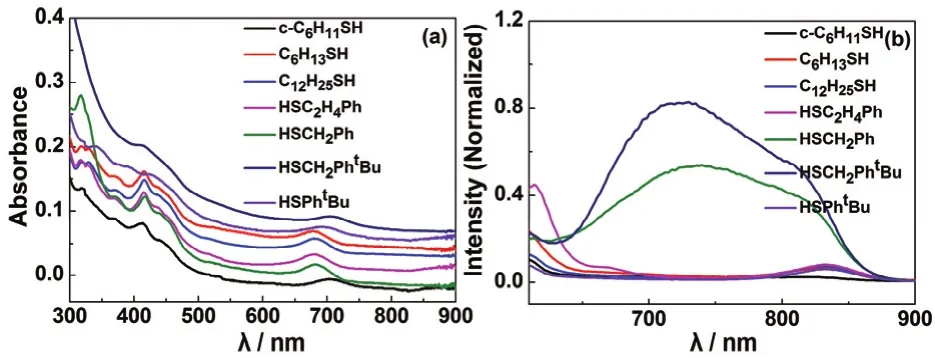
Fig. 7 UV-Vis-NIR (a) and luminescence (b) spectra of[Au25(PPh3)10(SR)5Cl2]2+ species with different thiolate ligands.Excitation wavelength: 514 nm.
4 Conclusions
In summary, we have demonstrated that thiolated gold nanoparticles have different compositions, structures, sizes and protecting thiolates can be transformed to [Au25(PPh3)10(SR)5Cl2]2+(SR: thiolate) through [Au11(PPh3)8Cl2]2+intermediate under the action of PPh3, i.e., PPh3acts as a universal converter for thiolated gold nanoparticles. But this transformation was not found in gold nanoparticles protected by other ligands (PVP, citrate) and Ag25, indicating the unique reactivity of thiolated gold nanoparticles with PPh3. The utility of this finding is that it provides ideal opportunity to investigate the ligand effect and screen ligand for speical applications of thiolated gold nanoparticles.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.
- 物理化学学报的其它文章
- Electronic Stability of Eight-electron Tetrahedral Pd4 Clusters
- Synthesis of High Yield Au21(SR)15 Nanoclusters
- Luminescence Emission of Copper Nanoclusters by Ethanol-induced Aggregation and Aluminum Ion-induced Aggregation
- Construction and NIR Luminescence Properties of Zn-Ln Rectangular Nanoclusters
- Ag7(MBISA)6 Nanoclusters Conjugated with Quinacrine for FRETEnhanced Photodynamic Activity under Visible Light Irradiation
- Synthesis and Structure Determination of Ag-Ni Alloy Nanocluster Ag4Ni2(SPhMe2)8 (SPhMe2 = 2,4-dimethylbenzenethiol)

