基于功能化6-甲氧羰基-2,2′-联吡啶配体的铜ガ铁ギ异金属双核配合物
黄 榕 曾雪花 王万曼 张梦丽 陈景林*, 廖金生 刘遂军 温和瑞
(1江西理工大学冶金与化学工程学院,赣州 341000)
(2赣州师范高等专科学校自然科学与计算机系,赣州 341000)
0 Introduction
Much effort has been devoted to the development of the metal-based luminophors with the emission wavelengths in the entire visible region,particularly the late transition-metal emissive complexes[1-3],and the most representative examples are d6-and d8-metal complexes of the third-row transition metal series such as Os,Ir,and Pt[4-6].However,because of the high cost and limited availability of these noble metals,more interest has been paid to explore inexpensive alternatives.Recently,copperガcomplexes have received considerable attention, due to their promising potential in materials science and the high relative abundance and low toxicityofcoppermetalas compared to noble metal,such as Ir,Os,and Pt[7-16].In this context,Cuガheteroleptic complexes have been most widely investigated,particularly Cuガ-diiminephosphine complexes. 1,1′-Bis(diphenylphosphino)ferrocene(dppf)has been extensively used in catalysis and materials science as a very useful chelating or bridging ligand,and the introduction of dppf can bring the metal-based system desirable stability,steric bulk,and oxidizability[17].Thus,we expect to synthesize new CuガFeギ heterobimetallic complexes using dppf and functionalized 6-methoxycarbonyl-2,2′-bipyridine ligands.Herein,we will describe the synthesis and characterization of two new Cuガ heterodinuclear complexes with 1,1′-bis(diphenylphosphino)ferrocene(dppf)and functionalized 6-methoxycarbonyl-2,2′-bipyridine ligands(Scheme 1).
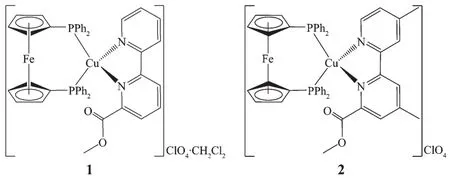
Scheme 1 Molecular structures of complexes 1 and 2
1 Experimental
1.1 Materials and measurements
Allreactions were performed under a N2atmosphere,using anhydrous solvents or solvents treated with an appropriate drying reagent.Commercially available reagents were used without further purification unless otherwise stated.[Cu(CH3CN)4]ClO4,6-methoxycarbonyl-2,2′-bipyridine(mbpy),and 6-methoxycarbonyl-4,4′-dimethyl-2,2′-bipyridine(mmbpy)were synthesized according to the literature methods[18-19].Infrared(IR)spectra were recorded on a Bruker Optics ALPHA FT-IR spectrometer using KBr pellets.C,H and N elemental analyses were conducted on a PerkinElmer model 240C elemental analyzer,where all the crystal samples are used after grinding and drying under vacuum.UV-Vis absorption spectra in CH2Cl2solution were measured on a Shimadzu UV-2550 spectrometer.Crystal structures were determined on a Bruker D8 QUEST diffractometer.
Caution!Perchlorate salts are potentially explosive and should be handled carefully in small amount.
1.2 Preparations of complexes 1 and 2
1.2.1 [Cu(mbpy)(dppf)]ClO4·CH2Cl2(1)
A solution of[Cu(CH3CN)4]ClO4(19.6 mg,0.06 mmol)and 1,1′-bis(diphenylphosphino)ferrocene(dppf)(33.3 mg,0.06 mmol)in CH2Cl2(3 mL)was stirred for 1 h at room temperature;mbpy (14.5 mg,0.06 mmol)was then added and this mixture was stirred for another 3 h.The solvent was evaporated to dryness at reduced pressure.The resultant residue was again dissolved in CH2Cl2,and slow diffusion of petroleum ether into the above solution gave yellow crystals of 1(51.8 mg,0.051 mmol,85%).Anal.Calcd.for C46H38ClCuFeN2O6P2(%):C,59.31;H,4.11;N,3.01.Found(%):C,59.01;H,4.23;N,3.21.IR(KBr,cm-1):3 454(m),3 056(w),2 952(w),1 732(s,-CO2CH3),1 633(w),1 592(m),1 480(m),1 436(s),1 322(m),1 284(m),1 250(m),1 198(w),1 157(m),1 093(vs,ClO4-),1 030(m),827(w),752(s),699(s),627(m),493(s).
1.2.2 [Cu(mmbpy)(dppf)]ClO4(2)
Complex 2 wasprepared according to the procedure for 1,using[Cu(CH3CN)4]ClO4(19.6 mg,0.06 mmol),dppf(33.3 mg,0.06 mmol),and mmbpy(14.8 mg,0.061 mmol).Yellow crystals were afforded by slow diffusion of petroleum ether into a CH2Cl2solution of 2(40.1 mg,0.048 mmol,80%).Anal.Calcd.for C48H42ClCuFeN2O6P2(%):C,60.08;H,4.41;N,2.92.Found(%):C,60.24;H,4.37;N,2.86.IR(KBr,cm-1):3845(w),3453(s),3055(w),2954(w),1735(s,-CO2CH3),1 479(m),1 436(s),1 389(w),1 344(m),1 267(m),1 222(s),1 161(m),1 094(vs,ClO4-),1 031(m),893(w),828(w),746(s),698(s),625(m),493(s).
1.3 X-ray crystallography
Single-crystal X-ray diffraction data for 1 and 2 were performed on a Bruker D8 QUEST diffractometer at room temperature using graphite-monochromated Mo Kα radiation(λ=0.071 073 nm).Structures were solved by direct methods and refined by full-matrix least-squares technique on F2using the SHELXL-97 software package[20].The heavy atoms were located from E-map and other non-hydrogen atoms were located in subsequent difference Fourier syntheses and refined with anisotropic thermal parameters on F2.The hydrogen atoms of the ligands and solvent molecules were generated theoretically onto the specific atoms and refined isotropically with fixed thermal factors.The crystallographic data and structure refinement details of 1 and 2 are provided in Table 1,and the selected bond lengths and angles are listed in Table 2.
CCDC:1835415,1;1835416,2.
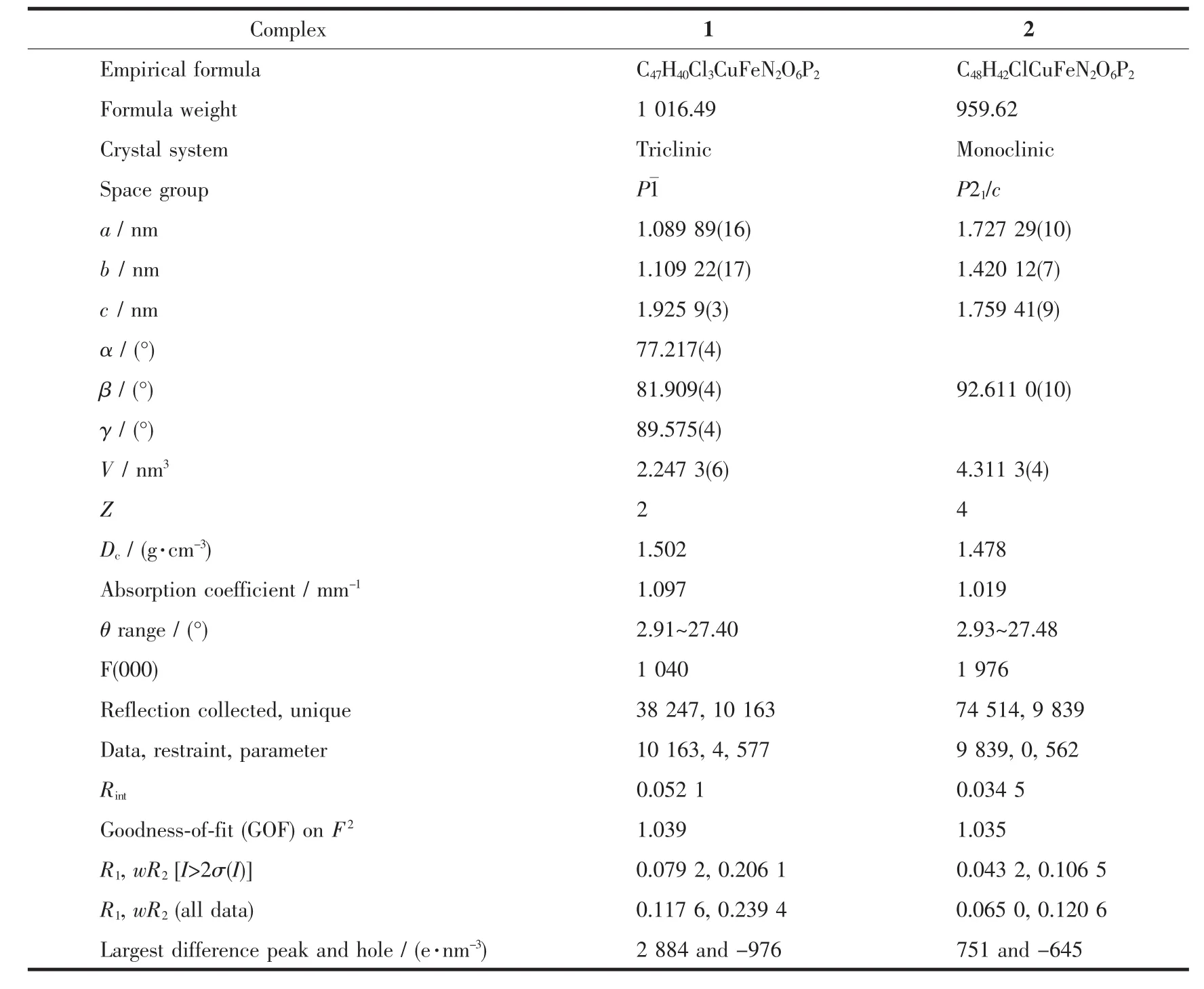
Table 1 Crystal data and structure refinement for 1 and 2
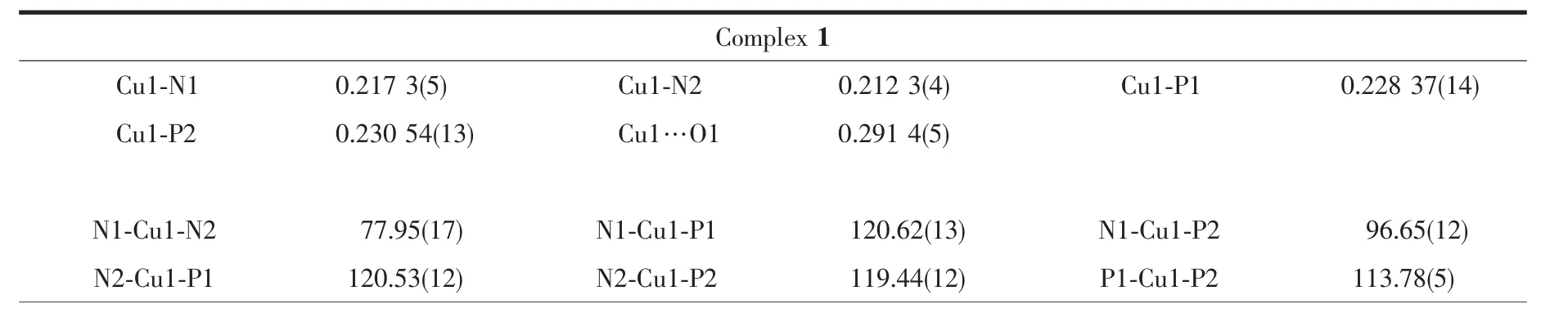
Table 2 Selected bond lengths(nm)and angles(°)for 1 and 2
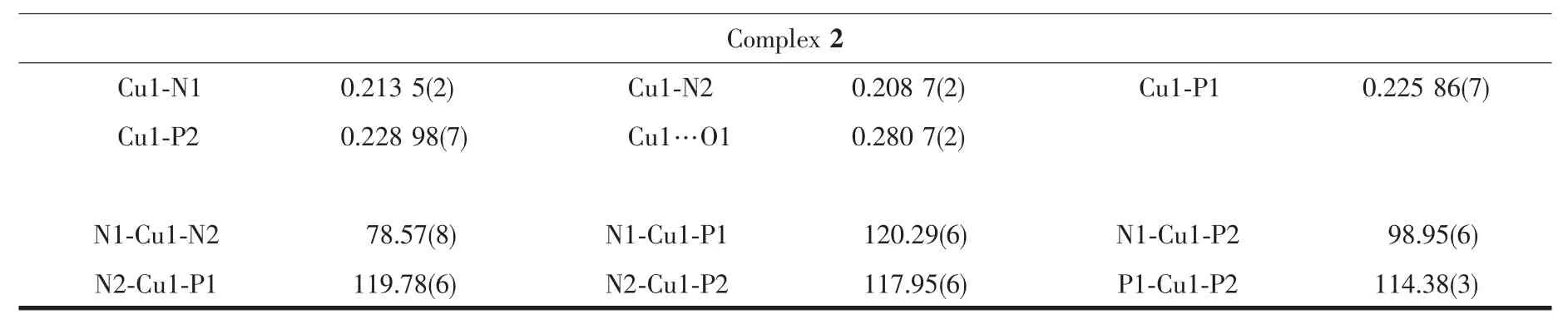
Continued Table 2
2 Results and discussion
2.1 Synthesis and characterization
To investigate the influence of 1,1′-bis(diphenylphosphino)ferrocene(dppf)and the introduction of two methyl groups into the 2,2′-bipyridyl ring on the structures and photophysical properties of Cuガcomplexes,two heterodinuclear Cuガcomplexes 1 and 2(Scheme 1)were synthesized by treating[Cu(CH3CN)4]ClO4with dppf and diimine (mbpy and mmbpy)ligands in a 1 ∶1 ∶1 molar ratio.In the IR spectra,a strong absorption peak is observed at 1 732 and 1 735 cm-1for 1 and 2,respectively,which originates from the carbonyl stretching vibration(νC=O)of the methoxycarbonyl group.Moreover,another strong absorption peak is also observed at 1 093 and 1 096 cm-1for 1 and 2,respectively,attributable to the Cl-O stretching vibration of the perchlorate anion.
The molecular structures of 1 and 2 were established by single-crystal X-ray crystallography.The molecular structures of the cations of 1 and 2 are shown in Fig.1.Complexes 1 and 2 crystallize in the P1 space group of triclinic crystal system and the P21/c space group of the monoclinic crystal system,respectively.In addition,complex 1 includes one disordered CH2Cl2solvent molecule,while complex 2 contains one disordered ClO4-anion.As depicted in Fig.1,the Cuガcations are all tetra-coordinated and in a distorted N2P2tetrahedral array formed by two N donors of the 2,2′-bipyridyl fragment and two P atoms of dppf,where the N-Cu-N bond angles are 77.95(17)°and 78.57(8)°and the P-Cu-P bond angles are 113.78(5)° and 114.38(3)°for 1 and 2,respectively,markedly deviating from the idealized bond angle of 109°28′.The Cu-N and Cu-P distances are within the normal range[21-31].The Cu1-N1 bond distances of 1(0.217 3(5)nm)and 2(0.213 5(2)nm)are slightly longer than the Cu1-N2 bond lengths of 1(0.212 3(4)nm)and 2(0.208 7(2)nm),owing to the addition of the methoxycarbonyl group.Moreover,the Cu-N bond lengths of 1(0.217 3(5)and 0.212 3(4)nm)are somewhat longer than those of 2(0.213 5(2)and 0.208 7(2)nm),consistent with the variation of the Cu1…O1 distance of 1(0.291 4(5)nm)and 2(0.280 7(2)nm),as a result of the introduction of two methyl groups into the 2,2′-bipyridyl ring.The Cu-N lengths of 1 and 2 are all longer than the Cu-N lengths of previously reported Cuガspecies with functionalized 6-alkoxycarbonyl-2,2′-bipyridine ligands[21-23],perhaps due to the significant influence of dppf introduced into the[Cu(N^N)]+unit.However,the Cu-P lengths of 1 and 2 are almost similar,implying that the introduction of the two methyl groups has a negligible influence on the[Cu(dppf)]+fragment.
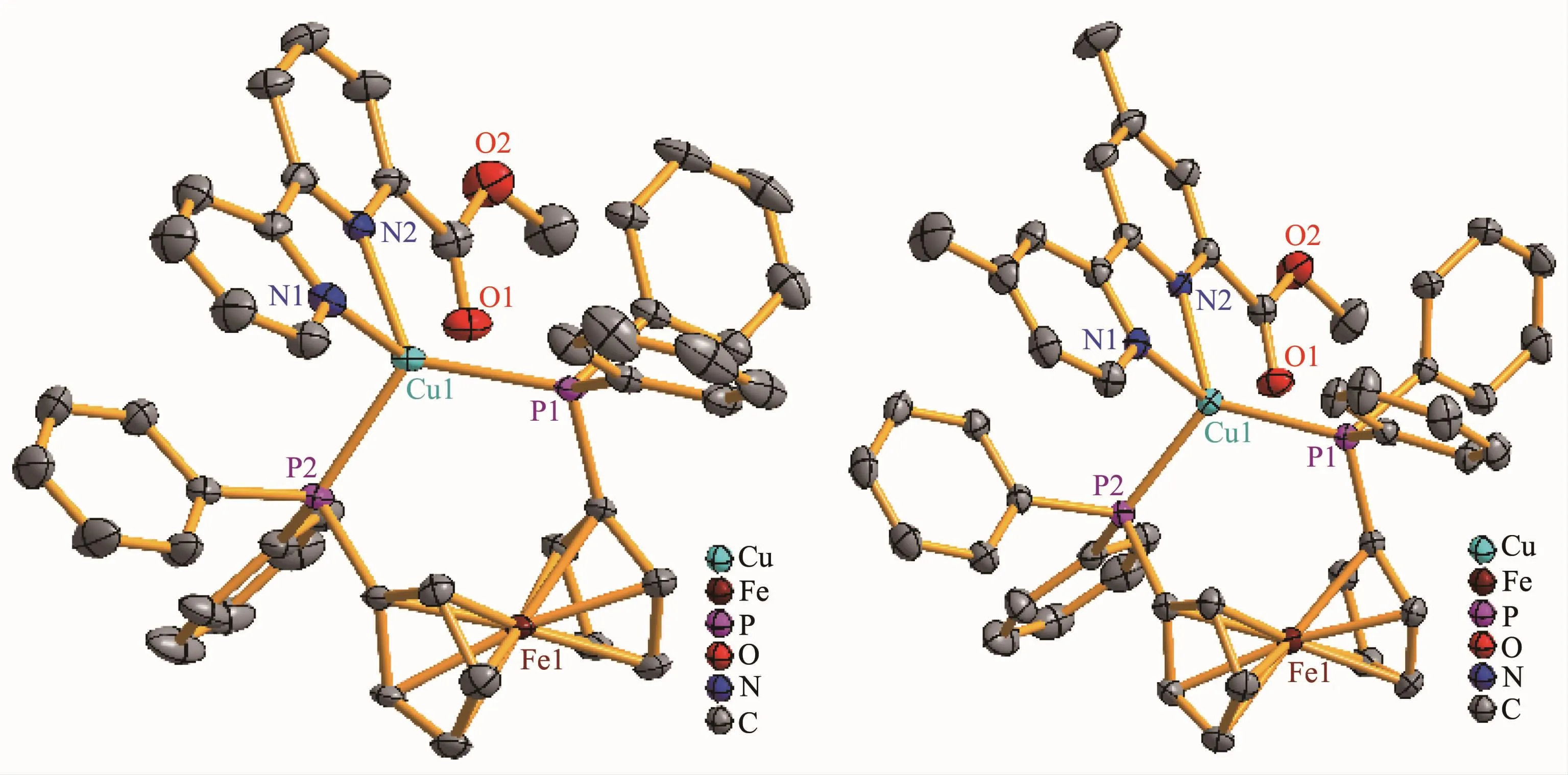
Fig.1 Molecular structures of the cations of 1(left)and 2(right)with 30%probability ellipsoids
2.2 Photophysical properties
The UV-Vis absorption spectra of 1 and 2 were measured in CH2Cl2solution at room temperature.As depicted in Fig.2,two CuガFeギ complexes exhibit a broad absorption band at 235~330 nm,which is assigned to the1π-π*transitions of functionalized 6-methoxycarbonyl-2,2′-bipyridine and dppf ligands.Moreover,a relatively weak broad absorption band(ε<104L·mol-1·cm-1)is also clearly observed in the range of 330~520 nm for 1 and 2,which can be tentatively ascribed to the charge transfer transitions with appreciable metal-to-ligand charge transfer(MLCT,Cuガ/Feギ→diimine)character[21-31].It is noted that the lowenergy absorption of 2(λmax=396 nm)is blue-shifted by 14 nm relative to that of 1(λmax=410 nm),which is attributable to the introduction oftwo electrondonating methyl groups into the 2,2′-bipyridyl ring,increasing the LUMO level and slightly affecting the HOMO level,and thus leading to a larger HOMOLUMO energy gap and a higher energy absorption of 2.Unfortunately,any detectable emission is unobserved in solution and solid states for 1 and 2 at room temperature,suggesting that the potentially luminescent MLCT state is possibly quenched by a photoinduced fast intramolecular energy transfer to the ferrocene unit[32].
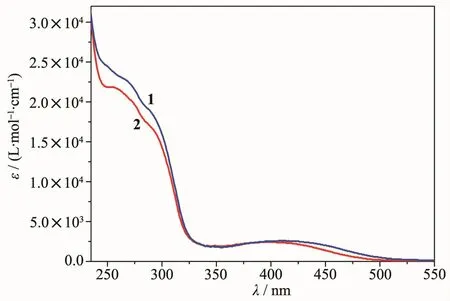
Fig.2 Absorption spectra of 1 and 2 in CH2Cl2solution
3 Conclusions
We have synthesized and characterized two new CuガFeギ heterobimetallic complexes with functionalized 6-methoxycarbonyl-2,2′-bipyridine and 1,1′-bis(diphenylphosphino)ferrocene ligands.It is revealed that a distorted tetrahedral N2P2geometry around Cuガis formed by two N donors of functionalized 6-methoxycarbonyl-2,2′-bipyridine and two P atoms of 1,1′-bis(diphenylphosphino)ferrocene.The two CuガFeギcomplexes are all fairly air-stable in solution and solid state at room temperature.No detectable emission is observed in solution and solid states at room temperature,possibly because the potentially emissive MLCT state is quenched by a photo-induced intramolecular energy transfer to the ferrocene unit.
Acknowledgements:This work was supported by the National Natural Science Foundation of China (Grants No.21561013,21501077),the Jiangxi Provincial Natural Science Foundation of China (Grants No.20171BAB203005,20161ACB21013,20171BCB23066),the MajorProjectof Jiangxi Provincial Education Department of China (Grant No.GJJ160597),the Foundation ofState Key Laboratory of Structural Chemistry (Grant No.20180019)and the Program for Qingjiang Excellent Young Talents of Jiangxi University of Science and Technology.

