4-(1,2,4-三唑-1-基)苯酚铜ギ/镍ギ配合物的晶体结构及荧光性质
赵红昆 丁 波 王修光 贾 芳 杨恩翠 赵小军
(天津师范大学化学学院,无机-有机杂化功能材料化学教育部重点实验室,天津市功能分子结构与性能重点实验室,天津 300387)
Transition metalcomplexesconstructed from inorganic metal ions and various organic ligands have attracted intense interest during the past decades,which is mainly attributed to their tailorable structures and promising properties in different fields[1-7].The successful preparations of the extended complexes with 1,2,4-triazole(Htrz)and its derivatives ligands have gained attractively considerable attention due to their novel framework topologies[8-10]and potential applications in gas storage and luminescence properties[11-15].Compared with the unsubstituted triazoles,4-(1,2,4-triazol-1-yl)phenol (hptrz)loses an active coordination site on the 1-positioned nitrogen due to the substitution of the H atom by a phenolic moiety,and can usually act as a terminal ligand to coordinate with the discrete metal ion in an unidentate mode.Therefore,the constructions of the extended structures with hptrz bridge are difficult and still challenging.Fortunately,hptrz-based Cdギcoordination polymers with aromatic polycarboxylate coligangds have been previously reported,which present strong hptrz-based fluorescence emissions[20].To the best of our knowledge,there exist few papers about Cuギ/Niギ-polymers based on hptrz,m-phenol-1,2,4-triazole(m-ptz)or pphenol-1,2,4-triazole(p-ptr)ligandswithNCSanion[16-17].As part of our continuing investigations on coordination chemistry and photophysically properties of 1,2,4-triazole[18-19]and its derivatives[20-21],herein,hptrz and aromatic polycarboxylate and ammonium thiocyanate co-ligands were selected as mixed ligands to carry out the self-assembly reactions with Cuギ/Niギ salts under controllable hydrothermal conditions.Asa result,two crystalline transition metal complexes,{[Ni(H2O)2(hptrz)2(tp)]·2DMF}n(1)and [Cu(hptrz)2(SCN)2]·2H2O(2),were successfully isolated and structurally characterized.Structural determinations reveal that the different crystal structures of the two complexes with 1D polymeric chain for 1 and mononuclear structure for 2 are significantly governed by the coligands (aromatic carboxylate group vs ammonium thiocyanate).Acting as a terminal ligand,the neutral hptrz ligand binds with the central Mギ ion in terminally monodentatemodethrough triazolylN donor,which is the common coordination mode of 1,2,4-triazole and its derivatives.Additionally,the luminescence behavior and thermal stability of the two resultant complexes are discussed in more details.
1 Experimental
1.1 Materials and physical measurements
All of the starting materials employed were commercially purchased (H2tp was purchased from Acros and other commercially analytical-grade reagents were from Tianjin Chemical Reagent Factory)and used as received without further purification.Doubly deionized water was used for the conventional synthesis.Elemental analyses for C,H and N were performed on a CE-440 (Leeman-Labs)analyzer.Fourier transform(FT)IR spectra(KBr pellets)were taken on an Avatar-370 (Nicolet)spectrometer in the range of 4 000~400 cm-1.Thermogravimetric analysis(TGA)experiments were carried out on Shimadzu simultaneous DTG-60A compositional analysis instrument from room temperature to 800℃under N2atmosphere at a heating rate of 5℃·min-1.Fluorescence spectra of the polycrystalline powder samples were performed on a Cary Eclipse fluorescence spectrophotometer(Varian)equipped with a xenon lamp and quartz carrier at room temperature.
1.2 Synthesis of{[Ni(H2O)2(hptrz)2(tp)]·2DMF}n (1)
To a mixed DMF-methanol(1∶1,V/V)solution(5.0 mL)containing hptrz (16.1 mg,0.1 mmol)and H2tp(16.6 mg,0.1 mmol)was slowly added an aqueous solution(5.0 mL)ofNi(NO3)2·6H2O(29.0 mg,0.1 mmol)with constant stirring.The pH value of the resulting mixture was adjusted to 7 by triethylamine.Then,the mixture was further stirred for half hour and filtered.Blue block-shaped crystals suitable for X-ray diffraction were grown by slow evaporation of the filtrate within five days.Yield:30%based on hptrz ligand.Anal.Calcd.for C15H18N4Ni0.50O5(%):C:49.54;H:4.99;N:15.41.Found(%):C:49.03;H:4.56;N:15.98.FT-IR (cm-1,pellet):3 434br,1 659s,1 562s,1 525s,1 439w,1 385s,1 279m,1 246w,1 145w,834m,752m,667m,629m.
1.3 Synthesis of[Cu(hptrz)2(SCN)2]·2H2O(2)
To a methanol solution(5.0 mL)containing hptrz(16.1 mg,0.1 mmol)and NH4SCN (15.2 mg,0.2 mmol)was slowly added to an aqueous solution (5.0 mL)of Cu(OAc)2·H2O(19.9 mg,0.1 mmol).The resulting mixture was further stirred vigorously for ca.30 min and filtered.Green block-shaped crystals suitable for X-ray diffraction were obtained by slow evaporation of the filtrate in five days.Yield:45%based on hptrz ligand.Anal.Calcd.for C18H18CuN8O4S2(%):C:40.18;H:3.37;N:20.83.Found(%):C:39.86;H:3.02;N:21.37.FT-IR (cm-1,pellet):3 464br,2 099vs,1 600m,1 525s,1 485w,1 438w,1 358w,1 280m,1 222w,1 144m,1 055w,980w,837m,671w,641w,468w.
1.4 X-ray crystallography
Diffraction intensities for 1 and 2 were collected on a a Bruker APEXⅡCCD diffractometer equipped with graphite-monochromated Mo Kα radiation with radiation wavelength of 0.071 073 nm by using a ω-φ scan technique at 296(2)K.There was no evidence of crystal decay during the data collection process.The program SAINT[22]was used for integration of the diffraction profiles.Semi-empirical absorption corrections were applied using SADABS program[23].The structures were solved by direct methods and refined with the full-matrix least-squares technique using the SHELXS-97 and SHELXL-97 programs[24].Anisotropic thermal parameters were assigned to all non-hydrogen atoms.The H atoms of the water molecules except for the splitting water were located from difference maps and refined with isotropic temperature factors.No attempts have been performed to locate hydrogen atoms of the splitting water molecules.The crystallographic data and experimental details for structural analyses are summarized in Table 1.Selected bond distances and angles are listed in Table 2.Hydrogen bond geometries are given in Table 3.
CCDC:746903,1;746904,2.
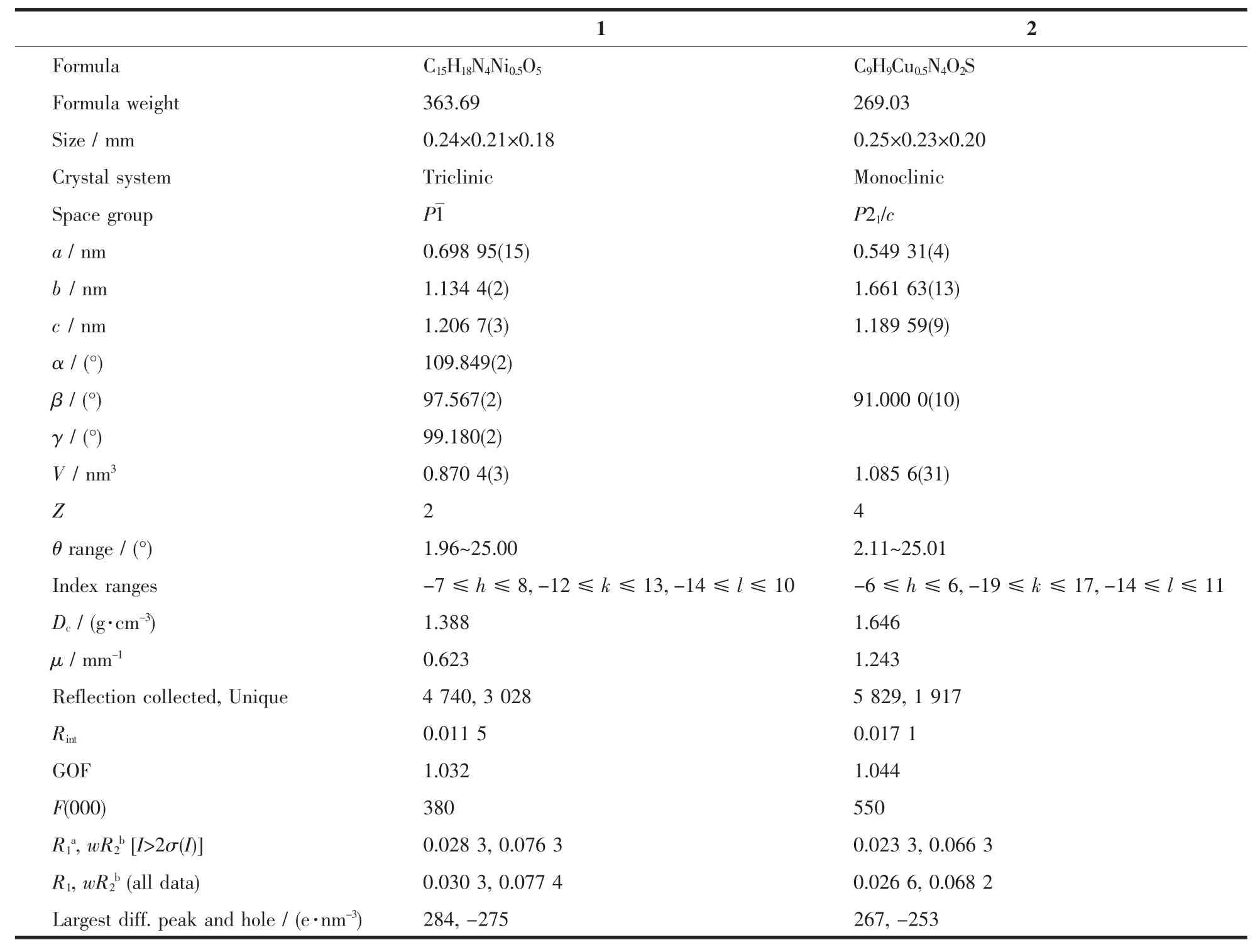
Table 1 Crystal data and structure refinement for 1 and 2
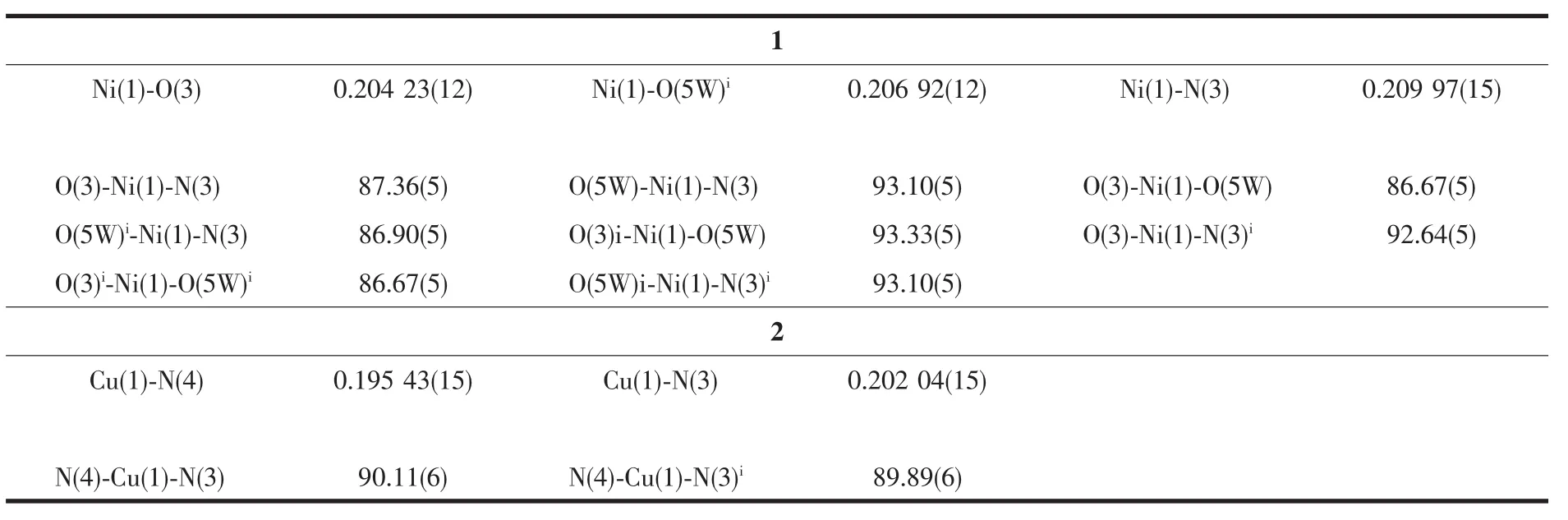
Table 2 Selected bond lengths(nm)and angles(°)for 1 and 2
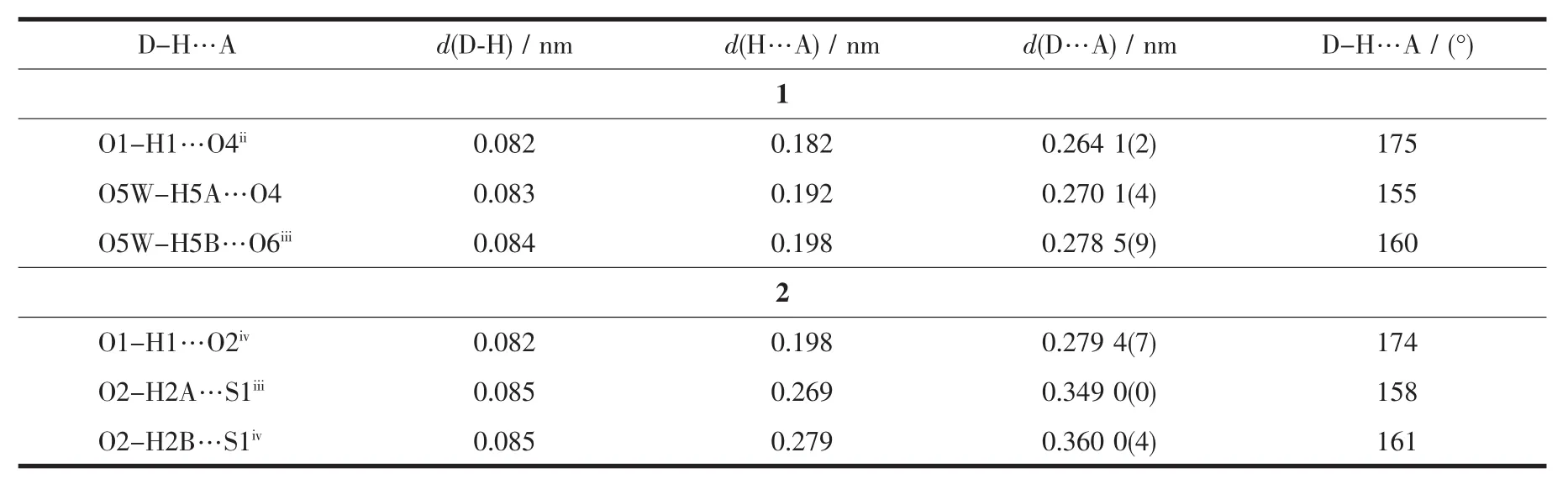
Table 3 Hydrogen-bonding parameters for 1 and 2
2 Results and discussion
2.1 Synthesis
Complexes 1 and 2 were successfully obtained as crystalline products by conventional evaporation method.Complex 1 was isolated in mixed methanol-DMF and the pH value of the resulting mixture was adjusted to 7.0 by triethylamine,while 2 was generated in mixed methanol-water solution without control the pH value.The lattice solvent molecule of 1 was slightly different from those in 2.Thus,it can be concluded that the reaction medium is highly important for preparation of the target crystalline samples.
2.2 Descriptions of crystal structures
Single crystalX-ray diffraction revealsthat complex 1 crystallizes in the P1 space group,consisting of a polymeric one-dimensional(1D)chain with slightly distorted Niギoctahedra bridged by doubly deprotonated tp2-anions.The asymmetric unit of 1 contains one hexa-coordinated Niギion,two neutral hptrz ligands,two terminally coordinated water molecules,one doubly deprotonated tp2-dianion and two lattice DMF molecules.
Asshown in Fig.1a,thecrystallographically unique Niギion in 1 is six-coordinated by two triazolyl N atoms from two distinct hptrz ligands in the axial sites,and four O atoms from two carboxylate groups of two trans-bound tp2-anions and two water molecules in the equatorial plane,adopting an axially elongated octahedral geometry with the axial Ni-N distances slightly longer by than those of Ni-O bonds in the equatorial plane(Table 2).
The fully deprotonated tp2-anions bridge the adjacent Niギions in a bis-unidentate binding fashion,leading to a 1D linear chain running along the crystallographic b-axis.The intrachain Niギ … Niギdistance is 1.134 4(2)nm separated by tp2-anion.Additionally,there exist pairs of intrachain O-H…O hydrogen-bondinginteractionsbetween coordinated water molecule and carboxylate O donor of tp2-dianion,which help to consolidate the 1D chain structure of 1(Fig.1b).
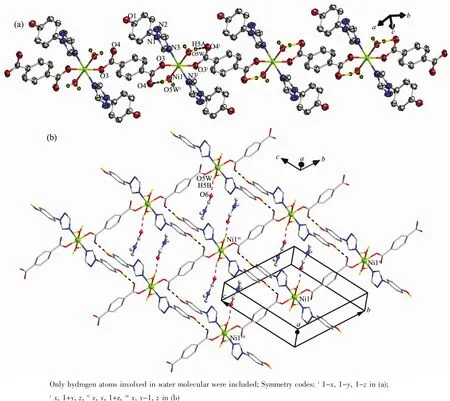
Fig.1 Crystal structure of 1:(a)Polymeric 1D chain of 1 with atomic labels in the asymmetric unit showing 60%probability displacement ellipsoids;(b)2D supramolecular sheet of 1 with lattice DMF molecules encapsulated in the grids
In addition to presenting the triazolyl N donor to coordinate with the Niギ atom in a commonly observed monodentate mode,the neutral hptrz ligand can also provide the uncoordinated phenolic OH group as a hydrogen-bond donor to produce interchain O-H…O H-bonding interaction with the carboxylate group of tp2-dianion from the adjacent chain,which assemble the 1D chains into a supramolecular 2D grid-based layer (Fig.1b and Table 3).Two lattice DMF molecules are encapsulated in the grids through O-H…O hydrogen-bonding interactions.
Complex 2 exhibits a centrosymmetric mononuclear structure,similar to that of[Cu(C2N3)2(C8H7N3O)2]reported previously[21e].As shown in Fig.2a,the sole Cuギatom in 2 is coordinated to four N atoms,in which two N donors are from two symmetry-related neutral hptrz and the others belong to a pair of isothiocyanate anions.The cis N-Cu-N bond angles are 89.89(6)°and 90.11(6)°,respectively,indicating a slightly distorted square planar environment.Possessing potentially versatile binding modes with different metal ions[25],the isothiscyanate anion herein acts simply as a terminal ligand to coordinate with Cuギcenter through nitrogen atom with the separations of Cu-N of 0.195 43(15)nm,which is shorter by 0.007 nm than that of Cu-Nhptrz(Table 2).The different bond lengths indicates the variable binding strength of the two mixed ligands upon coordinating with the Cuギion,which can influence the compositional stability of the resulting complex.Thus,the lattice water molecules play important roles for the periodic arrangement of the mononuclear entity,in which two types of hydrogenbonding interactions(O-H…O and O-H…S)involved the lattice water molecule were observed(Fig.2b).On one hand,lattice water molecule acts as H-bond acceptor,producing O-H…O H-bonding interaction with phenolic group formed with donor-acceptor distance of 0.279 4(7)nm.On the other hand,water molecule can also participate two O-H…S H-bonding interactions with two isothiscyanate anions with O…S distances of 0.349 0(0)and 0.360 0(4)nm,respectively.Thus,acting as a triple bridge,the lattice water molecules link the[Cu(hptrz)2(SCN)2]units into a 2D wave-like layer in the crystallographicbc plane,and the nearest nonbonding Cuギ…Cuギ distances are 1.021 7(8)nm and 1.189 5(9)nm along b and c directions.
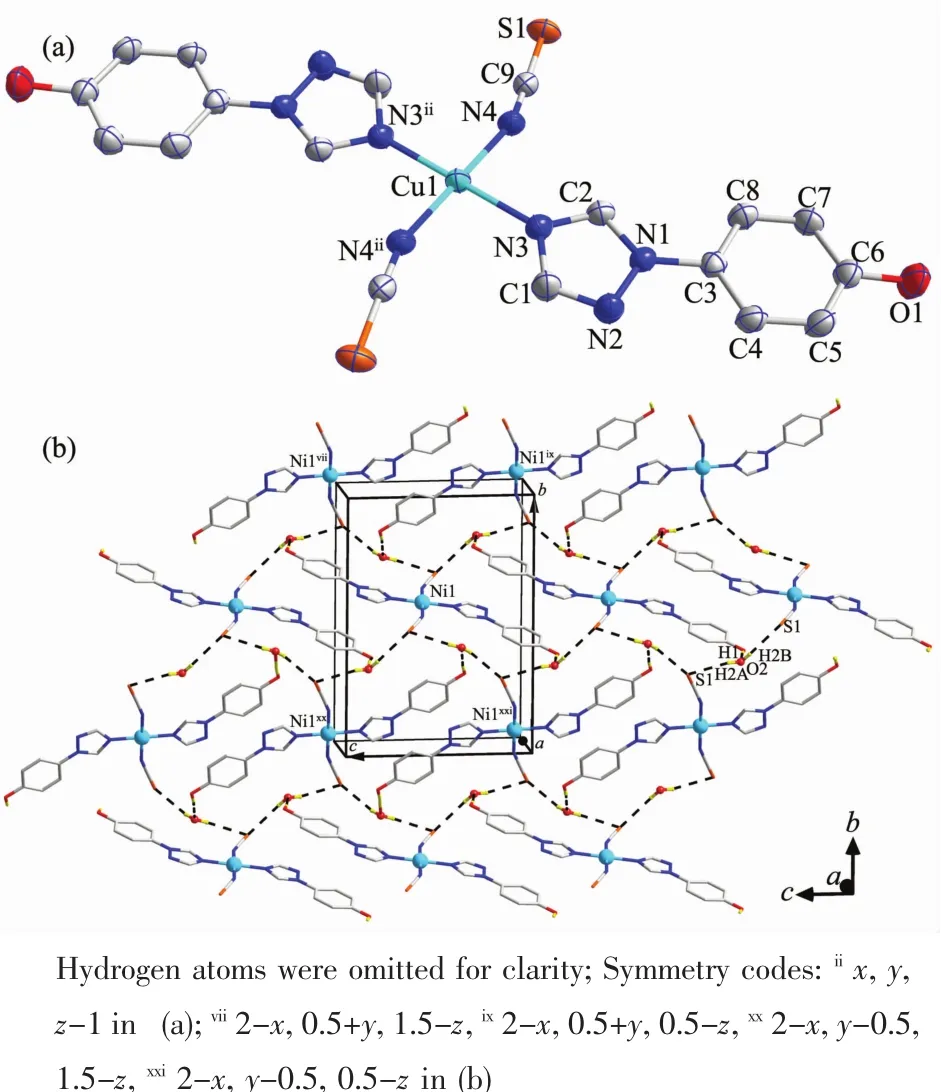
Fig.2 (a)Mononuclear structure of 2 showing 60%probability displacement ellipsoids;(b)2D layer of 2 formed by hydrogen-bonding interactions between mononuclear entities and lattice water molecules
2.3 IR spectra
In FT-IR spectra,the broad and strong absorption band appeared at 3 434 cm-1for 1 and 3 464 cm-1for 2 should be respectively ascribed to stretching vibration of the O-H,confirming the presence of water and phenolic group of hptrz ligand.The medium bands appeared at 800~1 400 cm-1in both 1 and 2 should be associated with triazole ring vibrations of hptrz ligand[26,34].The absence of the band at 1 681 cm-1in 1 suggests the full deprotonation of H2tp ligands[26].Strong characteristic bands for the asymmetric (1 659 and 1 562 cm-1)and symmetric stretching vibrations(1 439 and 1 385 cm-1)of the carboxylate group can be observed in the spectrum of 1.For 2,the very strong band centering at 2 099 cm-1and the weak band locating at 468 cm-1correspond to the N-bonded terminal isothiocyanate groups[27].Thus,the results of FT-IR spectra are in well agreement with those of crystal structure determinations.
2.4 Thermal analysis
To investigate the contribution of the co-ligand on the thermal stability of the resultant complexes,the thermogravimetric study of the complexes 1 and 2 were performed at an inert atmosphere from room temperature to 800℃ (Fig.3).Complex 1 can be thermally stable below 108℃and was followed by an obvious weight-loss stage between 108 and 426℃.Once the temperature was beyond 426℃,the decomposition of 1 finished completely,leaving NiO as final residue(Obsd.9.9%,Calcd.10.3%).The first weightloss stage of 2 was between 107 and 139℃,corresponding to the loss of lattice water molecules(Obsd.7.0%,Calcd.6.7%).Owing to the decomposition of hptrz and SCN ligands,the mononuclear entity of 2 began to decompose at 163℃,leaving CuO as final residue beyond 678℃(Obsd.14.0%,Calcd.14.8%).
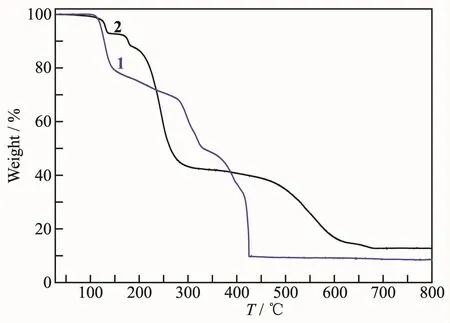
Fig.3 TGA curves for 1 and 2
2.5 Fluorescent emissions
Fluorescent emission spectra of 1 and 2 in aqueous solution were measured at room temperature.As presented in Fig.4,the complexes displayed similar broad emissions centered at 422 nm upon excitation at 370 nm.To understand the nature of the charge transition,the luminescence properties of the free hptrz ligand under the same measurement conditions was also recorded for comparison.As a result,the neutralhptrzligand displaysa relatively strong emission band centered at 421 nm.Thus,the analogous emissions between hptrz and the complexes indicated that the emission were originate from π-π*transfer of the core hptrz ligand,which is also similar with many fluorescentcomplexes based on the fluorescent ligands[10].
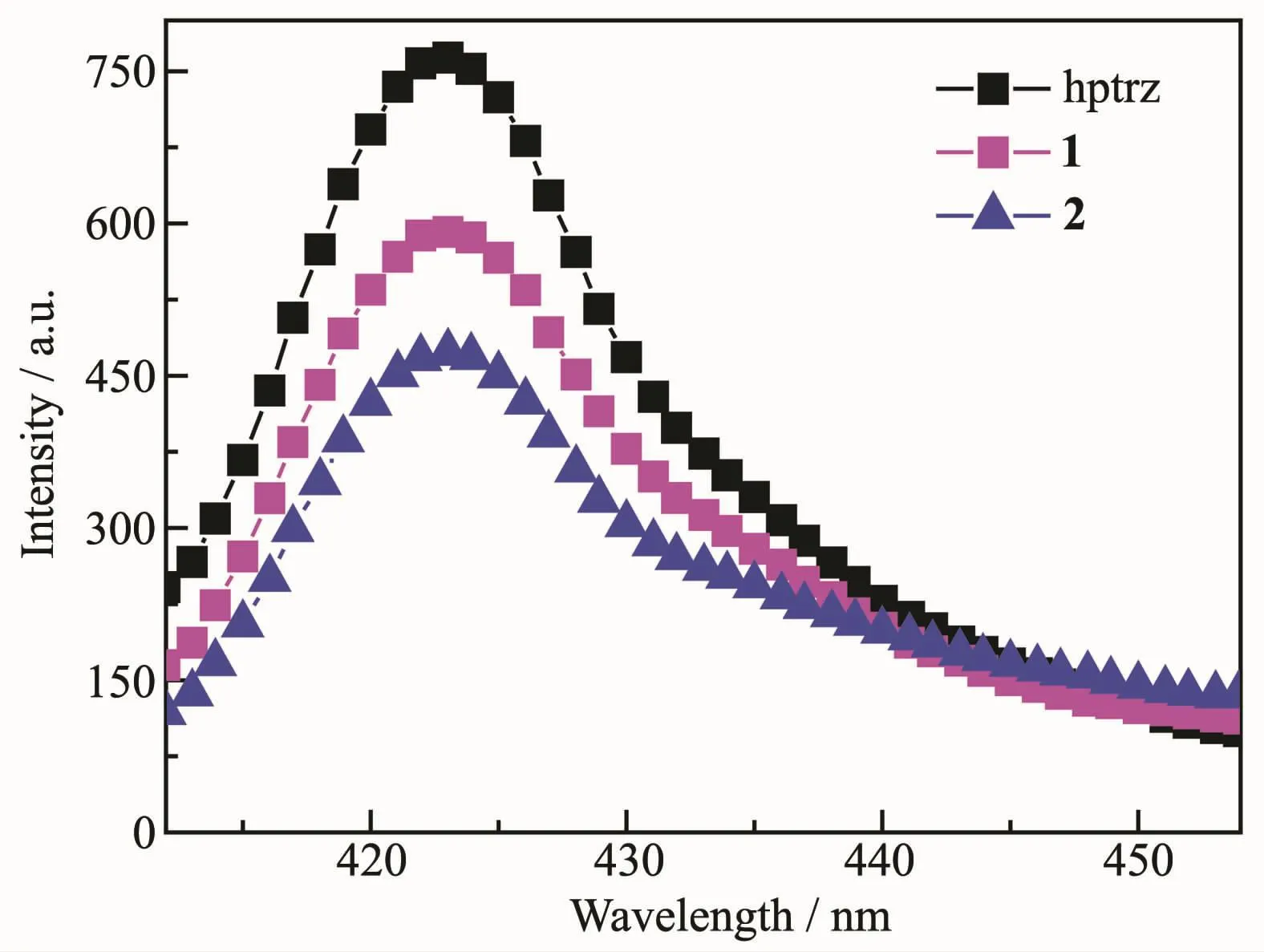
Fig.4 Fluorescence emission spectra of 1,2 and free hptrz in aqueous solution measured at room temperature
3 Conclusions
In summary,two new hptrz-based metal complexes were obtained by varying the transition metal ions and different co-ligands,exhibiting slightly bent 1D chain with slightly distorted Niギoctahedra extended by ditopic benzene-1,4-dicarboxylate connectors(1)and centrosymmetric mononuclear structure(2).Their structural difference is significantly directed by the tp2-and SCN-anion,rather than the terminal hptrz ligand.Owing to hptrz-based intraligand charge transfer,the two complexes show similar fluorescent emissions at room temperature.Thus,the high stability and strong emission of the two target complexes in the solid state firmly ensure their potential applications as fluorescent materials.
Supporting information is available at http://www.wjhxxb.cn

