两个基于苯并咪唑还原席夫碱的双核铜ギ配合物的合成、晶体结构和电化学性质
赵海燕 李 娜 杨晓东
(河北科技大学理学院,石家庄 050018)
0 Introduction
Binuclear copperギcomplexes are of ongoing interest due to their interesting magnetic,catalytic,electrochemical properties and a wide variety of biological applications as catechol oxidase,antibacterial species,DNA binding and cleaving agents[1-11].Among these binuclear complexes,the phenolate bridged binuclear copper complexes with tunable stereochemistry are often five-coordinated in which Cuギions are in distorted square pyramid with different Addison parameter(τ).Structural properties of the Cu2O2core,such as the coordination geometry of the copper ions,the Cu…Cu distances,Addison parameter(τ)and torsion angles have been postulated to influence the spectral and electrochemical properties of the binuclear copper complexes,which offer a great scope of design for species that are suitable for magnetic,catalytic properties and biological activities[3-4,12-14].Hence,it would be interesting to find a relationship between the geometries and properties of newly designed phenoxo bridged binuclear copper complexes.
Herein,we report the synthesis,crystal structures,and electrochemicalproperties oftwo binuclear copper complexes[CuL(CH3OH)]2(BF4)2(1)and[CuL(NO3)2]2(2)of the newly designed reduced Schiff base ligand HL(HL=2-(((2-(2-benzimidazyl)ethyl)aimino)methyl)phenol).The ligand HL is considered to be more flexible compared to the Schiff base due to the reduction of the rigid azomethine(-CH=N-)fragment to less constrained-CH2-NH-moiety[15]and therefore has the potential to form binuclear complexes through a bridging phenolate group.
1 Experimental
1.1 Materials and physical measurement
All chemicals were of reagent grade and used as received.2-(Aminoethyl)-benzimidazole dihydrochloride was performed by a method described by Cescon et al[16].FT-IR spectra(KBr pellet)were obtained on a FT-IR 170 SX (Nicolet)spectrometer in the range of 4 000~400 cm-1.Elemental analyses were taken using a Perkin-Elmer 240C analyzer.1H NMR was performed on a Brucker Avance 500MHz spectrometer using trimethyl silicon as internal standard.The electronic absorption spectra UV-Vis spectroscopy were recorded on a spectrophotometer using DMF as solvent.Cyclic voltammograms were run on a CHI model 750B electrochemical analyzer in a DMF solution containing tetrabutylammonium perchlorate (TBAP)as the supporting electrolyte.A three-electrode cell was used,which was equipped with a glassy carbonworking electrode,a platinum wire as the counter electrode and a saturated Ag/AgCl electrode as the reference electrode.
1.2 Synthesis of HL
The ligand was synthesized by a condensation reaction between 2-(aminoethyl)-benzimidazole dihydrochloride(3.16 g,13.5 mmol),previously neutralized with NaOH(1.08 g,27 mmol),and salicylaldehyde(1.4 mL,13.5 mmol)in 75 mL of methanol.The reaction mixture was refluxed for approximately two hours and then reduced by slow addition of NaBH4(0.5 g,13.5 mmol)at 0℃.The reaction mixture was concentrated under reduced pressure and the product was extracted with three portions of CHCl3(60 mL).The organic extracts were combined,washed with brine,dried over Na2SO4,filtered and concentrated underreduced pressure resulting in a white powder.Yield (2.56 g,68%).m.p.147~149 ℃.Anal.Calcd.for C16H17N3O(%):C,71.89;H,6.41;N,15.72.Found(%):C,71.52;H,6.37;N,15.96.1H NMR:δ 7.21~7.55(4H,CHbenzim),6.76~7.20(4H,CHar),3.99~4.04(2H,N-CH2),3.08~3.19(2H,CH2).IR(KBr,cm-1):3 316(m),2 855(w),1 598(s),1 455(s),1 275(s),1 094(m),1 031(m),922(m),751(s),617(w).
1.3 Syntheses of the complexes
1.3.1 Synthesis of[CuL(CH3OH)]2(BF4)2(1)
A solution of HL(26.6 mg,0.1 mmol)and NEt3(0.014 mL,0.1 mmol)in 7 mL methanol was added to a solution of Cu(BF4)2·6H2O(34.5 mg,0.1 mmol)in 3 mL water.The resulting dark green solution was stirred for two hours at room temperature.Crystals suitable for X-ray structural analysis were obtained by slow evaporation of the solvent at 4℃.Yield:65%.Anal.Calcd.for C17H20BCuF4N3O2(%):C,45.51;H,4.49;N,9.36.Found(%):C,45.62;H,4.52;N,9.49.IR(KBr,cm-1):3 647(m),3 120(w),1 599(s),1 545(m),1 483(s),1 457(s),1 419(w),1 394(w),1 293(s),1 251(s),1 083(s),880(m),854(m),764(s),753(s).
1.3.2 Synthesis of[CuL(NO3)2]2(2)
A solution of HL(26.6 mg,0.1 mmol)and NEt3(0.014 mL,0.1 mmol)in 5 mL methanol was added to a solution of Cu(NO3)2·3H2O(24.1 mg,0.1 mmol)in 5 mL methanol.The resulting dark green solution was stirred for two hours at room temperature.Crystals suitable for X-ray structural analysis were obtained by slow evaporation of the solvent at room temperature.Yield:47%.Anal.Calcd.for C16H16CuN4O4(%):C,49.05;H,4.12;N,14.30.Found(%):C,49.39;H,4.06,N,14.21.IR(KBr,cm-1):3 119(w),1 598(s),1 484(m),1 456(m),1 384(s),1 256(m),1 025(w),857(w),751(s).
1.4 X-ray crystallography
Diffraction intensities for complexes 1 and 2 were collected on a Bruker Smart 1000 CCD area detector using graphite-monochromatized Mo Kα radiation(λ=0.071 073 nm)with φ-ω scan mode at 293(2)K.Unit cell dimensionswereobtained with least-squares refinements and semi-empirical absorption corrections were applied using SADABS program[17].For complex 1 all fluorine atoms from BF4-are disordered and were refined isotropically with occupancy with 0.6 and 0.4.All the structures were solved by direct method and non-hydrogen atomswere obtained in successive difference Fourier syntheses.All the structures were solved by direct method and the refinements were performed by full-matrix least-squares methods on F2with SHELXTL program package[18].Hydrogen atoms were included in calculated positions and refined with fixed thermal parameters riding on their parent atoms.The crystal data and structure refinement parameters of complexes are listed in Table 1 while Table 2 lists selected bond distances and angles.
CCDC:2276305,1;2276307,2.
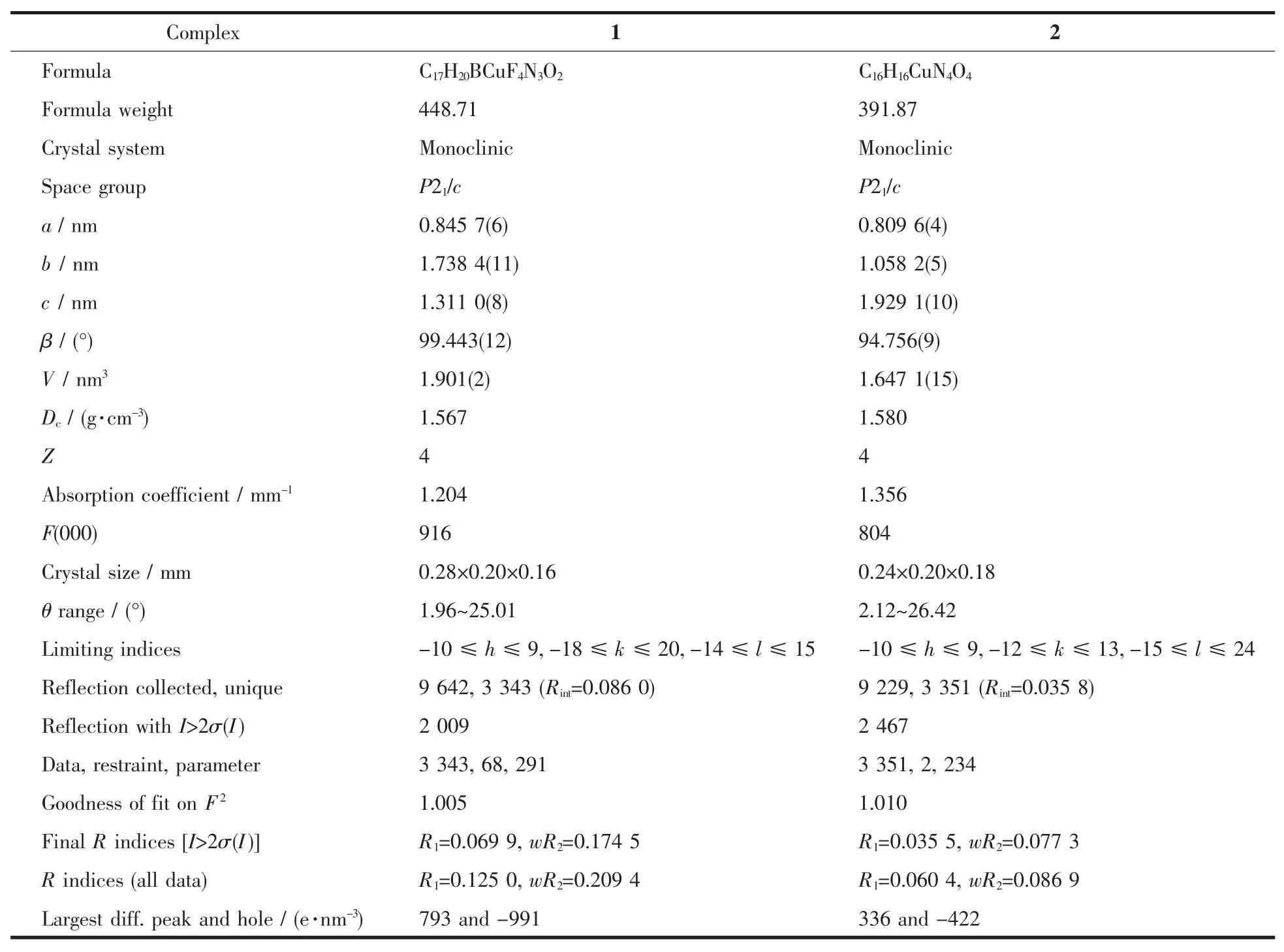
Table 1 Crystal data and structural refinement parameters for complexes 1 and 2
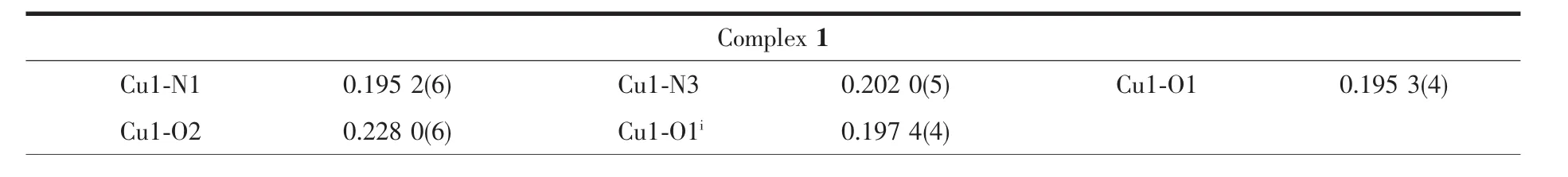
Table 2 Selected bond distances(nm)and angles(°)for 1 and 2
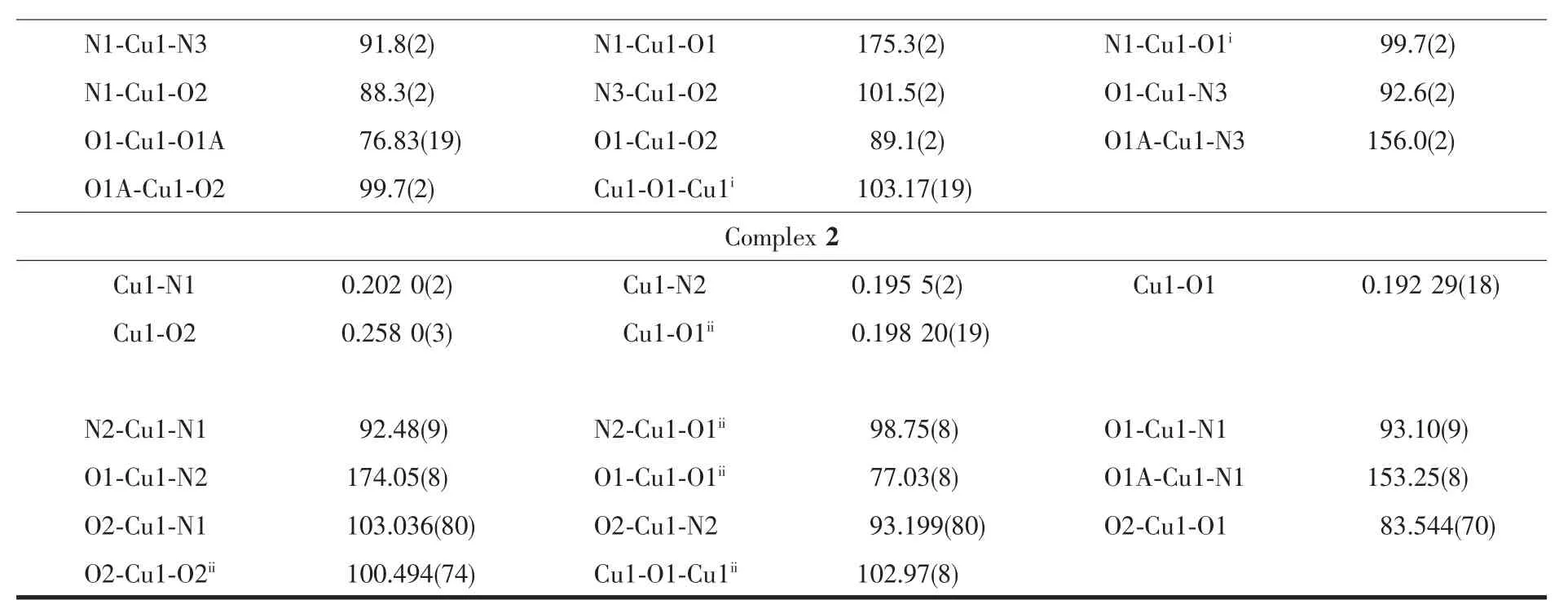
Continued Table 2
2 Results and discussion
2.1 Synthesis and characterization
The condensation of 2-(aminoethyl)-benzimidazole dihydrochloride in 1∶1 molar ratio with salicylaldehyde afforded the Schiff bases,N-salicylidine-2-aminoethylbenzimidazole which on reduction with sodium borohydride readily produced the reduced Schiff base,HL.HL on reaction with copperギtetrafluoroborate hexahydrate and copperギnitrate trihydrate in 1:1 molar ratios yielded complexes 1 and 2,respectively(Scheme 1).Unlike its Schiff base counterpart,the reduced Schiff base has flexible backbones.Two phenolic oxygen atoms bind two metals to form a dimer,in which one four-membered ring,four six-membered rings are formed as shown in Scheme 1.
The characteristic IR bands(4 000~400 cm-1)for the free ligand,when compared with those of its copperギcomplexes,provided positive indications with regard to the bonding sites of the ligand.The presence of one medium intensity broad band between 2 500 and 3 200 cm-1in the free ligand hints toward the existence of hydrogen bonding between NH of benzimidazole and other electronegative atoms[19].The broad bond remains almost unchanged in the two complexes,indicating that the N-H of benzimidazole ring does not participate in the coordination[20].In the spectrum of the free ligand the band of the stretching vibrational modes of the phenolic OH around 3 316 cm-1,disappeared from the spectra of the two complexes indicating the deprotonation of the ligand.Additionally,the bands originating from the C-O stretching vibrations at 1 205 cm-1,in the complexes exhibited positive shifts at 1 251 cm-1for complex 1 and 1 256 cm-1for complex 2,respectively,denoting coordination through the phenolic oxygen of the ligand[21].Furthermore,the reduction of the imine group is very clearly indicated by the absence of the strong band due to imine vibration which appeared in the region of 1 620~1 650 cm-1for the free ligand and the two complexes[22].Several new bands presented in the region 400~600 cm-1in the spectra of the complexes were assigned to the Cu-N and Cu-O stretching vibrations[23].
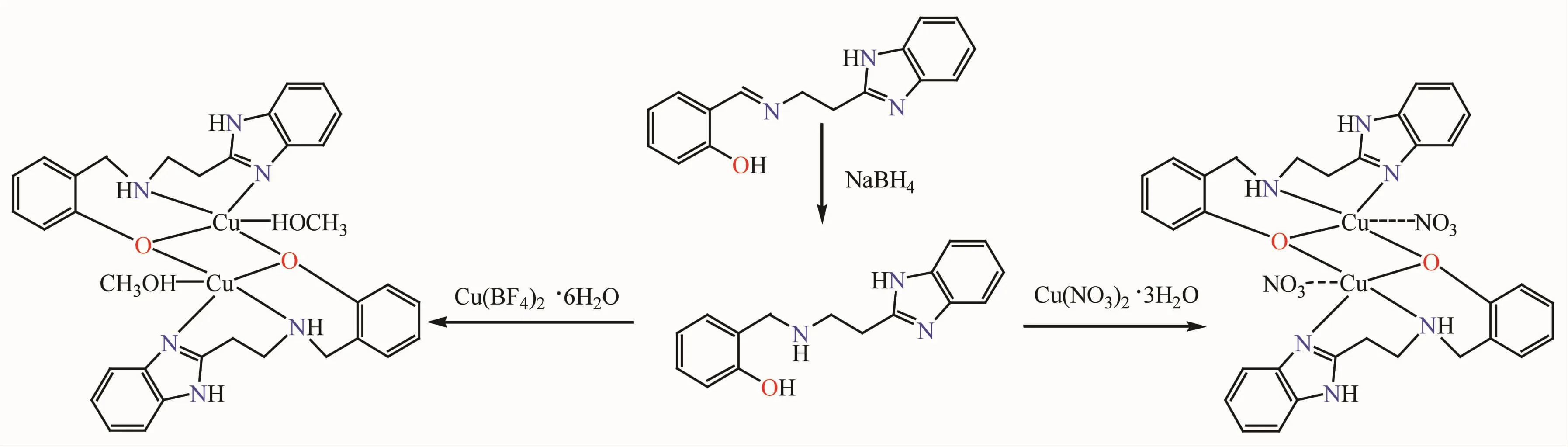
Scheme 1 Synthesis of the reduced Schiff base ligand and the complexes 1 and 2
Characteristic stretching frequencies for the anions are observed.The presence of the bands at near 1 100 and 522 cm-1in complex 1 confirmed the presence of non-coordinated tetrafluoroborate anions and point to a lack of strong deviation from tetrahedral symmetry[24].Furthermore,the BF4-group vibration near 1 083 cm-1splitting into a peak at 1 083 cm-1and a shoulder at 1 063 cm-1approximately,indicated BF4-anions involved hydrogen bonding,which is consistent with the crystal structures[25].Appearance of a strong sharp band at 1 384 cm-1demonstrated the presence of nitrate in complex 2[26].
The electronic absorption spectra of the ligand HL and the two complexes in DMF were recorded at room temperature.UV bands at 275 and 285 nm were observed for the free ligand HL,which are characteristic of the benzimidazole group and arise from a ππ*transition.These were blue-shifted upon coordination and were observed at 272 and 278 nm for the two complexes[27],respectively.The complexes display a broad d-d band in the region of 560~680 nm.The position of this band suggests that the geometry about each Cuギatom is best described as square pyramidal[28].In addition,one charger transfer band due to a LMCT transition between the bridging phenoxo and Cuギ atoms in the region of 400~420 nm was also observed.Similar UV-Vis features have been previously observed for several related phenolate bridged binuclear copperギcomplexes[29].
2.2 Crystal structure
Fig.1 gives the crystal structures of 1 and 2 with atomic labeling scheme,respectively.X-ray diffraction studies revealed that both complexes 1 and 2 crystallize in monoclinic system with P21/c space group.In complexes 1 and 2,two phenoxo groups bridge two Cuギatoms giving the binuclear structure,containing an exactly planar Cu2O2core owing to the crystallographic inversion symmetry.The stereochemistry around each Cuギis best described as a distorted square pyramidal with a value of τ being 0.31 for 1 and 0.35 for2,respectively.The equatorialpositionsare occupied by benzimidazolyl nitrogen atom,the amine nitrogen atom,phenolate oxygen atom and the bridging phenoxo oxygen atom from the symmetry related molecule.The Cu-O bridge distances are in the range of 0.192 29(18)~0.198 20(19)nm,with Cu1-O1-Cu1ibridging angles of 103.17(19)°for 1 and Cu1-O1-Cu1iiof 102.97(8)°for 2,falling within the normal range for diphenoxo-bridged copperギ complexes[3-4,12-14].The average Cu-Nimineand Cu-Nbenzimidazolebond lengths are 0.202 0 and 0.195 4 nm,which are similar to the Cu-N bond distance found for similar copper/reduced Schiff system and copper/benzimidazole system,respectively[27,30].In 1,the axial position is occupied by oxygen O2 of the monodentate coordinating methanol molecule with the Cu-O2 distance of 0.228 0(6)nm,suggesting a weak axial interaction.While in 2,the fifth position is occupied by a nitrate oxygen at a semi-coordination distance of 0.258 0 nm for Cu1-O2,Equatorial bond angles deviate from the expected value of 90°,the largest deviation being 13.17°,and the sum of the equatorial angles at Cuギ are nearly 360°for both 1 and 2.The Cu…Cu distances between the two metal ions are 0.308 5 nm for 1 and 0.305 6 nm for 2,which are almost close to other symmetric bis(μphenoxo)bridged binuclear copperギcomplexes[3-4,12-14].
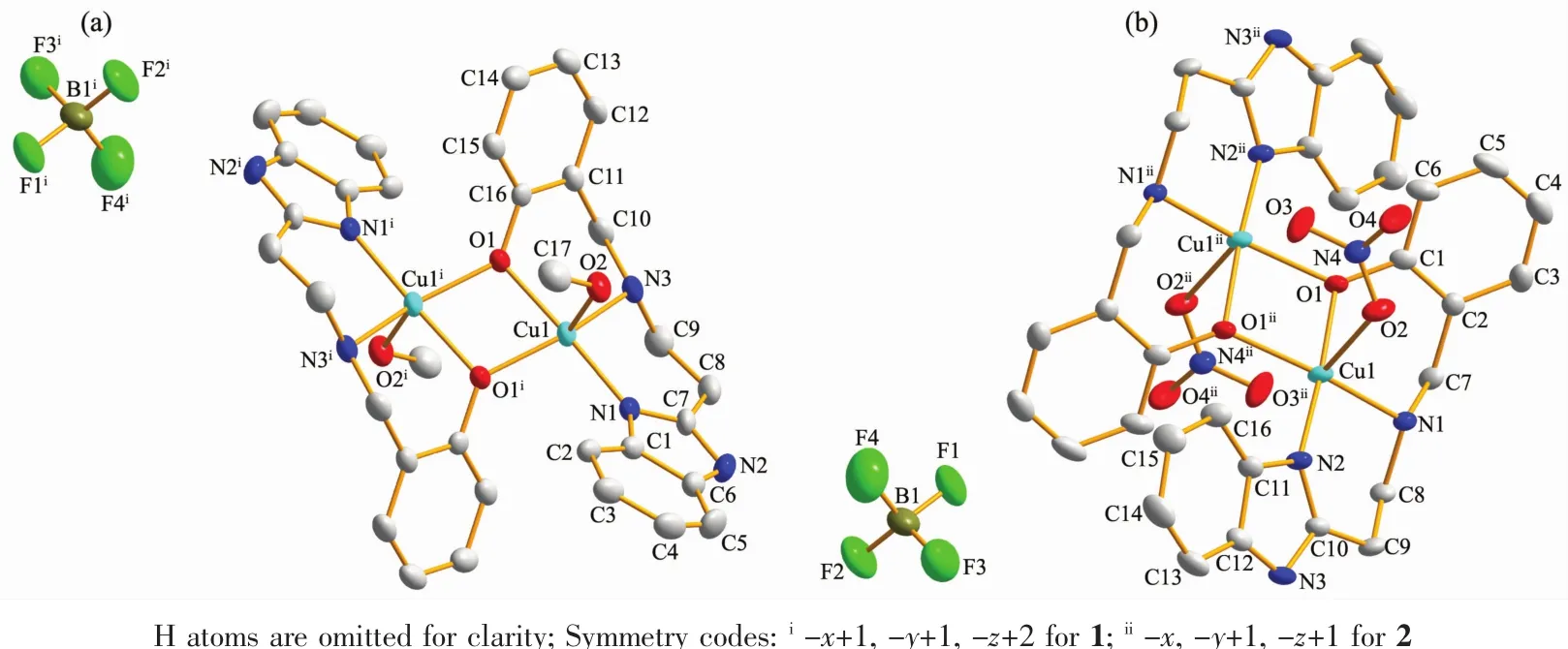
Fig.1 Molecular structures of 1(a)and 2(b)with 30%probability level along with the atom numbering scheme
In complex 1,the binuclear moieties are linked with pairs of tetrafluoroborate anions by hydrogen bonds into 1D chain (Fig.2).Each tetrafluoroborate acts as hydrogen bond acceptor and forms two hydrogen bonds with one methanol molecule and one benzimidazole NH group(N2-H2A…F4 and O2-H2…F1iii,Symmetry codes:iiix,-y+1,-z+1).The interatomic distances of N2…F4 and O2…F1iiiare 0.280 5 and 0.288 9 nm,respectively,both being in the range of moderate hydrogen bond distances.The H…F separations are 0.212 3 and 0.213 1 nm and hydrogen bond angles are 135.89°and 137.96°,respectively.
In complex 2,each nitrate acts as hydrogen bond acceptor and forms two hydrogen bonds with one amine group(N1-H1…O2iv,Symmetry codes:iv-x+1,-y+1,z+1)and one benzimidazole NH group (N3-H3A…O4v,Symmetry codes:vx,0.5-y,z-0.5)(Fig.3a).The interatomic distances of N1…O2ivand N3…O4vare 0.315 6 and 0.288 5 nm,respectively.The H…O separations are 0.238 2 and 0.204 9 nm and hydrogen bond angles are 151.03°and 165.33°,respectively.As a result,a 3D hydrogen-bonded network is formed(Fig.3b).
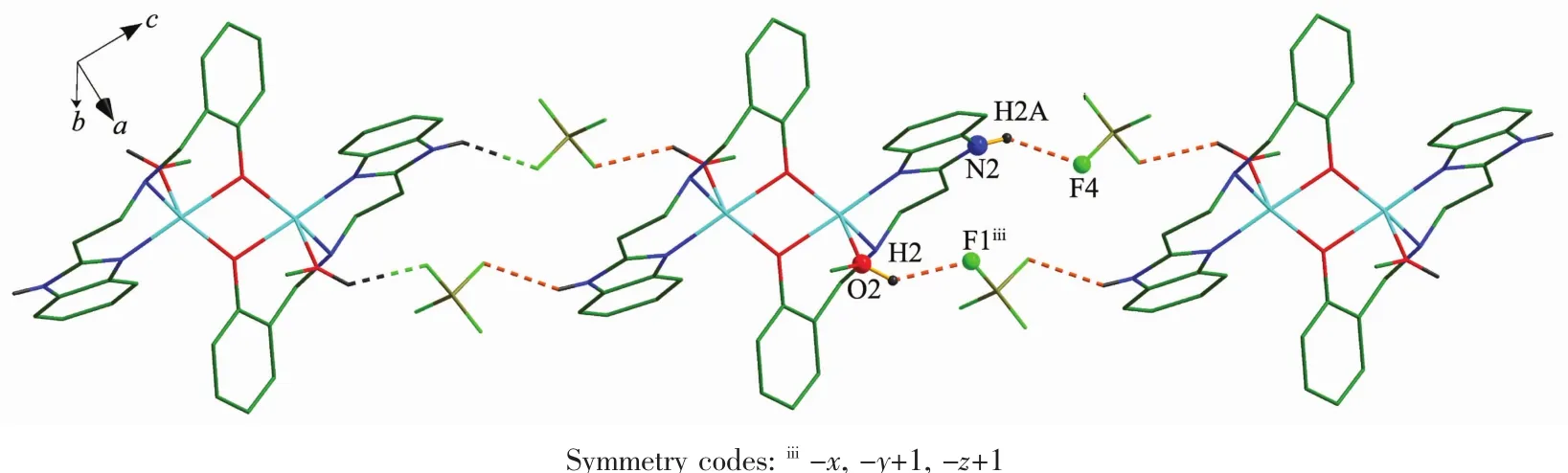
Fig.2 One dimensional chain in 1 formed through N-H…F and O-H…F hydrogen-bonding interactions along the a-axis
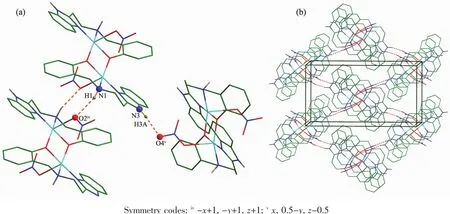
Fig.3 (a)Packing diagram showing the hydrogen-bonded interactions in 2;(b)3D framework for 2 built by hydrogen bond
2.3 Electrochemical characterization
Cyclic voltammetric studies were carried out for the copperギ complexes at 1 mmol·L-1concentration,dissolved in DMF containing 0.1 mol·L-1TBAP as a supporting electrolyte.The cyclic voltammetric data for the binuclear complexes 1 and 2 are shown in Table 3.The cyclic voltammograms (CV)of 1 and 2 were almost identical,suggesting similar environments around the Cuギions in 1 and 2 (Fig.4).This is consistent with the single crystal X-ray diffractionresults.The CV of the two complexes showed two quasi-reversible reduction waves at E11/2=-0.41 V and E21/2=-0.85 V for 1 and E11/2=-0.38 V and E21/2=-0.85 V for 2.The first process corresponds to the CuⅡCuⅡ⇌ CuⅡCuⅠreduction couple and the second process corresponds to the CuⅡCuⅠ⇌ CuⅠCuⅠreduction couple.The negative potentials observed in 1 and 2 are due to the factors such as the steric hindrance of the benzimidazole ring and the hard nature of the phenoxide atoms in the ligand,which will stabilize the copperギ oxidation state,making the Cuギ to Cuガconversion difficult.Similar observations are also reported for benzimidazole copper complexes and phenoxo copper complexes,which reduced in the range of-0.87~-1.40 V[31-33].
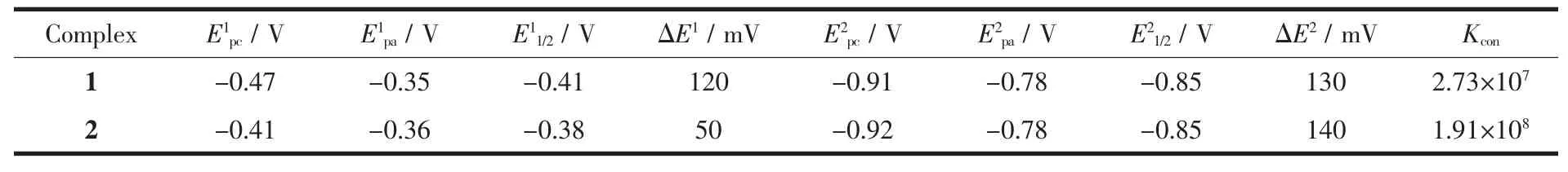
Table 3 Cyclic voltammetric data for 1 mmol·L-1solution of 1 and 2 in DMF containing 0.1 mol·L-1TBAP as supporting electrolyte
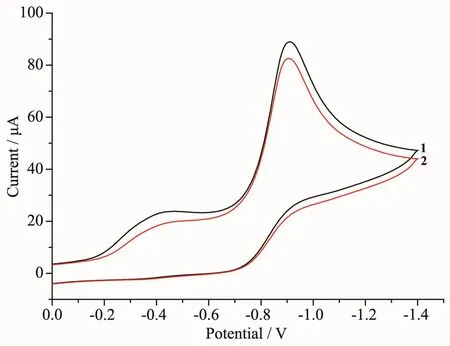
Fig.4 CV of the complexes 1 and 2 in DMF
The observed two well-separated one electron reductions for 1 and 2 can be substantiated from a stability consideration of the mixed-valence species,CuⅡCuⅠ.The stability of mixed valance form can be quantified by the conproportionation equilibrium constant(Kcon),lgKcon=16.9(ΔE1/2),where ΔE1/2=(E11/2-E21/2)[34].It is observed that the larger ΔE1/2is,the greater is the stability of the mixed-valence species with respect to conproportionation.The magnitude of the constant,Kconwas determined to be 2.73×107for 1 and 1.91×108for 2,respectively,which can be compared to the value of Kconfor phenoxo bridge copperギcomplexes[32].The large Kconvalues indicated that the addition of a second electron is more difficult than of the first electron and the CuⅡCuⅠmixed valence species is stable with respect to conproportionation[35].
3 Conclusions
In this work,one unsymmetrical tridentate Schiff base ligand has been reduced by NaBH4.The reduced ligand was more flexible compared to the Schiff base and used to form two phenoxo bridged binuclear copper complexes,which differ by the anions.The Addison parameters(τ)of both complexes indicated that the environment around the copper ions is a distorted square pyramidal geometry.The binuclear moieties are linked with pairs of tetrafluoroborate anions by the N2-H2A…F4 and O2-H2…F1iiihydrogen bonds in complex 1 while in complex 2 a 3D hydrogen-bonded network is formed by the N1-H1…O2ivand N3-H3A…O4vhydrogen bonds.The two complexes show two quasi-reversible one electron reduction processes in cyclic voltammetry.
———理学院

