Comparison of morphological and functional outcomes of mouse sciatic nerve repair with three biodegradable polymer conduits containing poly(lactic acid)
Fernanda Marques Pestana , Rui C.C. Domingues , Júlia Teixeira Oliveira Daniela F. P. A. Durço Camila Oliveira Goulart Henrique Rocha Mendonça , Anne Caroline Rodrigues dos Santos Natália Tavares de Campos Beatriz Theodoro da Silva
Cristina Cardoso Pereira4, Cristiano Piacsek Borges4, Ana Maria Blanco Martinez1, 2, 3, *
1 Pós Graduação em Ciências Morfológicas, Instituto de Ciências Biomédicas-UFRJ, Rio de Janeiro, RJ, Brazil
2 Anatomia Patológica - Faculdade de Medicina – HUCFF -UFRJ, Rio de Janeiro, RJ, Brazil
3 Laboratório de Neurodegeneração e Reparo - Faculdade de Medicina – HUCFF-UFRJ, Rio de Janeiro, RJ, Brazil
4 Programa de Eng. Química, COPPE-UFRJ, Rio de Janeiro, Brazil
5 Polo Universitário de Macaé, Laboratório Integrado de Produtos Bioativos e Biociências, Macaé, UFRJ, Brazil
Funding: This work was supported by Grant 465656/2014-5 from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq),Brasil, and also supported by CAPES and FAPERJ, Brasil.
Abstract Poly(lactic acid) (PLA)-containing nerve guidance conduits (NGCs) are currently being investigated for nerve repair as an alternative to autograft, which leads to permanent functional impairment in the territory innervated by the removed nerve. Combination of polymers modifies the physical properties of the conduits, altering their nerve-guidance properties. Conduits made from PLA-only or combined with other polymers have been used successfully for nerve repair, but their efficiency has not been compared. We compared the morphological and functional outcomes of peripheral nerve repair by using NGCs made of poly(lactic acid) and combined or not with polycaprolactone (PLA/PCL) or polyvinylpyrrolidone (PLA/PVP). To assess the functional recovery, we employed a mechanical hyperalgesia analysis, sciatic functional index (SFI), and electroneuromyography. The mechanical hyperalgesia analysis showed that the PLA group improved more rapidly than the PLA/PVP and PLA/PCL groups; similarly, in the electroneuromyography assay, the PLA group exhibited higher amplitude than the PLA/PCL and PLA/PVP groups. However, the SFI improvement rates did not differ among the groups. Morphologically, the PLA group showed more vascularization, while the nerve fiber regeneration did not differ among the groups. In conclusion, the PLA-only conduits were superior to the other NGCs tested for nerve repair.
Key Words: sciatic nerve; tubulization; biodegradable conduit; poly(lactic acid); regeneration
Introduction
Peripheral nerve injury is a common clinical problem, leading to temporary or permanent disability. Nerve guidance conduits (NGCs) made of biocompatible, bioresorbable, and biodegradable polymers have been investigated as an alternative to autograft in cases where nerve tissue is lost after lesion,making direct neurorraphy impossible without considerable stretching. In the NGC approach, the ends of a sectioned nerve are connected by a polymer conduit, i.e., a hollow-fiber polymer membrane, in order to guide the axon growth and enhance the recovery of sensory and motor functions(Pabari et al., 2010; Daly et al., 2012; Gu et al., 2014). This approach avoids several problems that are experienced with the gold-standard autograft technique, such as reduction of morbidity, requirement for only one surgical procedure, and the possibility of connecting larger nerve gaps (Daly et al., 2012;Steed et al., 2011; Marquardt and Sakiyama-Elbert, 2013).
NGCs can provide a favorable microenvironment for nerve regeneration and can act as a support for directing the axonal growth from the proximal stump toward the distal stump(Heath and Rutkowski, 1998; Maquet et al., 2000). NGCs have several ideal features, including mechanical properties such as flexibility, as well as morphological properties that are important for nerve regeneration. Parameters such as the tubular lumen diameter and wall thickness, and the presence, distribution and diameter of pores are important factors (Konofaos and Ver Halen, 2013). Structural, mechanical and morphological properties are important characteristics of NGCs that can limit their production and useful application. NGCs must bear stresses during handling and suturing in the surgical procedure and during implantation, in order to maintain integrity with the movements of the patient. It is recommended that mechanical properties should be comparable to in vivo tissue at the transplant site (Stamatialis et al., 2008), where Young modulus (E)values are in the 8–16 MPa range, ultimate stress of 6.5–8.5 MPa and elongation of 0.6–1.6 mm/mm (Chiono and Tonda-Turo, 2015). A wall thickness of 200 to 600 µm allows acceptable nutrient diffusion flux (Chiono and Tonda-Turo,2015). The inner diameter of the NGC must be tailored to fit the nerve size, avoiding nerve compression caused by degradation-induced swelling and inducing nerve damage.
Pore size, distribution and conduit morphology are reported as one of the main features affecting the success of NGC application. Some investigators consider that a pore size greater than 20 µm will cause fibroblast infiltration, and the optimal pore size should be within the 10–20 µm range (Goulart et al., 2016). Other authors report that NGCs should exhibit an approximately 50 kDa molecular weight cut-off (Adamus et al., 2012). Also, the NGC must allow retention of Schwann cells and growth factors secreted by nerve ends inside the lumen of the NGC. Regarding NGC morphology, hollow poly(-lactic acid) (PLA) fibers can be synthesized as spongelike and fingerlike structures. Domingues et al. (2017) reported that the sponge-like and finger-like morphology of the hollow PLA fibers significantly affects their biodegradability and mechanical properties, which can be crucial to the success of the NGC application. Synthetic and biological polymeric resorbable biomaterials are being investigated for biomedical and NGCs applications, such as collagen, chitosan, polycaprolactone(PCL), polyvinylalcohol (PVA), polyglycolic acid (PGA), and PLA. Their copolymers such as poly(lactic co-glycolic acid)(PLGA) and poly(lactic acid co-caprolactone) are the most often studied, since combinations of these might increase biodegradability, biocompatibility, thermal plasticity, and mechanical properties (Nectow et al., 2011; Gu et al., 2014; Chiono and Tonda-Turo, 2015).
PLA is an aliphatic polyester that is widely used as a biomaterial in hemodialysis (Gao et al., 2014), tissue engineering, and drug delivery applications (Adamus et al., 2012; Minbu et al.,2015; Uzun et al., 2015). It is also being considered as a promising material for NGC application (Bell and Haycock, 2011;Nectow et al., 2011; Chiono and Tonda-Turo, 2015), with excellent outcomes in the functional recovery of sciatic-nerve injury in mice (Goulart et al., 2016). Also, its mechanical, thermal and degradation properties are described as suitable for this application (Domingues et al., 2017).
Axon regeneration in peripheral nerves occurs spontaneously across short gaps, but regeneration across larger gaps is still challenging and requires further investigation. It has been reported that results similar to an autograft were rarely observed using commercial NGCs (Kehoe et al., 2012). The limitations of this approach are currently associated with the requirements for structural, trophic, and morphological features of NGCs (de Ruiter et al., 2009; Pabari et al., 2010; Daly et al.,2012; Adamus et al., 2012; Marquardt and Sakiyama-Elbert,2013; Gu et al., 2014; Chiono and Tonda-Turo, 2015). However, Goulart et al. (2016) showed that the use of a PLA-only conduit for mouse nerve repair led to improved recovery of motor function, correlated with a higher count of nerve fibers within the optimal g-ratio range for the sciatic nerve.
A detailed analysis of the application of NGCs containing PLA (alone or combined with other polymers) might assist in the clinical choice of the most suitable conduit for nerve repair. To address this question, we compared the physical properties of NGCs made in our laboratory from PLA alone or combined with PVP or PCL, and the morphological and functional outcomes of peripheral nerve repair using these in mice.
Materials and Methods
Materials
PLA (molecular weight (MW) = 350 kDa, amorphous) was kindly supplied by the Macromolecules Institute of the Federal University of Rio de Janeiro, Brazil. PCL (MW = 320 kDa) and bovine serum albumin (BSA, MW = 67 kDa) were purchased from Sigma (St. Louis, MO, USA). Polyvinylpyrrolidone (PVP)k90 (MW = 360 kDa) (Sigma) was used as an additive in some of the solutions. The solvents n-methylpyrrolidone (NMP),dioxane and dimethyl sulfoxide (DMSO) were acquired from Vetec (Rio de Janeiro, Brazil). Water, previously microfiltered and distilled, was used as non-solvent in all experiments. All solvents were used as received.
Liquid-induced phase separation (LIPS) technique for membrane synthesis
All membranes were prepared by the liquid-induced phase-separation technique. The technique consists of immersing a polymer solution (PLA + solvent) in a water bath,causing mass transfer of water to the polymer solution and of the solvent to the water bath simultaneously. At some point this will induce precipitation of the polymer, forming a pure polymer solid phase, which is the type of membrane used as NGCs in this study. In the case of the PCL-containing membranes, a 1:4 PCL:PLA blend was used as an optimum proportion, as reported by (Tanaka et al., 2006). In all the remaining membranes, only PLA was used.
The membrane synthesis was carried out as previously described (Domingues et al., 2017). Briefly, the polymer solution is extruded toward the spinneret at 65 g/min by using nitrogen. The spinneret has inner and outer diameters of 0.7 and 1.5 mm respectively. Water was used as the bore fluid and the external precipitation bath. The air gap between the spinneret and the water bath was set to zero in all experiments.The polymer solution, storage tank, and spinneret were kept at 60°C. After spinning, the resulting hollow fibers were immersed in a copious volume of water for 72 hours, in order to remove all residual solvent. The prepared membranes were tested and characterized for water permeation and for BSA rejection in dialysis tests, as described (Domingues et al., 2017).The formulation and solvents of the prepared membranes are described in Table 1.
Animals and experimental groups
Twenty-four adult C57Bl/6 mice, males and females, at 8 weeks of age, weighing 20–24 g, were obtained from the Federal University of Rio de Janeiro (BIORIO, RJ, Brazil) and housed under a 12-hour light/dark cycle with free access to food and water. The mice were randomly divided into four experimental groups: normal (n = 6) orexposed to sciatic-nerve transection and repair through the tubulization technique,with one of three types of conduit: PLA/PCL (n = 6), PLA/PVP (n = 6), or PLA (n = 6). Animals were euthanized after a post-surgery period of 8 weeks. Each animal was identified by a numeric code before an experiment was performed, and the code was accessed only after data acquisition and statistical analyses, to prevent bias. All animal procedures were approved by the Ethics Committee for the Use of Experimental Animals of the Center of Health Sciences of the Federal University of Rio de Janeiro, Brazil (Protocol DHE003).
Surgical procedures
Under anesthesia (Anasedan®xylazine, 15 mg/kg and Dopalen®Ketamin, 100 mg/kg, intraperitoneal), the left sciatic nerve was exposed and transected at mid-thigh level. One millimeter each of the proximal and distal stumps of the nerve was inserted into each end of the conduit (5 mm long) through an epineural suture (10-0, mono nylon suture, Ethicon, Blue Ash, Ohio, USA), maintaining the original neural alignment and leaving a 3-mm gap to stimulate the regeneration process.After the tubulization procedure, the muscle above the nerve was repositioned and the skin sutured with 6-0 silk suture (6-0,mono nylon, Ethicon).
Eight weeks after surgery, the mice were anesthetized for the electroneuromyography procedure, followed by transcardiac perfusion with a fixative solution containing 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Subsequently,the regenerated segments of the left (injured) sciatic nerves obtained from the mid-portion of the conduits were harvested and processed for light microscopy.
Scanning electron microscopy
The ultrastructural characteristics of the membranes were investigated prior to the in vivo experiment. The conduits were fixed by immersion for 2 hours in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4), then washed in 0.1 M phosphate buffer (pH 7.4) and 0.1 M cacodylate buffer (pH 7.4),post-fixed for 2 hours in 1% osmium tetroxide containing 0.8% potassium ferrocyanide and 5 nM calcium chloride in 0.1 M cacodylate buffer (pH 7.4). Then, the samples were washed in 0.1 M cacodylate buffer (pH 7.4) and dehydrated in a series of solutions with increasing concentrations of ethanol. For the cross-section analysis, the samples were fractured at low temperatures through immersion in liquid nitrogen, avoiding mechanical deformation of the sample. The photomicrographs were obtained by scanning electron microscopy (FEI,model Quanta 200, Hillsboro, Oregon, USA) with voltage acceleration of 20 keV was used. The samples were previously covered with a thin layer of gold in an Emitech sputter coater.The membrane thickness, internal diameter, and mean pore diameter of the inner and outer surfaces were measured and evaluated by image analysis, with the aid of ImageJ software.
Light microscopy
After perfusion, the segments of the regenerated nerves obtained from the mid-portion of the conduits were fixed by immersion for 2 hours in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4), then washed three times in the same buffer followed by 0.1 M cacodylate buffer (pH 7.4), and postfixed for 1 hour in 1% osmium tetroxide containing 0.8% potassium ferrocyanide and 5 nM calcium chloride in 0.1 M cacodylate buffer (pH 7.4). After this procedure, the regenerated nerve segments were washed three times in 0.1 M cacodylate buffer (pH 7.4), washed once in distilled water, and then immersed in 1% aqueous uranyl acetate solution overnight. After that, the sample was washed in distilled water and then dehydrated in increasing concentrations of acetone (50%, 60%,70%, 80%, 90% and 100%). Finally, the sample was infiltrated with Poly/Bed®812 resin (Polysciences, Inc., Washington,PA, USA) and polymerized at 60°C for 48 hours. Semi-thin crosssections with a thickness of 500nm were cut (RMC MT-6000 ultramicrotome, Tucson, Arizona, USA), stained with 1% toluidine blue, and the images were acquired with a light microscope (Zeiss Axioskop 2 plus, San Diego, CA, USA).
Morphometric analyses
The semi-thin crosssections were photographed under the light microscope at magnifications of 400× and 1000×,using Axiovision Rel. 4.5 (Carl Zeiss Microimaging, Thornwood, NY, USA). Histology slides were identified by codes and the photomicrographs were acquired by an independent experimenter who did not know which codes represented which groups, to avoid bias. The acquired images were used to count the number of myelinated nerve fibers and the number of blood vessels. The parameters of axonal area, fiber area,myelin area, and g-ratio were calculated using five samples per nerve, observed under a light microscope at 1000× magnification. Myelin area was obtained by subtracting the area of the axon from the area of the nerve fiber. The g-ratio was calculated by dividing the axon diameter by the fiber diameter, and the results were stratified in the ranges of 0.0–0.4, 0.4–0.5,0.5–0.6, 0.6–0.7, 0.7–0.8 and 0.8–0.9. Quantification was performed manually, using Image J version 1.42q (NIH,Bethesda, MD, USA).
Functional analyses
In order to evaluate if the intrinsic characteristics of each NGC affected the regeneration process, functional parameters of the animals were analyzed weekly by means of a test of motor function (sciatic functional index, SFI) and a test of tactile sensitivity (mechanical hyperalgesia test). In addition, at the end of the experiment (8 weeks), an electroneuromyography was performed.
SFI was performed weekly as previously described (Inserra et al., 1998). Briefly, the mice had their feet painted with India ink, and were then allowed to walk through a wooden corridor, 45 cm long × 6.5 cm wide, lined with white paper (Canson A4 140 g/m2). The paw prints were analyzed considering two different parameters: the toe spread (TS), the distance between the first and fifth toes; and the print length (PL), the distance between the tip of the third toe and the heel. Both measurements were taken from both the injured (E) and uninjured side (N).
The SFI was calculated as follows:
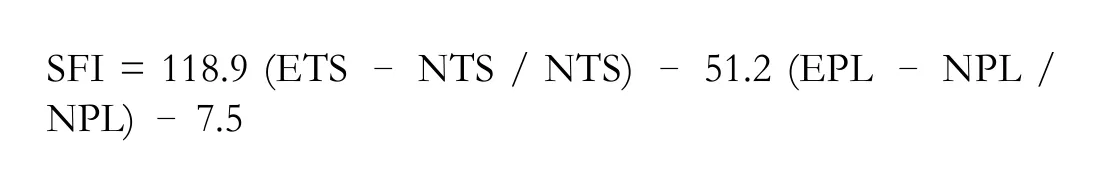
An SFI value close to 0 indicates normal nerve function,and a value close to –100 indicates total dysfunction.
Mechanical hyperalgesia was analyzed in the mice as pre-viously reported (Cunha et al., 2004). The paw-withdrawal threshold, in grams, was evaluated weekly by inducing gradual pressure on the plantar surface of the hind paws. A digital analgesimeter (electronic von Frey analgesimeter, Insight Equipment Ltda., Ribeirão Preto, SP, Brazil) was used. During the experiment, the animals were contained in small cages with a wire-mesh floor, through which the sensor pin (0.5 mm2 polypropylene tip contact area) was inserted into the medial plantar surface of the hind paw. The operator applied the tip perpendicularly to the bottom of the paw, producing an increase in the linear force applied. At the moment that the mouse withdrew the stimulated paw, the transducer recorded the pressure in grams. Soon after the paw was withdrawn, the mouse manifested the pain by flicking the paw. Three measurements were taken and a mean was obtained.
Electroneuromyography analysis
Eight weeks post-injury, the animals were anesthetized as described above and the sciatic nerve and gastrocnemius muscle of the injured hind limb were exposed. The electroneuromyography study was performed as in a previous report(Stipp-Brambilla et al., 2010), using a PowerLab 4/35 data acquisition device (ADInstruments, New South Wales, Australia). A 10-V electrical stimulation was applied to the proximal portion of the sciatic nerve (proximal to the injury) through a bipolar hook electrode, with the cathode 2 mm from the anode. To record the compound-muscle action potential, needle electrodes were used. The recording electrode was inserted into the belly of the gastrocnemius muscle, and the reference electrode was positioned in the calcaneus tendon. The grounding electrode was positioned under the skin. The parameters analyzed were amplitude (mV) and latency [the length of time from the stimulation (red line) to peak amplitude (ms)] of the compound-muscle action potential. The action potentials evoked were displayed in appropriate settings for measuring the amplitude from the baseline to the peak of the response,from which one can infer the number of re-innervated muscle fibers and the latency as a unit of time from the stimulus to the peak of the response.
Statistical analysis
All analyses were performed using GraphPad Prism 5.01(GraphPad Software, Inc., San Diego, CA, USA). The normality of the data was confirmed by the Kolmogorov-Smirnov test. To compare the results between the different groups with only one variable, as in the case of the amplitude and latency analysis of electroneuromyography and morphometry, oneway analysis of variance was used, followed by the Bonferroni post-test for paired comparison between the groups, 8 weeks after surgery. For analysis of behavioral tests, two-way analysis of variance (ANOVA) was used to compare groups at different times. For multiple comparisons between means, the Bonferroni post hoc test was used. Data are presented as mean ±standard deviation (SD). A value of P < 0.05 was considered significant.
Results
Structural organization of NGCs
The SEM photomicrographs are presented in Figure 1. After the conduits were prepared, they were processed for SEM, for their ultrastructural features and qualitative analysis (Figure 1)and quantification of the thickness of the wall, inner and outer diameter, and inner and outer diameter of the pores (Table 1). The cross sections of the conduits were analyzed from the electronmicrographs (Figure 1A–C). The thicknesses of their walls were: 0.22 mm in the PLA/PCL conduit, 0.21 mm in the PLA/PVP conduit, and 0.13 mm in the PLA conduit (Table 1). The inner and outer diameters of the conduits were also measured: 0.74 mm and 1.18 mm in the PLA/PCL conduit,0.79 mm and 1.2 mm in the PLA/PVP conduit, and 0.67 mm and 0.92 mm in the PLA conduit, respectively (Table 1).The PLA/PCL conduit showed higher porosity on the outer surface, and the pores were smaller compared to those on the outer surface of the PLA/PVP; PLA conduits, showed no external pores (Figure 1D–F). The inner surfaces of the PLA/PCL and PLA conduits showed much less porosity than the PLA/PVP conduit, which in turn showed the largest pore diameter (Figure 1G–I). Regarding the diameters of the inner and outer pores, we found 0.25 µm and 0.29 µm in the PLA/PCL conduit, 19.51 µm and 4.45 µm in the PLA/PVP conduit respectively; and 0.15 µm for the inner pore diameter in the PLA conduit (Table 1).
PLA conduits for sciatic nerve repair enhance morphological outcome
After 8 weeks, the surviving animals were euthanized. Their regenerated sciatic nerves were harvested and processed for histomorphometric analysis, which consisted of counting the total number of myelinated nerve fibers and blood vessels(Figure 2). Although the PLA and PLA/PVP groups showed a tendency toward higher numbers of myelinated fibers com-pared to the PLA/PCL group, there was no significant difference among the lesioned groups, all showing fewer myelinated fibers than the normal group (Figure 2I). Regarding the total count of blood vessels, the PLA group had more blood vessels compared to the PLA/PCL group, but did not differ from the PLA/PVP group or from normal animals (Figure 2J). Myelin area (Figure 2K), fiber area (Figure 2L), and axon area (Figure 2M) did not differ among groups. Concerning g-ratio analysis,no differences among the NGC-treated groups were found,and all groups showed fewer fibers in the 0.7–0.8 interval compared to normal animals (Figure 2N).
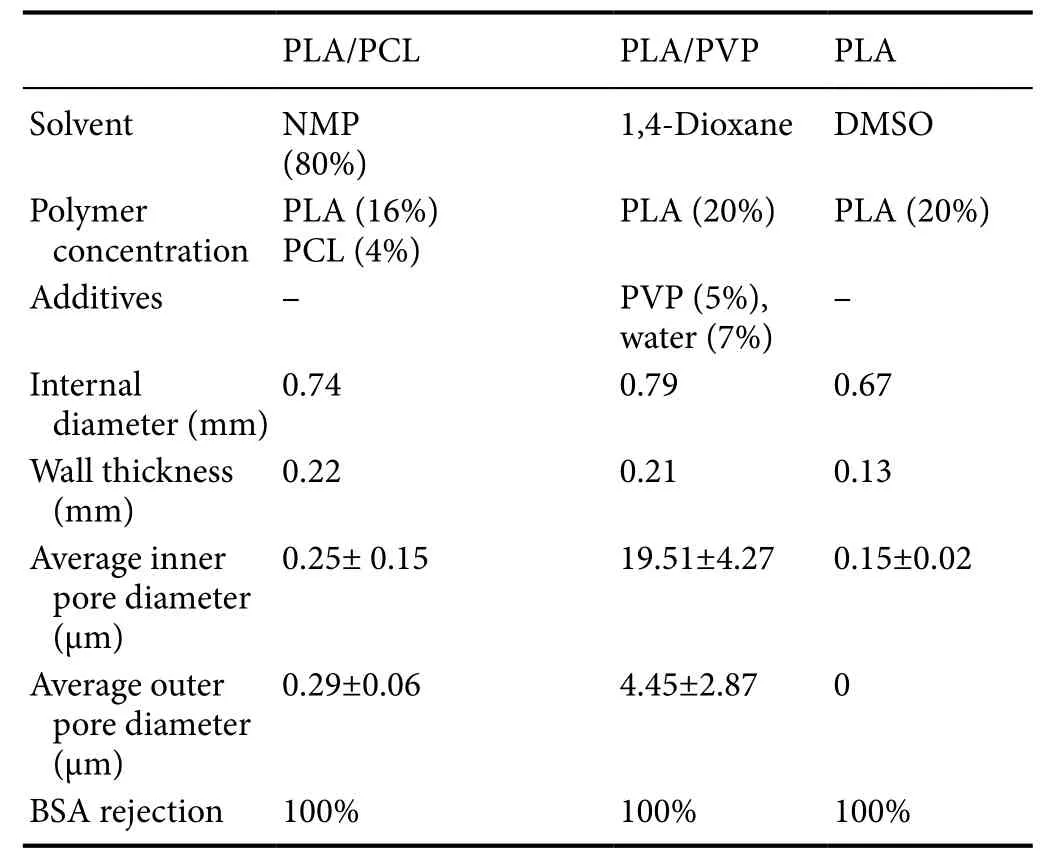
Table 1 Formulation, dimensions and transport properties of tubes used in this work
PLA conduits for sciatic nerve repair improve sensory but not motor outcome
The tactile sensitivity was analyzed weekly by an analgesimeter until the end of the experiment (56 days post-injury). All experimental groups showed an increase in the withdrawal threshold on day 7 post-injury compared to the normal group(P < 0.001) (Figure 3A). However, the PLA group achieved values that were not significantly different from normal animals after 14 days post-injury, whereas the PLA/PCL and PLA/PVP groups only achieved normal values after 42 and 28 days, respectively. The motor performance was analyzed weekly by SFI until the end of the experiment (56 days post-injury). The PLA/PCL, PLA/PVP and PLA groups progressively improved over the period, reaching scores of 84.1, 77.4, and 73.6, respectively.There was no statistical difference among groups at any time point (P > 0.05) (Figure 3B).
PLA conduits for sciatic nerve repair enhance gastrocnemius muscle activation
Eight weeks post-surgery, all animals underwent electroneuromyography (ENMG) to analyze the action potential of the compound muscle. ENMG traces show the electrical pattern of the compound-muscle action potential, from which the amplitude and latency parameters were measured (Figure 4A–D).The quantitative data showed that the PLA/PCL and PLA/PVP groups showed a decrease in amplitude compared to both the normal (P < 0.01) and PLA (P < 0.05) groups (Figure 4E). However, there was no difference in amplitude between the normal and PLA groups (P > 0.05). In addition, there were no differences among all groups regarding latency (P >0.05; Figure 4F).
Discussion
Strategies to improve peripheral nerve regeneration are essential and clinically demanding, due to the poor nerve regeneration observed in cases of long and challenging gaps or in lesions situated far from the target organ, leading to permanent motor and sensory disabilities. Functional recovery is often improved when the reinnervation time is short, since the neurostimulatory potential of Schwann cells declines over time (Goulart et al., 2014). In the presence of a nerve defect,the placement of an autologous nerve graft is the current gold standard for nerve repair. However, nerve autografts have some disadvantages, such as the limitation of donor tissue availability, the possibility of painful neuromas forming in the donor area, and the loss of nerve function at the donor site(Bockelmann et al., 2010; Moroder et al., 2011). The clinical use of NGCs may be an alternative to nerve autografts. The effectiveness of various synthetic biodegradable materials has been tested using the tubulization technique. Among these,PLA and PCL have shown positive effects on peripheral nerve regeneration (Maturana et al., 2013; Oliveira et al., 2014).Advancement in the production of biodegradable conduits is an important research area that eventually may meet clinical needs; and, in the future, biodegradable conduits may be an advantageous alternative to the autograft technique. In the present study, we compared the morphological and functional outcomes of three different PLA polymer-containing NGCs after sciatic-nerve transection and repair by tubulization, in mice.
Polymers are macromolecules formed by the union of several units of monomers, and copolymers are polymers formed by more than one type of monomer. In this study we used a combination of poly(L-co-D, lactic acid) and also another combination with polycaprolactone. Among biomaterials, poly-L/D-lactide combines the best characteristics of poly-L-lactic acid and poly-D-lactic acid, i.e., the mechanical properties of the first and the short degradation time of the second.These characteristics have made poly-L/D-lactide a highly important bioengineering compound (Ciambelli et al., 2013). The polycaprolactone polymer is often used as one of the components in copolymers to improve the properties of the biomaterials, for example by increasing flexibility. Previous studies have shown that the rate of biodegradation of nerve guides should conform to axonal growth rates (Robinson, 1989). The efficacy of poly (DL-lactic-caprolactone) copolymers has previously been demonstrated in vitro (Meek et al., 1997) and in vivo experiments (Den Dunnen et al., 1995). However, direct comparisons between different types of polymers or copolymers employed in nerve repair were lacking. Our results did not show an improvement in regeneration, neither in functional nor in morphometric parameters, when the copolymer(DL-lactic-caprolactone, PLA/PCL group) was compared to poly(lactic acid) (PLA/PVP and PLA groups). These results may be explained by the structural differences among the groups, such as differences in thickness and mean diameter,and in the pore distribution on the external and internal surfaces of the tubes, showing that in this study, the copolymers provided no advantages over the PLA polymer alone.
The thickness of the wall is an important characteristic of NGCs. If the NGC wall is too thick, it will degrade very slowly, thus extending the time of possible immunological reaction to the biomaterial and affecting the rates of nutrient diffusion(Konofaos and Ver Halen, 2013). On the other hand, a thinwalled NGC can degrade very quickly, resulting in loss of the supporting structure and collapse of the tubular lumen during the regenerative process (Mobasseri et al., 2015). Previous studies have shown that biodegradable nerve guides with a wall thickness of approximately 0.3 mm have provided optimal results for peripheral-nerve regeneration (Konofaos and Ver Halen, 2013). Other studies have shown that PCL and PLA conductors with a wall thickness of approximately 0.17 mm collapse completely, diverting all regenerative axons from the canal (Meek et al., 1997). Other NGCs with a mean thickness of 0.23 mm did not collapse after surgery (Den Dunnen et al., 1995). On the other hand, another study compared the regeneration in ducts with wall thicknesses between 0.07 and 21 mm, and found that 0.07-mm walls gave excellent regenerative results, equivalent to the results obtained with the gold-standard autograft technique (Mobasseri et al., 2015). Accordingly,our research showed better results in the electroneuromyography, in the test of mechanical hyperalgesia, and in the morphometric results (total number of vessels) for the PLA group,which had a thinner wall (0.13 mm) than the PLA/PVP (0.21 mm) and PLA/PCL (0.22 mm) groups.
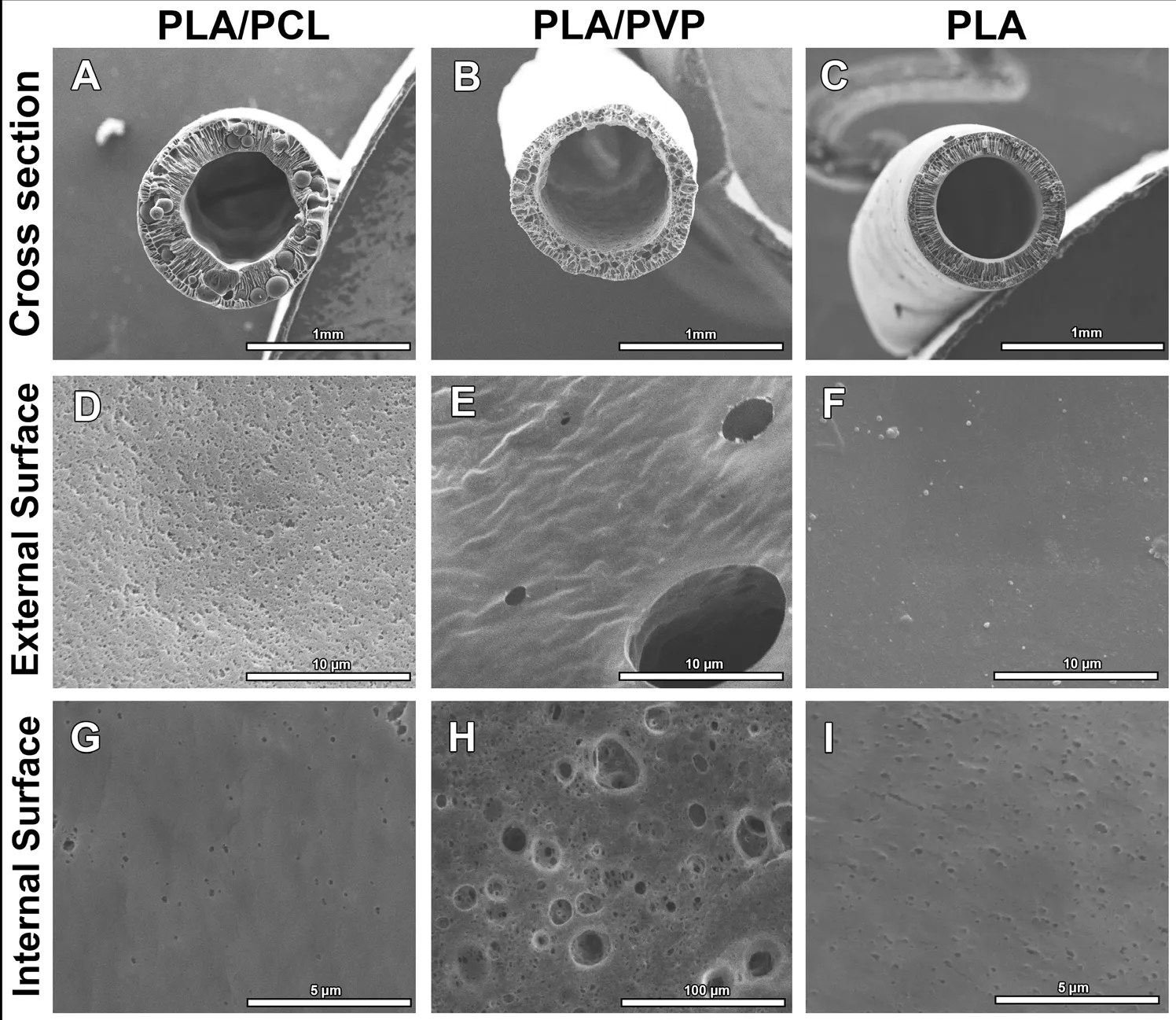
Figure 1 Morphological features of the NGCs.
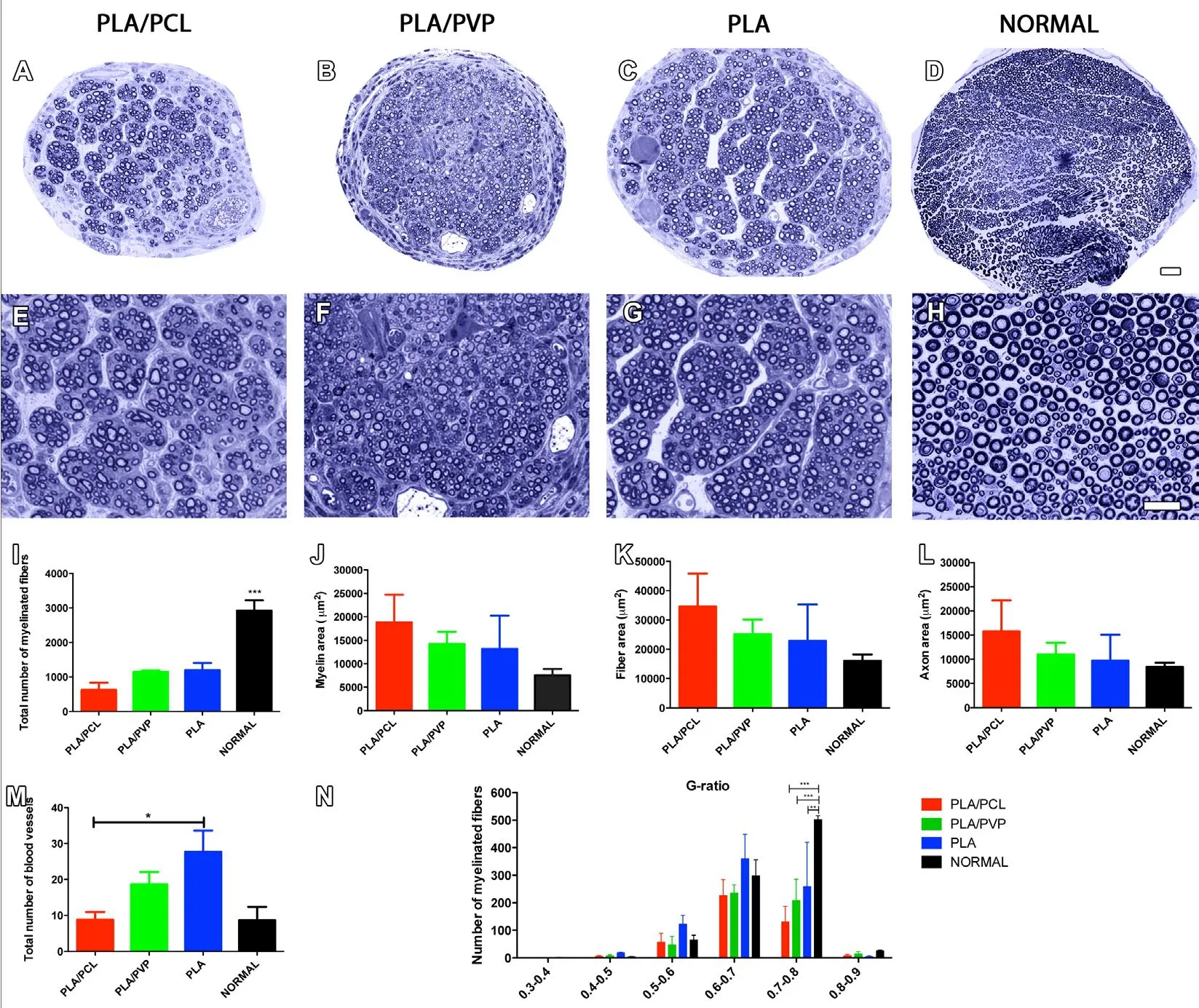
Figure 2 PLA conduits enhances morphological outcome of mice.
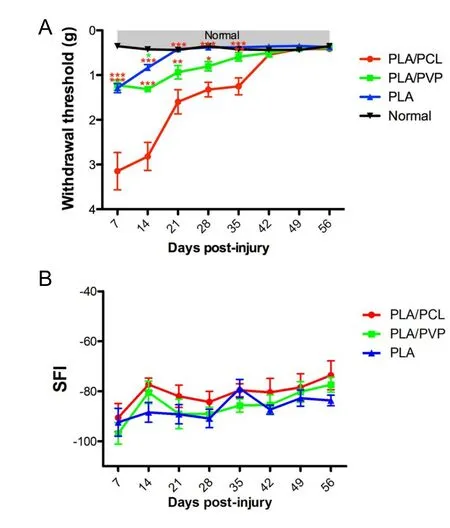
Figure 3 PLA conduits improves sensory but not motor outcome of mice.
The permeability of an NGC is also important, because nutrients and oxygen must diffuse to the regeneration site until the regenerated nerve becomes vascularized again (Konofaos and Ver Halen, 2013). The benefits of permeable NGCs can be attributed to different ratios, including metabolic exchange through the tube wall, diffusion into the lumen of the channel of the factors generated in the external environment, retention of trophic factors secreted by the nerve stumps, or a combination of these (Aebischer et al., 1988). Therefore, the pore size of the tube wall and its stability over time appear to determine the flow of different substances that can promote or inhibit regeneration (Busscher et al., 1983). Although the effect of NGC pores on regeneration has been studied by several research groups, the results are contradictory. On one hand, it has been shown that porous conduits favor nerve regeneration(Wang et al., 2015; Hsieh et al., 2016; Santos et al., 2017); but on the other hand, studies have failed to demonstrate regenerative differences when comparing porous and nonporous tubes (Ezra et al., 2013). Other researchers continue to correlate the size of the pore diameter with the efficacy of the repair (Oh et al., 2012), where nanopores (diameter 0.1 μm)were found to be more beneficial than micropores (diameter 200 μm). Indeed, it was recently shown that the hypoxia with-in the distal stump generated by sciatic-nerve lesion activates macrophages, which in turn secrete VEGF-A, stimulating vascular chord formation which guides Schwann cell migration and alignment, which in turn guides nerve regeneration(Cattin et al., 2015). Similarly, in the present study the best results were shown by the PLA group which showed no external pores. The absence of external pores, besides inducing lowoxygen content within the conduit, also prevents fibroblast infiltration, avoiding fibrosis, and promotes Schwann cell retention within the conduit. Schwann cells, in turn, may secrete trophic factors, such as nerve growth factor, neurotrophin-3 and neurotrophin-4, which promote nerve regeneration and reinnervation (Goulart et al., 2014).
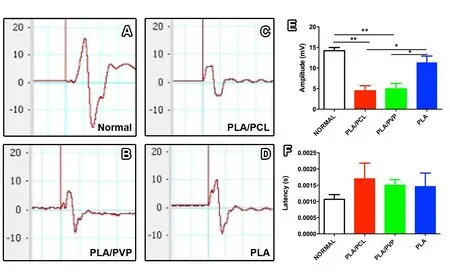
Figure 4 PLA conduits enhance gastrocnemius muscle activation of mice at 8 weeks post-surgery.
Of the functional evaluations (except the SFI) and morphological evaluations (except the total number of myelinated fibers) performed, the PLA group gave the best results, possibly because it had the thinnest wall and the smallest mean pore diameter. The PLA/PVP group had moderate results, and the PLA/PCL group performed worst. We believe that these differences are correlated with the NGC structure, since the PLA/PCL group had the thickest wall, while the PLA/PVP group had the largest pore diameter.
Since the EMG compound-muscle action potential amplitude correlates with the amount of muscle fibers activated by nerve stimulation, our results suggest that although nerve fiber number and characteristics in the mid-portion of the NGCs were the same in the nerve segment analyzed in all the lesioned groups, muscle reinnervation was considerably higher in the PLA group. This result agrees with the analgesymeter test, which indicated an improved sensory-motor response,leading to the spinal cord-withdrawal reflex. The unimproved SFI results of the PLA group may be related to greater impairment of motor control. Indeed, a peripheral lesion disorganizes the barrel cortex representation (Oliveira et al., 2014), and sensory-motor integration within the neocortex participates in the hind-limb posture. It remains to be determined whether PLA NGCs are related to enhancement of regeneration in the distal portion of the nerve or in neuromuscular synaptogenesis.
Since our study compared crude conduits, the regeneration and functional outcome were not expected to be similar to normal animals. However, our experimental design allowed the best comparison of a single variable, i.e., the NGC structure. We conclude that of the three NGCs analyzed, the most efficient for nerve repair was the PLA NGC, probably due to its structural characteristics, which seem to be even more important than its composition. Combination of PLA NGC with injection of trophic factors genes and/or grafted Schwann or stem cells might enhance regeneration and the functional outcome after peripheral nerve lesion.
Author contributions: FMP, RCCD, JTO, DFPAD, COG, HRM, ACRS,NTC, BTS, CCP, and CPB contributed to the study implementation.FMP, RCCD, JTO, HRM, COG, and AMBM wrote the paper. AMBM was responsible for design of the study and supervising the experiement,and revised the manuscript critically for important intellectual content.All authors approved the final version of the paper.
Conflicts of interest: There is noconflict of interestto declare in this work.
Financial support: This work was supported by Grant 465656/2014-5 from Conselho Nacional de Desenvolvimento Científico e Tecnológico(CNPq), Brasil, and also supported by CAPES and FAPERJ, Brasil.None of the funding bodies play any role in the study other than to provide funding.
Institutional review board statement: All animal procedures were approved by the Ethics Committee for the Use of Experimental Animals of the Center of Health Sciences of the Federal University of Rio de Janeiro, Brazil (Protocol DHE003).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer: Carolyn Tallon, Johns Hopkins University, USA.
Additional file: Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Validity and reliability of the Ocular Motor Nerve Palsy Scale
- Mitogen-activated protein kinase phosphatase 1 protects PC12 cells from amyloid beta-induced neurotoxicity
- High-frequency (50 Hz) electroacupuncture ameliorates cognitive impairment in rats with amyloid beta 1–42-induced Alzheimer’s disease
- Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia
- Combined VEGF/PDGF improves olfactory regeneration after unilateral bulbectomy in mice
- Transcriptome analysis of adherens junction pathwayrelated genes after peripheral nerve injury