Atomically Precise Zr-Oxo and Zr/Ti-Oxo Nanoclusters by Deep Eutectic-Solvothermal Synthesis
NARAYANAM Nagaraju , CHINTAKRINDA Kalpana , FANG Weihui , ZHANG Lei ,*, ZHANG Jian State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, P. R. China.
2 International College, University of Chinese Academy of Sciences, 100049 Beijing, P. R. China.
Abstract: Atomically precise nanoclusters form an important class of functional materials that have recently attracted research interest for their unique properties and easily tunable surface functionalities. Core-shell nanomaterials with precise structural information can be produced to better understand the structure–property relationships for different applications. Polyoxo-titanium clusters (PTCs) are such a kind of nanomaterial for different functional applications in catalysis, photovoltaics, ceramics, etc.However, the high bandgap of semiconductive PTCs is the limiting factor in their practical solar application in the visible region of sunlight. The development of PTCs with different surface-bound ligands is an emerging area of research in the design and synthesis of core-shell nanoclusters with reduced bandgaps. It has been extensively reported that the polynuclear growth of PTCs requires molecular-level water supply in reactions. Moreover, it is important to identify more environment-friendly synthetic methods. Deep eutectic-solvothermal (DES) synthesis is an emerging green method for the synthesis of different crystalline materials. The hygroscopic nature of DES should enhance the provision of water during polynuclear growth of nanoclusters. Hence, we chose to synthesize different kinds of PTCs using DES as solvent. Two nanoclusters, Zr-oxo (PTC-65) and Zr/Ti-oxo (PTC-66) clusters having surface-bound 1,10-phenanthroline (1,10-phn) and phenol ligands, were successfully synthesized using this approach; 1,10-phn was employed as the precursor in the synthetic reaction, and phenol was not employed directly in the chemical reaction,but was supplied from the DES solvent used in the reaction. In the presence of chromophoric ligands, 1,10-phn and phenol are believed to enhance the light absorption properties of the resulting functional nanomaterials. Their crystal structure revealed that they form core-shell mimics with Zr-oxo and Ti/Zr-oxo core units having surface-bound shell ligands. Based on their different structural characteristics, photocatalytic hydrogen evolution studies were performed on these two functional materials using an aqueous solution of H2O (50 mL)/triethanol amine (10 mL). Interestingly,PTC-65 formed a turbid solution, whereas PTC-66 formed a clear solution. The possible reasons for their different dispersion behaviors are widely discussed, with emphasis on their structure–property relationships. This study provides a potential tool for the homogenization of Ti-O materials to improve their photocatalytic activities. Moreover,the success of our work confirms that deep eutectic-solvothermal synthesis can be an effective method for cluster preparation. Many other interesting polynuclear complexes like polyoxometalates, chalcogenides, and noble-metal clusters could be obtained by this synthetic methodology.
Key Words: Nanocluster; Titanium; Zirconium; Deep-eutectic solvothermal; H2 evolution
1 Introduction
The possibility to create atomically precise nanoparticles and nanoclusters with surface bound ligands has recently led to a plethora of applications to design novel functional devices1–5.These types of structural materials might also be regarded as core-shell nano structures6–10. The development of polyoxo titanium clusters (PTCs) with different surface bound ligands is an emerging area of research to design and synthesize such kind of core-shell nanoclusters11–13. Such kind of structural arrangements are believed to enhance their thermal and solution stabilities making them more rigid architectures suitable for a range of applications in photocatalytic water splitting, catalysis, and photovoltaic cells14,15. Therefore,intensive research in the past decades has been devoted to PTCs as they can also provide precise structural information for thorough understanding of the binding modes of sensitizers to Ti-O surfaces16–18. But the biggest challenge lies in understanding their structure–property relationships with correlation to structural arrangements of core and shell bounded ligands and their internal energy transfer dynamics.Hence, precise structural information with clear-cut boundaries concerning the core and shell units is highly required.
Deep eutectic solvents (DES) consisting of an ionic compound (quaternary ammonium salts) and a molecular compound (amides, amines, phenols, or carboxylic acids), has found applications in many chemical syntheses19–23. The highly hygroscopic nature of DES provides more water and help to grow high nuclearity functional molecular metal oxide analogues during solvothermal reactions24. Recently, deep eutectic solvothermal synthesis is explored by our group to synthesize highly precise titanium-oxo clusters with core-shell like arrangements of π-conjugate chromophore ligands and their interesting photocatalytic water splitting behaviors are also evaluated25. In continuation of that work, we herewith synthesized two novel poly-oxo molecular clusters with Zr- and Ti/Zr-mixed metal core units (Scheme. 1). The synthesized clusters include [Zr6(µ3-O)8(PhenO)6(1,10-phn)6] (PhenO)2(PTC-65) and H2 [Ti11Zr4(µ3-O)14(µ2-O)10(PhenO)11(1,10-phn)8]Cl3∙PhenOH (PTC-66) (1,10-phn = 1,10-phenanthroline; PhenOH = Phenol). Zr6and Ti11Zr4cluster cores in this work are decorated on their surfaces with high density of light absorbing π-conjugate chromophoric (1,10-phn and phenol) ligands. The phototcatalytic hydrogen evolution experiments carried out shows different activities. This study highlights a novel design strategy to identify and synthesize novel atomically precise core-shell nanoclusters with different chemical environments.
2 Experimental
2.1 Materials and instrumentation
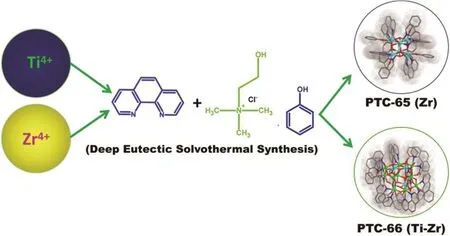
Scheme 1 Schematic representation of synthesis of PTC-65 & -66 using deep-eutectic solvothermal synthesis.
All the reagents and solvents employed are commercially available and are used as received without further purification.Elemental analyses are performed using a Perkin-Elmer 240C elemental analyzer. Powder X-ray diffraction (PXRD) analysis is performed on a Mini Flex-II diffractometer with Mo Kαradiation (λ = 0.154056 nm) in the 2θ range of 5°-50° with a scanning rate of 1 (°)∙min-1(Supporting Information, Figs. S1 and S2). IR spectra (KBr pellets) were recorded on an ABB Bomem MB102 spectrometer over a range of 400–4000 cm-1(Supporting Information, Figs. S3 and S4). Thermal stability studies are performed using a NETSCHZ STA-449C thermoanalyzer with a heating rate of 10 °C∙min-1under a N2gas flow (Supporting Information, Figs. S5 and S6). Optical absorbance of PTC-65 and PTC-66 is measured by a solid-state UV-Vis diffuse reflectance measurement method at room temperature (Supporting Information, Figs. S7 and S8).The composition of PTC-66 is studied with an Oxford X-max energy-dispersive spectrometer (EDS) equipped on the JSM6700-F FESEM (Supporting Information, Fig. S9). Particle size analysis is measured using Dynamic Light Scattering(DLS) technique with a NanoBrook Zetaplus Zeta Potential Analyzer and analyzed in ZetaPALS Particle Sizing Software version 5.32.
2.2 General methods for X-ray crystallography
Suitable single crystals are carefully selected under an optical microscope and glued to thin glass fibers. Crystals are found to be air stable at room temperature. Thereafter, single crystal X-ray diffraction analyses are performed on Super Nova diffractometer at room temperature. The structures are solved by direct methods and refined on F2by full matrix least-squares using new SHELXL program26,27. All of the nonhydrogen atoms are located from Fourier maps and are refined anisotropically. The crystallographic data is listed in Table 1.CCDC numbers 1542118 & 1542119 (PTC-65 & -66) contains the supplementary crystallographic data for this paper.Supplementary single crystal XRD data, including structure factors, is available free of charge from the Cambridge Crystallographic Data Centre (CCDC) via www.ccdc.cam.ac.uk/data_request/cif.
2.3 Synthesis of DES
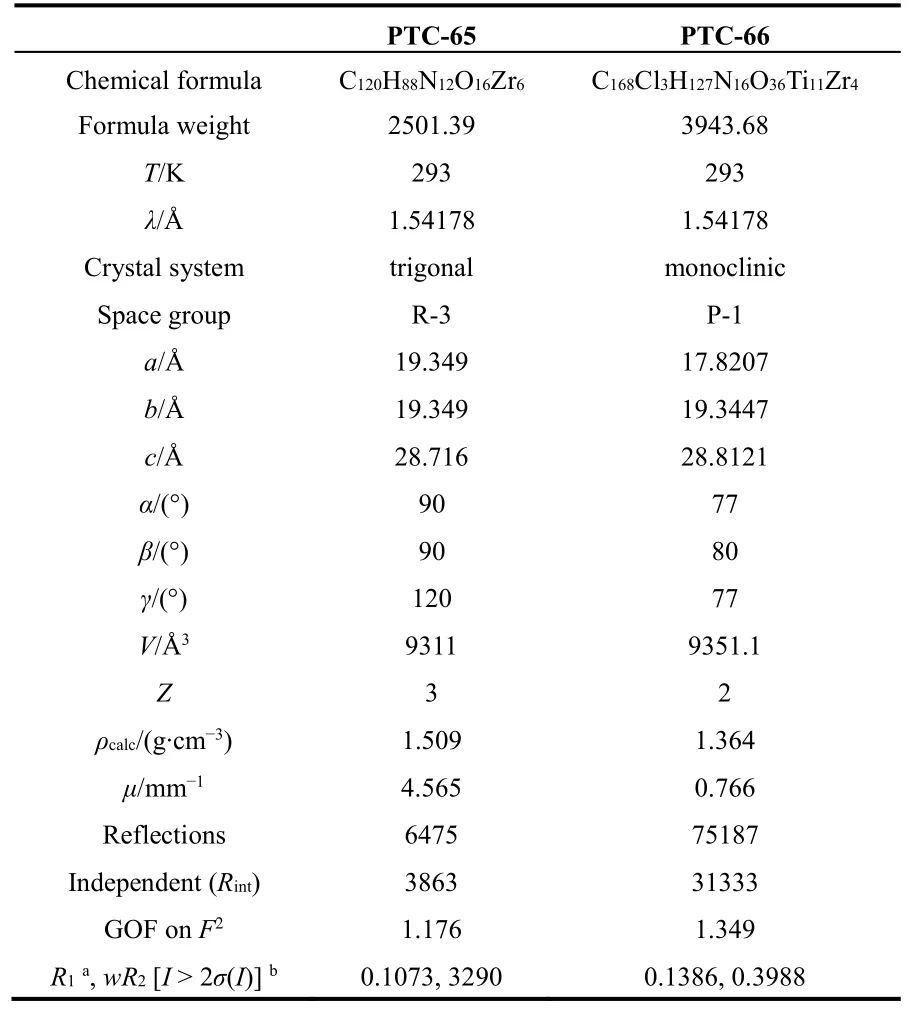
Table 1 Crystal structure parameters of PTC-65 & -66.
Deep eutectic solvent is prepared as reported earlier by mixing solids of choline chloride (13.9 g, 0.1 mol∙L-1) and phenol (18.8 g, 0.2 mol∙L-1) thoroughly for 30 minutes at room temperature to form colorless solvent and used without any further treatment25.
2.4 Synthesis of PTC-65
A mixture of 1,10-phn (0.368 g, 2.0 mmol and Zr(OBut)4(2 mL) are dissolved in 2 mL of DES in a 20 mL scintillation vial,heated at 100 °C for 120 h, and then cooled to room temperature. Colorless cube-shaped crystals of PTC-65 are obtained, washed with excess amount of 2-propanol and dried in air (65% yield, based on 1,10-phn ligand). Elemental analysis for C120H88N12O16Zr6, Calcd (%): C, 57.62; H, 3.55;N, 6.72. Found (%): C, 58.01; H, 3.98; N, 6.59.
2.5 Synthesis of PTC-66
A mixture of 1,10-phn (0.729 g, 2.0 mmol), Ti(OBut)4(1 mL), and Zr(OBut)4(0.5 mL) are dissolved in 2 mL of DES in a 20 mL scintillation vial, heated at 100 °C for 120 h, and then cooled to room temperature. Dark yellow coloured block-shaped crystals of PTC-66 are obtained, washed with excess amount of 2-propanol and dried in air (75% yield,based on 1,10-phn ligand). Elemental analysis for C168Cl3H127N16O36Ti11Zr4, Calcd (%): C, 51.17; H, 3.25; N,5.68. Found (%): C, 51.71; H, 3.48; N, 6.05.
2.6 Photocatalytic hydrogen evolution experiments
The sample for photoinduced hydrogen production was located in a closed gas circulation system (Perfect Light Company Labsolar-III (AG). The 300 W Xe lamp is used as the light source. The gas in the system is analyzed by online-GC to determine the amount of hydrogen generated at each hour.Typically, 50 mg of sample is dispersed in 50 mL of H2O with 10 mL of triethanol amine (TEOA) as sacrificial agent, and then 33 µL 1.0% (w) H2PtCl6∙xH2O is added as co-catalyst.Hydrogen gas evolved after each hour is determined for a period of 6 h.
3 Results and discussion
Many metal-ligands core-shell nanoparticles synthesized earlier lack precise structural information as they were isolated as powdered materials7. Even though many PTCs are reported with surface bound ligands, like core-shell materials, most of their titanium-oxo cluster cores are decorated with carboxylates or phosphonate acids12. It is interesting to note that titanium-oxo cluster cores decorated with light absorbing chromophores or sensitizers will act as active photocatalyst materials18. But it is extremely difficult to synthesize such kind of chromophores decorated functional materials using conventional synthesis techniques.Recently, we explored deep-eutectic solvothermal synthesis to synthesize titanium-oxo clusters decorated and protected by π-conjugated light absorbing chromophores. That study highlights the protecting effect of highly chromophore distributed titanium-oxo clusters to behave like homogeneous reaction solutions for photocatalytic hydrogen evolutions25.
In order to test the generality of DES synthetic method,we herewith explored similar kind of study on two novel molecular clusters to synthesize as Zr-oxo and Ti/Zr-oxo mixed metal clusters decorated with a high density of chromophores. The reaction of zirconium butoxide and 1,10-phenanthroline in DES produced colorless cubic shaped crystals with a symmetrical Zr6cluster in PTC-65 (Figs.1a,b). Although similar Zr6core structure has already been reported28, herein for the first time its shell is completely functionalized by π-conjugate phenol and 1,10-phenanthroline ligands. Furthermore, a mixed metal reaction was carried out with titanium, zirconium and 1,10-phenanthroline to produce (yellowish-) red crystals and forms a heterometallic Ti11Zr4cluster in PTC-66 (Fig. 1c,d).It is constructed by connecting the 11 Ti and 4 Zr atoms through 14 µ3-O and 10 µ2-O bridges. Interestingly, two pentagonal {Ti4Zr2(µ3-O)5} units are observed in PTC-66(Fig. S10). It is worth of noting that isostructural {Ti5Ti}pentagon has found to be essential building units of highly symmetrical Ti-O cages29,30. And as expected, the core of this mix-metal Ti11Zr4cluster is also fully surrounded by conjugated π-bond system consisting of eight 1,10-phenanthroline and 11 phenol ligands.
Hence it can be understood that deep-eutectic solvothermal synthesis can be effectively used to synthesize different types of high nuclearity and heteronuclear materials with core-shell like structural arrangements. PTC-65 and PTC-66 show good water stability as they show unchanged PXRD patterns after immersed in water for 24 h (Supporting Information, Figs. S11 & S12). The solid state optical absorbance spectra of the samples show lower optical bandgaps attributed to the penetration of the highest occupied phenols levels into the bandgap of Zr/O or Ti-Zr/O cores (Supporting Information, Figs. S8 & S9). Additionally,the 1,10-phenanthroline ligands might also believe to contribute in lowering the bandgaps.
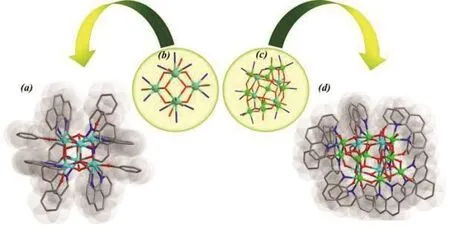
Fig. 1 Structures of the Zr-oxo and Ti/Zr-oxo clusters decorated with 1,10-phn and phenol chromophores synthesized in DES.(a) PTC-65 & (d) PTC-66; Core units of (b) PTC-65 and (c) PTC-66(Color codes: Zr-Pale Blue; Ti-Pale green; N-Blue; O-red; C-Pale black.)
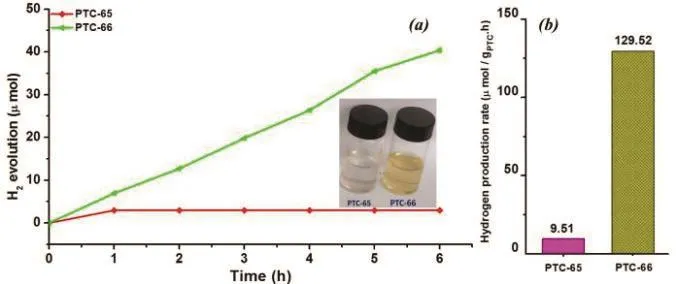
Fig. 2 Comparative photocatalytic hydrogen evolution studies(Solutions are shown inset) (a); comparative hydrogen evolution rates of PTC-65 & PTC-66 (b).
Photocatalytic hydrogen evolution studies were performed on these two functional materials using an aqueous solution of H2O (50 mL)/triethanol amine (TEOA, 10 mL). It is interesting to note that PTC-65 forms turbid solution and contrarily PTC-66 forms a clear solution. This huge difference in their dispersive behaviors might be attributed to the difference in their structural arrangements (Fig. 2a). In PTC-65, zirconium was bonded with 1,10-phn and phenol chromophores and failed to get activated to produce very low amounts of hydrogen. While, on the other hand, the presence of titaniums attached with more numbers of 1,10-phn and phenol chromophores in the cluster activated the catalyst and believed to produce more number of charge carriers responsible for hydrogen generations. Hence there observed such huge difference in their hydrogen evolution behaviors with PTC-65 producing only 9.51 mol∙g-1∙h-1and PTC-66 producing 129.52 mol∙g-1∙h-1hydrogen (Fig. 2b).
It is worth to understand that the optical excitation kinetics of the constituent components is very important while designing novel functional materials. When results of present and previous studies are compared, all the highly dispersible Ti-oxo clusters are functionalized by 1,10-phenanthroline which is believed to form stronger coordination complexes with transition metals for the additional delocalized π-bond.Furthermore, presence of Zr atoms would possibly reduce the disperstiveness of clusters.
To identify the sizes of the highly dispersed cluster containing particles, dynamic light scattering (DLS) study was carried out for clear solution of PTC-66 and the mean particle size diameter of 270.1 nm is obtained (Fig. S13). It is indicating the aggregation of small number of molecular clusters in the solution when compared with its cluster size of 2.0 nm. Hence it can be concluded that the above opposite kind of dispersive behaviors shows great influence on the photocatalytic activities confirmed by the highly dispersed PTC-66 producing higher hydrogen and the poorly dispersed PTC-65 (Fig. S14) producing lower hydrogen.
4 Conclusions
In summary, two crystalline Zr-oxo and Ti/Zr-oxo molecular clusters are synthesized successfully with deep-eutectic solvothermal synthesis. The highly hygroscopic nature of DES helps to grow polynuclear clusters decorated with more numbers of surface bound chromophoric ligands (1,10-phn and phenols). The presence of these chromophoric groups on titanium is believed to improve the photocatalytic hydrogen evolution activities.The materials with such kind of structural arrangements are also believed to improve their dispersions during photocatalysis and help to improve their functional activities.Therefore, this study provides a potential tool for the homogenization of Ti-O materials to improve their photocatalytic activities. Moreover, the success of our work also confirms that deep eutectic solvothermal synthesis can be a very effective method for cluster preparation. It is believed that many other interesting polynuclear complexes such as polyoxometalates, chalcogenides, and even noble-metal clusters will be obtained by this synthetic methodology.
Acknowledgment: We acknowledge the support of The World Academy of Sciences (TWAS) for the award of CAS-TWAS President’s Research Fellowship to the student.
Supporting Information: available free of charge via the internet at http://www.whxb.pku.edu.cn.
- 物理化学学报的其它文章
- Electronic Stability of Eight-electron Tetrahedral Pd4 Clusters
- Synthesis of High Yield Au21(SR)15 Nanoclusters
- Luminescence Emission of Copper Nanoclusters by Ethanol-induced Aggregation and Aluminum Ion-induced Aggregation
- Construction and NIR Luminescence Properties of Zn-Ln Rectangular Nanoclusters
- Ag7(MBISA)6 Nanoclusters Conjugated with Quinacrine for FRETEnhanced Photodynamic Activity under Visible Light Irradiation
- Synthesis and Structure Determination of Ag-Ni Alloy Nanocluster Ag4Ni2(SPhMe2)8 (SPhMe2 = 2,4-dimethylbenzenethiol)

