Relationship of calcitonin gene-related peptide with disease progression and prognosis of patients with severe traumatic brain injury
Li-Xiong Chen, Wei-Feng Zhang, Ming Wang, Pi-Feng Jia,
1 Department of Critical Care Medicine, North Hospital of Ruijin Hospital, Shanghai, China
2 Department of Neurosurgery, North Hospital of Ruijin Hospital, Shanghai, China
Abstract Calcitonin gene-related peptide (CGRP) has been implicated in multiple functions across many bioprocesses; however, whether CGRP is associated with severe traumatic brain injury (TBI) remains poorly understood. In this study, 96 adult patients with TBI (enrolled from September 2015 to December 2016) were divided into a mild/moderate TBI group (36 males and 25 females, aged 38 ± 13 years) and severe TBI group(22 males and 13 females, aged 38 ± 11 years) according to Glasgow Coma Scale scores. In addition, 25 healthy individuals were selected as controls (15 males and 10 females, aged 39 ± 13 years). Radioimmunoassay was used to detect serum levels of CGRP and endothelin-1 at admission and at 12, 24, 48, 72 hours, and 7 days after admission. CGRP levels were remarkably lower, but endothelin-1 levels were obviously higher in the severe TBI group compared with mild/moderate TBI and control groups. Levels of CGRP were remarkably lower, but endothelin-1 levels were obviously higher in deceased patients compared with patients who survived. Survival analysis and logistic regression showed that both CGRP and endothelin-1 levels were associated with patient mortality, with each serving as an independent risk factor for 6-mont h mortality of severe TBI patients. Moreover, TBI patients with lower serum CGRP levels had a higher risk of death. Thus, our retrospective analysis demonstrates the potential utility of CGRP as a new biomarker, monitoring method, and therapeutic target for TBI.
Key Words: nerve regeneration; calcitonin gene-related peptide; severe traumatic brain injury; prognosis biomarkers; endothelin-1; mortality;dynamic serum levels; critical care medicine; neural regeneration
Introduction
Use of biomarkers to monitor and predict patient conditions is critical for prognosis in the field of critical care medicine.Traumatic brain injury (TBI), a common cause of death and disability among children and young adults in industrialized countries, often results from motor vehicle collisions, falls, or violence (Roozenbeek et al., 2013; Carney et al., 2017; Honeybul et al., 2017). TBI often involves a primary brain injury compounded by secondary brain injury that can cause uncoupling of blood flow and metabolism, leading to cerebral ischemia or hyperemia (Morales et al., 2005; Hirschi et al., 2017).Most TBI patients with blunt head trauma experience mild TBI, leading to transient confusion, disorientation, or loss of consciousness lasting for 30 minutes or less, and resulting in a mortality rate of less than 1% (Pomschar et al., 2013; Margulies et al., 2016). However, in severe TBI, which is defined as head trauma associated with a Glasgow Coma Scale (GCS)score ranging from 3 to 8, the mortality rate can be up to 40%(Andriessen et al., 2011; Cheng et al., 2017). As a result of the severity of symptoms and poor prognosis, management for severe TBI remains a challenge in the clinic.
During recent years, roles for inflammatory and vasoactive mediators in development of TBI have attracted scholarly interest. Indeed, altered levels of many small molecules in the brain or serum have been associated with TBI, such as endothelin-1 (ET-1) (Armstead and Kreipke, 2011), substance P(Carthew et al., 2012), and tumor necrosis factor (Khuman et al., 2011). Calcitonin gene-related peptide (CGRP), a 37-amino acid regulatory neuropeptide, has been implicated in multiple functions across a diverse array of bioprocesses. For example, CGRP has been shown to elicit beneficial cardiovascular effects including potent vasorelaxation and protection of cardiomyocytes and endothelial cells (Zhang et al., 2012).In addition, CGRP could reduce brain injury in a rat model of focal cerebral ischemia (Holland et al., 1994). Moreover, decreased levels of CGRP were also observed in cerebral blood vessels of patients who died from subarachnoid hemorrhage(Edvinsson et al., 1991).
However, as no previous study has focused on the clinical significance of CGRP in severe TBI, it has been unclear whether CGRP is associated with severe TBI. The present study investigated dynamic alterations of CGRP and ET-1 in severe TBI patients and demonstrated their correlation with patient mortality.
Subjects and Methods
Subjects
In this prospective observational cohort study, 96 adult patients diagnosed with TBI at North Hospital of Ruijin Hospital of China were included from September 2015 until December 2016. All patients were admitted to North Hospital of Ruijin Hospital of China within 12 hours after TBI and diagnosis of TBI was confirmed by computer tomography (Ip et al., 2015).Patients were divided into a mild/moderate TBI group with GCS scores ranging from 9 to 15, and a severe TBI group with GCS scores from 3 to 8 (Ozyurt et al., 2015). Patients who had other severe diseases (such as severe cardiac, liver, brain or renal diseases, or severe infection) or were pregnant were excluded from the study. Additionally, 25 healthy individuals (as confirmed by physical examination) were enrolled as healthy controls. Written informed consent was obtained from all participants or their families within 24 hours of admission. The present study was approved by the Ethics Committee of North Hospital of Ruijin Hospital of China.
Measurement and data collection
Demographic data, such as age and sex,and clinical variables,including mechanism of injury and GCS score, were recorded.Peripheral elbow vein blood samples (10 mL) were collected at admission (0 hours), and 12, 24, 48, 72 hours, and 7 days after admission. Serum levels of CGRP and ET-1 were determined by radioimmunoassay using commercially available CGRP and ET-1 radioimmunoassay kits (Huaying Biological Engineering,Beijing, China) according to the manufacturer’s instructions.For analysis of survival, 6-month survival rates were analyzed.All causes of patient deaths during hospitalization were considered and recorded, and follow-up lasted for 6 months after admission if the patient left the hospital. Survival time was considered from the time of admission until death or last follow-up.
Statistical analysis
Measurement data, expressed as the mean ± SD, were analyzed using SPSS 18.0 software (SPSS, Chicago, IL, USA). Chi-square test was used to compare basic patient data and rates, such as sex ratio, mechanism of injury, and mortality rate. Comparison between two groups of continuous data was performed using Student’s t-test. Comparison among three or more groups was conducted using one-way analysis of variance followed by Tukey’s post hoc test. For survival analysis, length of survival was calculated from the date of admission to the date of death or up to 6 months, and a Kaplan–Meier curve was generated.Relationships between serum levels of CGRP/ET-1 and 6-month patient mortality were analyzed separately using a logistic regression model with a stepwise method. Values of P < 0.05 were considered statistically significant.
Results
Basic clinical information of TBI and control participants
As shown in Table 1, the present study included 96 cases of mild/moderate or severe TBI. Among all patients, 61 individuals (63.5%) exhibited mild/moderate TBI with a mean GCS score 11 ± 2 (9–15), while 35 individuals (36.5%) exhibited severe TBI with a mean GCS score 6 ± 1 (3–8). During hospitalization and 6-month follow-up, 13 (37.1%) patients died,all members of the severe TBI group. No patient in the mild/moderate TBI group died and no significant difference was found in other characteristics such as age or sex.
Dynamic alteration of serum CGRP and ET-1 levels in TBI patients from different groups
To investigate alterations of CGRP and ET-1 levels in TBI patients, serum levels of CGRP and ET-1 were determined at admission (0 hours), and 12, 24, 48, 72 hours, and 7 days after admission. Figure 1A and B demonstrated that at the time of admission, CGRP levels in both mild/moderate and severe TBI groups were significantly lower than in the control group(P < 0.05); whereas ET-1 levels in both mild/moderate and severe TBI groups at admission were significantly higher than in the control group (P < 0.05). At 12 hours after admission,decreased CGRP and increased ET-1 levels in patients reached their minimum and peak values, respectively, and started to recover to levels observed at admission. From 12–48 hours after admission, serum levels of CGRP were significantly lower,but serum levels of ET-1 were significantly higher in all TBI patients compared with controls (P < 0.05). At 72 hours and 7 days, levels of both CGRP and ET-1 were not significantly different between mild/moderate TBI groups and the control group. CGRP levels in the severe TBI group were still significantly lower and ET-1 levels were still significantly higher compared with the control group (P < 0.05). Additionally, at all time points, CGRP levels were significantly lower and ET-1 levels were significantly higher in all members of the severe TBI group compared with the mild/moderate TBI group (P< 0.05). Finally, mean values of CGRP and ET-1 levels in all patients at different time points were calculated and found to be negatively correlated (R = 0.9133; Figure 1C).
Dynamic alteration of CGRP and ET-1 in deceased and surviving patients
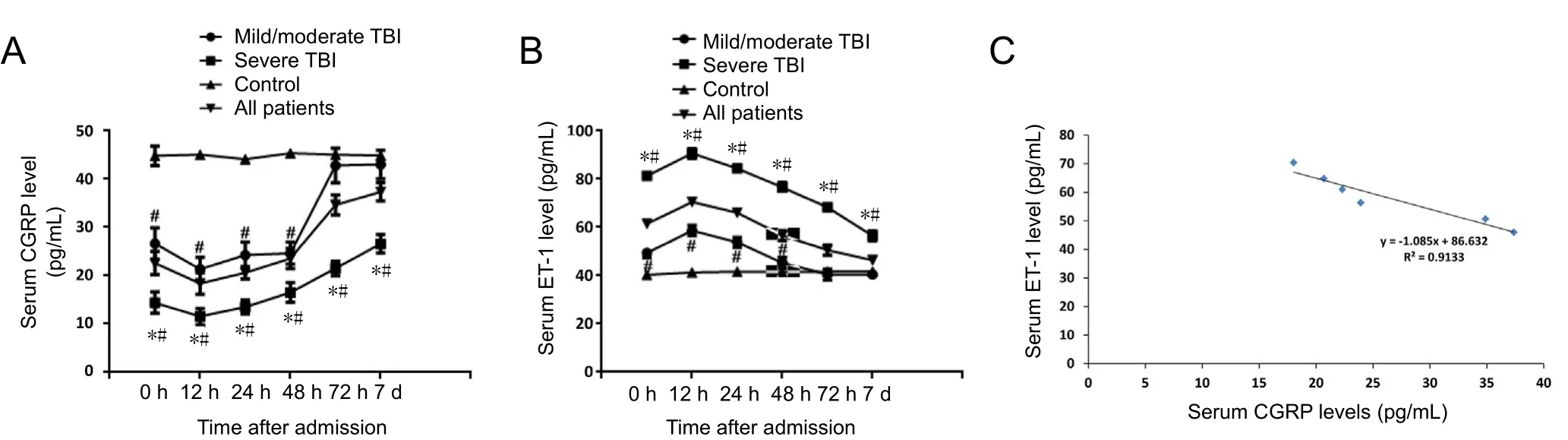
Figure 1 Dynamic alteration in serum levels of CGRP and ET-1 in diあerent groups.
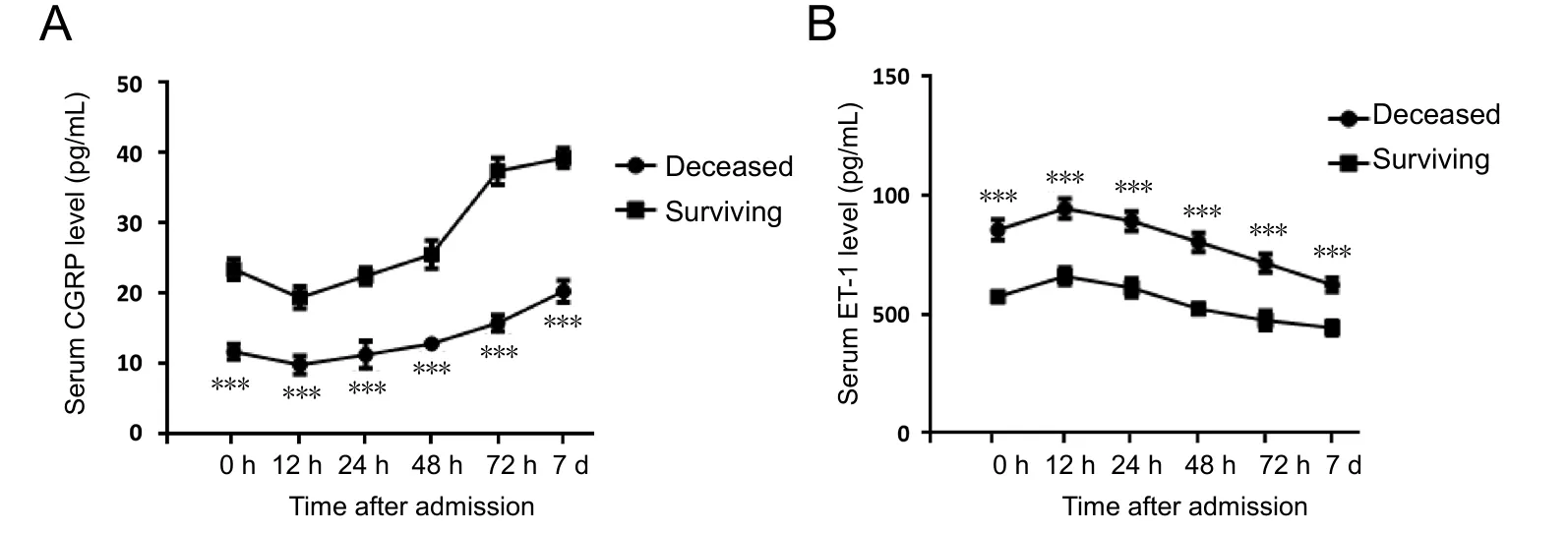
Figure 2 Dynamic alterations in serum levels of CGRP and ET-1 in deceased and surviving patients.

Figure 3 Kaplan-Meier curves for patients with high and low CGRP (A) and ET-1
To further investigate the role of CGRP and ET-1 in TBI patients, levels of both CGRP and ET-1 in deceased and surviving TBI patients were compared. As shown in Figure 2, at all time points, levels of CGRP in deceased patients were significantly lower than those in surviving patients; whereas ET-1 levels exhibited a contrasting trend (P < 0.001), indicating that serum levels of both CGRP and ET-1 might be associated with mortality in severe TBI patients.
Correlation of CGRP and ET-1 with 6-month mortality in severe TBI patients
Among all deceased patients, 8 (61.5%) died within 12 hours after admission. We analyzed relationships between serum CGRP and ET-1 levels at admission and mortality of severe TBI. First,all patients were divided into different groups according to median values of CGRP (24 pg/mL) and ET-1 (56 pg/mL) levels at admission. A Kaplan-Meier survival analysis was used to compare 6-month mortality in severe TBI patients with high/low levels of CGRP or ET-1, respectively. As displayed in Figure 3, survival times of patients with high and low CGRP/ET-1 levels were significantly different (P < 0.05). Moreover, additional logistic regression showed that both serum CGRP and ET-1 levels at admission were independent risk factors for 6-month mortality in severe TBI patients (Table 2).
Discussion
In the United States alone, over 1.7 million individuals are annually affected by TBI (Brophy et al., 2011). Unlike other organ-based injuries or diseases, no rapid diagnosis employing biomarkers has proven invaluable for TBI, which limits the accuracy and timeliness for its diagnosis (Mondello et al., 2012).Previous studies have reported potential biomarkers for TBI(Lin et al., 2016). For example, Daoud et al. (2014) reviewed eighteen different biomarkers in TBI and found that interleukin(IL)-6, IL-8, IL-1β, S100β, nerve growth factor, neuron-specific enolase, doublecortin, ET-1, high-mobility group box 1, and cy-tochrome c in cerebrospinal fluid, as well as glial fibrillary acidic protein, hyperphosphorylated neurofilament, ubiquitin C-terminal hydrolase L1, αII-spectrin breakdown product 145 kDa, and leptin in blood, were remarkably correlated with TBI outcomes(Daoud et al., 2014). However, further studies are still necessary, as contrary or unclear results still exist. Zetterberg et al.(2013) also reviewed biomarkers of mild TBI in cerebrospinal fluid and blood. They found that although a large number of biomarkers can be detected in cerebrospinal fluid and peripheral blood in TBI patients, it remains difficult to identify detailed diagnostic algorithms (Zetterberg et al., 2013). Neher et al.(2014) demonstrated potential treatment value of complement receptors CR2/CR1 in animal models; however, the diagnostic value of those factors is unclear.

Table 1 Basic clinical information for all participants
Although several early studies demonstrated potential effects of CGRP on brain injury (Edvinsson et al., 1991; Holland et al., 1994), few studies have advanced these findings. The present study, for the first time, demonstrated dynamic alteration of serum levels of CGRP in mild/moderate and severe TBI patients, and found that decreased serum levels of CGRP, as well as increased serum levels of ET-1, were associated with 6-month mortality in severe TBI patients.
Our results showed that serum CGRP levels in all TBI patients were dramatically lower than those observed in the control group during 0–48 hours after admission. Notably, serum levels of ET-1 exhibited opposite changes. CGRP is a 37-amino acid neuropeptide encoded by the calcitonin gene. A previous study showed that CGRP has potent regulatory effects on brain and heart functions. Zheng et al. (2012) found that CGRP up-regulation could protect streptozotocin-induced diabetic hearts from ischemia/reperfusion injury. Wiklund et al. (2011)found that CGRP could redistribute in brain and heart tissues after cardiac arrest. In an animal study, CGRP within the brain of trout was shown to potentially act as a potent neurotransmitter and/or neuromodulator in the regulation of cardio-ventilatory functions (Le Mével et al., 2012). In another animal study,Song et al. (2012) verified that increased CGRP levels in serum could accelerate fracture healing following TBI. ET was first sequenced in 1988, with ET-1 and ET-3 being most commonlyfound in the brain (Rubanyi and Polokoff, 1994). Many studies have demonstrated increased ET in TBI patients. Moreover,Maier et al. (2007) demonstrated that ET-1 was elevated in both cerebrospinal fluid and plasma after TBI in human patients.Armstead and Kreipke (2011) found that ET-1 is up-regulated after TBI in a cross-species, cross-model analysis.
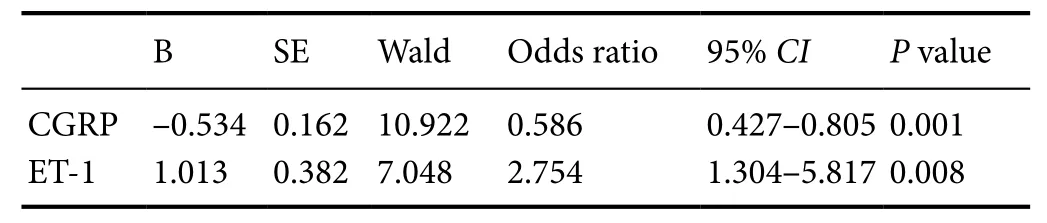
Table 2 Correlation between serum CGRP and ET-1 levels with 6-month mortality in severe traumatic brain injury patients by logistic multivariate regression analysis
The results of the present study confirm decreased serum CGRP levels in TBI patients, especially severe TBI patients.Observed alterations of ET-1 were consistent with previous related studies. Furthermore, we demonstrated that both serum CGRP and ET-1 levels were associated with 6-month mortality in severe TBI patients; indeed, both were confirmed as independent risk factors for mortality of severe TBI patients. ET-1 was previously found to be associated with myocardial injury and death in septic shock patients (Brauner et al., 2000; Lundberg et al., 2016). Rey et al. (2013) found that carboxy-terminal pro-endothelin-1 was associated with increased prediction of mortality risk scores of pediatric patients in intensive care units. However, no similar studies have shown a relationship between serum CGRP levels and TBI mortality rate or other brain injuries in recent years. This study has some limitations. Most notably, the sample size was small, all patients were Chinese, and we did not investigate mechanisms underlying how CGRP influenced the TBI process. Thus, further studies should be performed to confirm these results; indeed, animal or in vitro studies should be done to attain deeper insights for CGRP in TBI.
In conclusion, we conducted a prospective study to analyze relationships between CGRP and prognosis of patients with severe TBI. Serum levels of CGRP were markedly lower in severe TBI patients compared with mild or moderate patients,and decreased serum levels of CGRP and increased serum levels of ET-1 were associated with 6-month mortality of severe TBI patients. These results yield new insight into the role of CGRP in severe TBI, and may provide a new potential biomarker for severe TBI.
Author contributions: LXC wrote the paper. WFZ, MW and PFJ participated in discussion and comments on an early version of the paper. All authors approved the final version of the paper.
Conflicts of interest: There are no conflicts of interest associated with this papaer.
Financial support: None.
Institutional review board statement: The study was approved by Ethics Committee of North Hospital of Ruijin Hospital of China.
Declaration of patient consent: The authors certify that they obtained all appropriate patient consent forms. In the form, the patients or their guardians have given the patients’ consent for their images and other clinical information to be reported in the journal. The patients or their guardians understand that the patients’ names and initials have not been published and due efforts have be made to conceal their identity, but anonymity cannot be guaranteed.
Reporting statement: This manuscript was prepared and modified according to the Strengthening the Reporting of Observational Studies in Epidemiology(STROBE) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of North Hospital of Ruijin Hospital, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Data of participants, including data dictionaries, in the study can be obtained from the authors; the individuals’ data including in the manuscript without personal labels can be shared; study plain and statistical data can be shared; the data can be shared after 3 months for purification of the manuscript and the sharing will end 5 years after publication; scholars can obtain the data by only reasonable data sharing proposal; data can be only shared for approved purpose; data sharing proposal should be sent to corresponding author, and an agreement should be signed.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers: Francesco Mattia Rossi, Universidad de la Republica Uruguay Facultad de Ciencias, Uruguay; Fernando J. Álvarez Cervera, Universidad Autonoma de Yucatan, Mexico; Isaac G. Onyango, Gencia Biotechnology, USA.
Additional file: Open peer review reports 1–3.
- 中国神经再生研究(英文版)的其它文章
- Validity and reliability of the Ocular Motor Nerve Palsy Scale
- Mitogen-activated protein kinase phosphatase 1 protects PC12 cells from amyloid beta-induced neurotoxicity
- High-frequency (50 Hz) electroacupuncture ameliorates cognitive impairment in rats with amyloid beta 1–42-induced Alzheimer’s disease
- Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia
- Combined VEGF/PDGF improves olfactory regeneration after unilateral bulbectomy in mice
- Comparison of morphological and functional outcomes of mouse sciatic nerve repair with three biodegradable polymer conduits containing poly(lactic acid)