Motor imagery training induces changes in brain neural networks in stroke patients
Fang Li , Tong Zhang , , Bing-Jie Li , Wei Zhang , Jun Zhao , Lu-Ping Song
1 Capital Medical University School of Rehabilitation Medicine, Beijing, China
2 Neurorehabilitation Center, Beijing Boai Hospital, China Rehabilitation Research Center, Beijing, China
Funding: This study was supported by the National Natural Science Foundation of China, No. U1613228; a grant from the Sub-Project under National “Twelfth Five-Year” Plan for Science & Technology Support Project in China, No. 2011BAI08B11; a grant from the Beijing Municipal Science & Technology Commission in China, No. Z161100002616018; the Special Fund for Basic Scientific Research Business of Central Public Scientific Research Institutes in China, No. 2014CZ-5, 2015CZ-30.
Abstract Motor imagery is the mental representation of an action without overt movement or muscle activation. However, the effects of motor imagery on stroke-induced hand dysfunction and brain neural networks are still unknown. We conducted a randomized controlled trial in the China Rehabilitation Research Center. Twenty stroke patients, including 13 males and 7 females, 32–51 years old, were recruited and randomly assigned to the traditional rehabilitation treatment group (PP group, n = 10) or the motor imagery training combined with traditional rehabilitation treatment group (MP group, n = 10). All patients received rehabilitation training once a day, 45 minutes per session, five times per week,for 4 consecutive weeks. In the MP group, motor imagery training was performed for 45 minutes after traditional rehabilitation training, daily.Action Research Arm Test and the Fugl-Meyer Assessment of the upper extremity were used to evaluate hand functions before and after treatment. Transcranial magnetic stimulation was used to analyze motor evoked potentials in the affected extremity. Diffusion tensor imaging was used to assess changes in brain neural networks. Compared with the PP group, the MP group showed better recovery of hand function, higher amplitude of the motor evoked potential in the abductor pollicis brevis, greater fractional anisotropy of the right dorsal pathway, and an increase in the fractional anisotropy of the bilateral dorsal pathway. Our findings indicate that 4 weeks of motor imagery training combined with traditional rehabilitation treatment improves hand function in stroke patients by enhancing the dorsal pathway. This trial has been registered with the Chinese Clinical Trial Registry (registration number: ChiCTR-OCH-12002238).
Key Words: nerve regeneration; stroke; hand function; motor imagery; brain neural network; motion evoked potential; dorsal pathway; ventral pathway; diffusion tensor imaging; neural regeneration
Introduction
In the United States each year, more than 690,000 adults suffer an ischemic stroke (Kernan et al., 2014), and most victims cannot take care of themselves. Approximately 2 million stroke patients are newly diagnosed each year in China, of which 70–80% cannot live independently because of disability (Chinese Society of Neurology et al., 2017). Although stroke centers are able to promptly detect stroke symptoms and provide treatment, only a small number of acute stroke patients receive intravenous injection of recombinant tissue-type plasminogen activator for thrombolysis, and many patients still suffer from residual dysfunctions (Winstein et al., 2016). Therefore, there is an urgent need for effective neurorehabilitation to alleviate dysfunction, especially of the upper extremity, in survivors(Winstein et al., 2016).
Over the past 30 years, many new rehabilitation methods have emerged, such as constraint-induced movement therapy(Zarantonello et al., 2017), motor relearning training, music therapy (Yakupov et al., 2017), and motor imagery therapy(Herrador Colmenero et al., 2018). These methods can improve motor function in a short period of time by inducing neural plastic changes. Among these, motor imagery therapy, also termed mental practice (Cunha et al., 2017; Park et al., 2017), is a very promising therapy, particularly because it involves a complete cognitive process (Vry et al., 2012).Furthermore, it resembles motor execution in behavioral and physiological studies (Kato and Kanosue, 2017; Tong et al.,2017). Motor imagery is the mental representation of an action without any overt movement or muscle activation (Jeannerod,2001; O’Shea and Moran, 2017). Numerous studies have shown that the brain regions activated by hand-related motor execution and those activated by motor imagery are very similar (Vry et al., 2012; Pilgramm et al., 2016). These regions include the bilateral primary motor cortex, supplementary motor area, posterior parietal cortex and bilateral cerebellar regions.The posterior parietal cortex consists of the supra-marginal gyrus, secondary sensory cortex and intraparietal sulcus (Vry et al., 2012). Compared with motor execution, motor imagery activates a wider area in the prefrontal cortex and posterior parietal lobe, covering the left ventral and dorsolateral prefrontal cortex, the other frontal lobe and the left medial intraparietal sulcus (Vry et al., 2012). The neural networks linking the brain regions related to motor execution and motor imagery processes can be divided into the dorsal and ventral pathways,which functionally connect different cortical networks involved in motor imagery and motor execution.
The dorsal fibers are mainly composed of the superior longitudinal fasciculus and the arcuate fascicle. The superior longitudinal fasciculus II–III connects the parietal lobe and the prefrontal cortex, while the arcuate fascicle links the prefrontal and temporal lobes (Makris et al., 2005). The dorsal pathway conveys sensory information about motion planning and control, which appears to be associated with motor execution.The ventral pathway is the ventral fiber bundle through the extreme capsule, which connects the imagery-specific brain regions of the prefrontal and parietal lobe regions. Some studies have shown that the ventral pathway is involved in motor cognition (motor perception) and semantics during motor imagery (Vry et al., 2012), language comprehension (Saur et al., 2008), spatial attention (Umarova et al., 2010), arithmetic(Willmes et al., 2014), and motor perception (Hoeren et al.,2013). However, the neural changes in the dorsal and ventral pathways induced by motor imagery training remain unclear.
Some studies (Liu et al., 2014a, b) have shown that motor imagery training combined with traditional treatment can substantially improve motor function compared with traditional treatment alone. Fujisawa et al. (2011) found that motor imagery reduced the suppression of motor evoked potentials(MEPs) and F waves induced by relaxation, suggesting that the excitability of the spinal cord and cerebral cortex are enhanced by imaging tasks. However, the changes induced by motor imagery on MEPs are unclear.
In the present study, stroke patients were treated with traditional rehabilitation treatment or motor imagery training combined with traditional rehabilitation treatment. We evaluated motor function of the upper extremity and investigated neural plastic changes before and after treatment using diffusion tensor imaging (DTI) and transcranial magnetic stimulation.
Subjects and Methods
Design
This randomized controlled pilot study was performed in the Beijing Boai Hospital, China Rehabilitation Research Center,China. A total of 20 stroke patients who were admitted to the China Rehabilitation Research Center for rehabilitation from March 2014 to March 2016 were randomly divided into two groups of 10 patients each, as follows: one group was given motor imagery training combined with traditional rehabilitation treatment (MP group), while the other was given traditional rehabilitation treatment (PP group). The study was approved by the ethics committee of the China Rehabilitation Research Center (approval number: 2012-045-1). Participants provided informed consent according to the Declaration of Helsinki. This trial has been registered in the Chinese Clinical Trial Registry (registration number: ChiCTR-OCH-12002238)(Protocol version (1.0)). Sample size calculation is shown in Additional file 1. Clinical data for the 20 patients are given in Table 1.
Inclusion criteria
Patients who met all of the following criteria were considered for study inclusion:

Table 1 Clinical characteristics of the subjects
(1) Age of 18–80 years, either sex, right-handed (Oldfield,1971).
(2) Confirmation by computed tomography or magnetic resonance imaging (MRI) of cerebral infarction accompanied by right limb motor dysfunction, meeting the diagnostic criteria for stroke of the Fourth National Cerebrovascular Disease Conference of 1995 (Chinese Society of Neurology and Chinese Society of Neurosurgery, 1996).
(3) Stable vital signs, stable condition, and no disease progression for over 48 hours.
(4) Brunnstrom stage III or higher of the affected upper limb and hand, and the elbow can perform a few stretching movements (Liu et al., 2014b).
(5) Normal cognitive function, normal vision or normal after correction, and capable of coping with the experiment.
(6) Total score in the Kinesthetic and Visual Imagery Questionnaire-10 of ≥ 25 points (Malouin et al., 2007), accuracy rate of mental rotation experiment ≥ 75%, can accurately perform simple and complex finger opposition tasks, while the motor execution time (t1) > motor imagery time (t2) (Simmons et al., 2008).
(7) Provision of informed consent.
Exclusion criteria
Patients who met one or more of the following conditions were excluded from the study:
(1) Multiple stroke, cerebellar infarction, cerebral hemorrhage.
(2) Severe upper extremity pain or spasm.
(3) Post-stroke depression.
(4) Severe aphasia, memory disorders, attention disorders, visual disturbances and communication disorders or other neural symptoms that may interfere with this study.
(5) MRI contraindications, such as metal in the body.
(6) Severe heart, lung, liver or renal disorders.
(7) Cognitive impairment.
(8) History of other neurological or psychiatric illnesses or epilepsy.
Training methods Traditional rehabilitation training methods
Traditional rehabilitation was performed in both the MP and PP groups. During traditional rehabilitation, motor dysfunction rehabilitation programs were developed based on the patients’motor function. Therapists and patients took one-on-one initiative treatment, including occupational therapy. Patients received traditional rehabilitation twice a day, 45 minutes per session, 10 times a week, for 4 consecutive weeks.
Motor imagery training
In the MP group, MP training was performed after traditional rehabilitation training once a day, 45 minutes per session, five times a week, for 4 consecutive weeks.
Environment and posture for motor imagery training: In a quiet and spacious room, the patient sat in a chair with a backrest, with the hips, knees and ankles at 90°, with the neck and spine erect, and the forearm in front of the treatment table. For motor imagery training, the patient had the forearm in the pronated, supinated or neutral position, according to the task.
Tasks of motor imagery training: To improve hand function, the purely functional tasks recommended by Simmons et al. (2008) were used, including 11 small tasks. These included thumb flexion-extension movement (pronation), index finger flexion-extension (pronation), thumb circling, index finger circling, adduction and abduction of all digits, flexion and extension of all digits, making a fist and then spreading the fingers,wrist lateral movement (five fingers unbent), wrist flexion and extension (five fingers unbent), wrist circling, and hand flip.Five tasks were randomly selected for each training session.
Procedure for motor imagery training: The patient was required to imagine each task in a first-person perspective, without undertaking any physical action.
Adduction and abduction of all fingers were used as an example.
(1) The patient was asked to watch a video of the task with two repetitions. The movement of the hands in the video was recorded in the first perspective.
(2) The therapist demonstrated adduction and abduction of all digits, repeating twice.
(3) The patient used the healthy hand to perform adduction and abduction of all digits, repeating twice.
(4) The therapist instructed the patient, “Please close your eyes and focus on your unaffected hand (motor area), and do not move your fingers or arms. Now, imagine the healthy hand opening fingers wide and then closing them flat together;remember the feeling of your muscles and skin moving. Finish two repetitions, then open eyes and describe your experience.”
(5) Following the instructions, the patient imagined adduction and abduction of all digits, with two repetitions, each carried out in the first perspective without physical action.
(6) As in steps (4) and (5), the patient imagined three repetitions of adduction and abduction of all digits, but this time of the affected hand.
(7) The patient attempted to use the affected hand to perform adduction and abduction of all digits, three times.
(8) This process for each task was repeated twice. Whether actual physical action occurred during the motor imagery training was carefully observed and recorded.
Evaluation of upper extremity function
Pre- and post-rehabilitation, upper extremity function of each patient was evaluated with the Action Research Arm Test(Yozbatiran et al., 2008) and the Fugl-Meyer Assessment of the upper extremity (See et al., 2013).
Transcranial magnetic stimulation
We employed the Keypoint portable type EMG potentiometer produced by Dantec Corporation (Skovlunde, Denmark), and the Magstim Rapid transcranial magnetic stimulator and circular coil produced by Magstim Company (Dwyfed, UK).Static task of the paralyzed hand was as follows:
(1) In a quiet, comfortable room with no sound or light stimuli, the subject sat relaxed in a comfortable chair. The lower jaw and forehead were fixed to prevent the head from moving and affecting the experimental procedure and results.The subject did not conduct any psychological activity.
(2) For transcranial magnetic stimulation, single-pulse stimulation was chosen, and the intensity of stimulation was adjusted.
(3) Before electrode placement, the region was scrubbed with 75% alcohol to remove keratinocytes. The circular recording and reference electrodes were pasted on the muscle belly and tendon of the musculus abductor pollicis brevis in the thenar eminence, respectively. The spacing between the recording and reference electrodes was at least 1 cm. The ground electrode was connected to the contralateral wrist.
(4) According to the standard anatomy atlas, we found the best stimulation site. First, 60% of the maximum output intensity was selected. Ten sham stimuli were applied (the stimulus was not set on the scalp), and the amplitude was observed.The procedure was continued if the amplitude was 0 and there was no detectable spike. The axis perpendicular to the coil plane was determined. The center of the coil was used as the axis of the coil-induced magnetic field focus, which was made through the target area. The contralateral primary motor cortical hand motor area was stimulated using the center of the stimulation coil (where the magnetic field was strongest).The coil was close to the scalp. The direction of the current in the coil was clockwise from the top view. The coil point was manually moved in the hand motor area, and the MEP waveform in the contralateral musculus abductor pollicis brevis was observed. The point with the largest amplitude, the shortest latency and highest repeatability was selected as the best stimulation point for transcranial magnetic stimulation. If the waveform was not induced, the output strength was increased by 5% and the procedures were repeated until the best stimulation point was found.
(5) Determination of motor threshold: The subject was completely relaxed. After determining the optimal stimulation point, the coil was fixed. The motor threshold was the minimum stimulus intensity that induced at least five MEPs, peakto-peak, at 50 μV, for 10 consecutive stimuli (5–10-second intervals between stimuli) at the optimal stimulation point. The initial stimulus intensity was 30%, with 10 stimulations at the optimal stimulation point. In the absence of significant amplitude changes, the stimulus intensity was increased by 5% until at least 5 MEPs, peak-to-peak, at 50 μV, in 10 consecutive stimuli were observed. This stimulus intensity was the motor threshold. Continuous visual monitoring of myoelectric activity showed that no background myoelectric activity occurred.In a formal experiment, the single pulse stimulus intensity was set to 110% of the motor threshold.
(6) Recording myoelectric activity. After determining the optimal stimulus point and stimulus intensity, myoelectric activity was recorded until the EMG stabilized before the experiment.The subject performed the tasks in the order described above,and the muscle contraction of the hand was recorded.
(7) The measurement was repeated five times. The parameter with the largest amplitude and the shortest latency was selected and recorded.
fMRI scanning
fMRI scanning was performed using the Philips Achieva 3.0T TX Magnetic Resonance Scanner (Philips Healthcare, Best,The Netherland) with a 32-channel head dedicated quadrature coil. The 3D T1 and DTI were scanned. During scanning, the patient was supine in the examination bed. The operator used a sponge pad to help immobilize the patient’s head. 3M anti-noise elastic ear plugs were used to reduce machine noise.Before scanning, the operator communicated with the patient with the microphone, requiring the patient to maintain the eyes closed, and asking the subject how they felt before each scan to ensure the effectiveness of data collection.
The scanning parameters for the high-resolution 3D T1-weighted images were as follows: layer = 180, layer thickness = 1 mm, layer spacing = 0, repetition time = 8.2 ms, echo time = 3.8 ms, field size = 240 mm × 240 mm, resolution =240 × 240, flip angle = 8° .
The DTI scanning parameters were as follows: slice thickness = 2 mm, layer distance = 0 mm, 70 layers covering the whole brain, diffusion sensitive gradient direction number 32,scanning time of approximately 10 minutes. DTI scan was conducted using a single excitation plane echo sequence. An image of 32 non-collinear diffusion gradients (b = 1000 s/mm2) and two images without diffusion weighting (b = 0 s/mm2) were acquired. A total of 70 cross-sections were collected per subject. The relevant parameters were as follows: field of view = 224 mm × 224 mm, acquisition matrix = 112 ×109, number of averages = 1, slice thickness = 2 mm, interlayer spacing = 0, echo time = 71 ms, and repetition time = 9331 ms.
MRI data processing and statistical analysis
The DTI data were processed using SPM 12 (Statistical Parametric Mapping 12) and FSL (FMRIB Software Library,Oxford Center for Functional MRI of the Brain, UK) based on matlab2012a (Mathworks, Inc., Natick, MA, USA). First,the quality of the image data was determined and artifacts were eliminated. The diffusion-weighted image was registered to the corresponding b = 0 images, and the head movement and eddy current were corrected. Based on the FSL diffusion toolbox, the white matter fiber integrity of the main indicators fractional anisotropy (FA) and mean diffusivity (MD)were calculated. In total, 12 spheres in the frontal lobe and 6 spheres in the parietal lobe with a radius of 4 mm were selected as seed points for fiber probability tracing according to the brain regions activated by motor execution and motor imagery (seed points are shown in Table 2). Subsequently, the ventral/dorsal pathways in the left/right brain were tracked by the method of fiber probability tracking. Finally, the white matter integrity of the left/right and ventral/dorsal sides of each group was evaluated in the two scans.
Statistical analysis was performed using SPSS 20.0 (IBM,Armonk, NY, USA). Variables are shown as the mean ± SD.FA, MD and amplitude and latency between the two groups were analyzed by independent sample t-test. The difference between pre- and post-training was analyzed by paired samplet-test. The differences in the FA and MD between pre- and post-training were analyzed by independent sample t-test. P <0.05 was considered statistically significant.
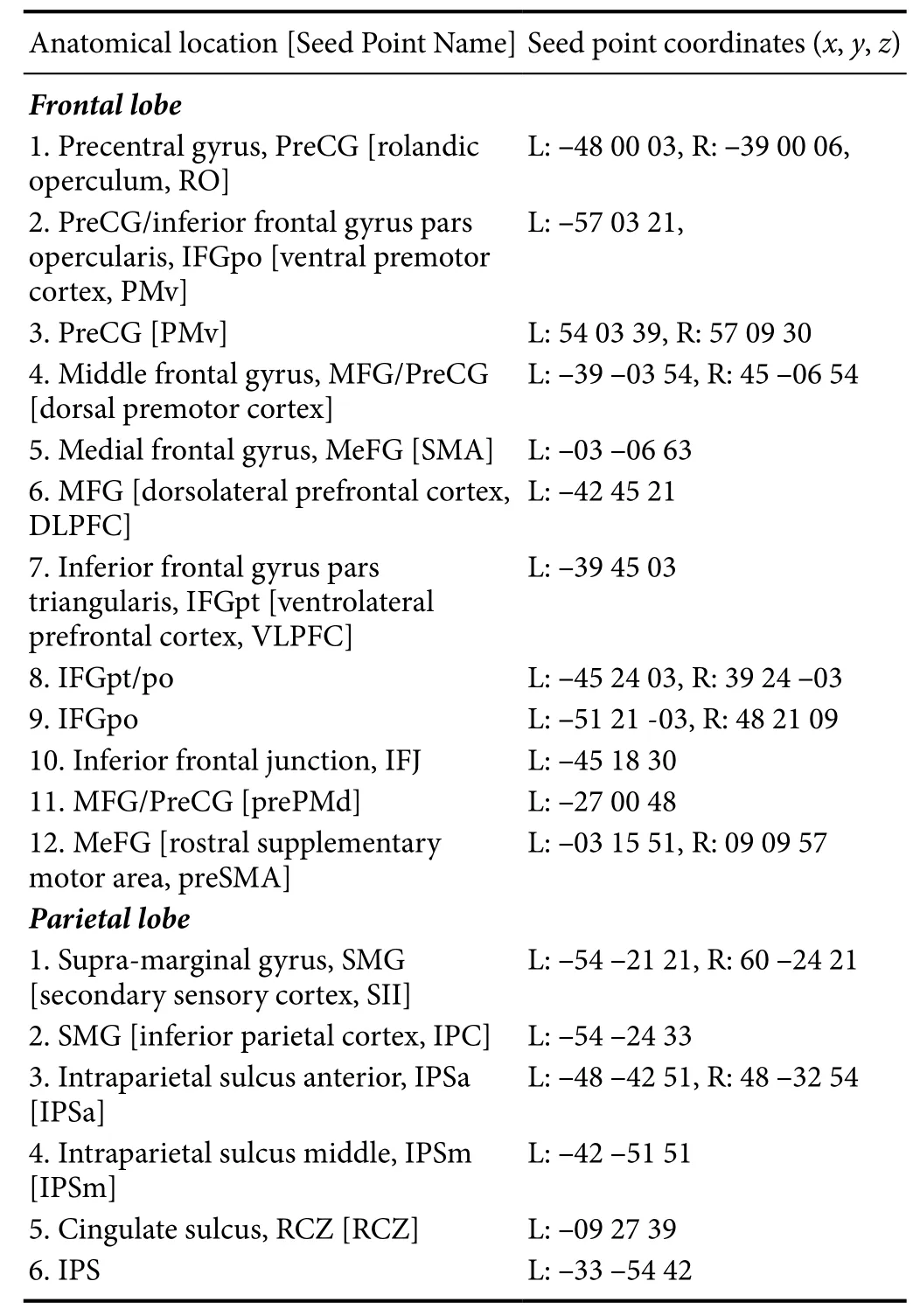
Table 2 Diあusion tensor imaging seed points
Results
Effect of motor imagery training combined with traditional rehabilitation treatment on hand function in stroke patients
Before training, the Action Research Arm Test scores in the MP and PP groups did not significantly differ (t = 0.270, P =0.790). Before training, the Fugl-Meyer Assessment scores in the MP and PP groups did not significantly differ (t = 0.272, P= 0.789) (Figure 1).
After training, the Action Research Arm Test score in the MP group was higher than that in the PP group (t = 6.48, P =0.00). Post-training, the Fugl-Meyer Assessment score in the MP group was significantly higher than that in the PP group (t= 4.7, P = 0.001) (Figure 1).
Effect of motor imagery training combined with traditional rehabilitation treatment on MEPs in the abductor pollicis brevis muscle in stroke patients
The amplitude and latency of MEPs in the two groups werenormally distributed before and after the training, and the independent sample t-test was used for comparison (Figure 2). MEP amplitudes in the MP (P = 0.0000) and PP groups (P = 0.0017)both significantly improved after training compared with before training. Post-training, MEP amplitude was higher in the MP group compared with the PP group (P = 0.0181). However,there was no significant difference in MEP latency pre- and post-intervention in the MP or PP group.

Table 3 Eあect of motor imagery training combined with traditional rehabilitation treatment on FA of the bilateral dorsal/ventral pathway in stroke patients
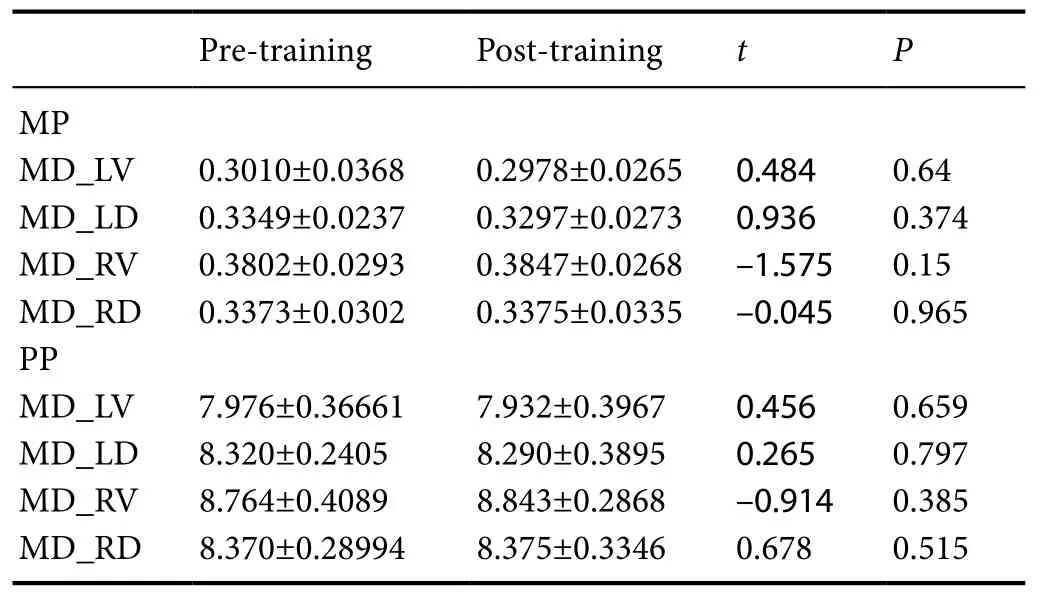
Table 4 Eあect of motor imagery training combined with traditional rehabilitation treatment on MD of the bilateral dorsal/ventral pathway (× 104) in stroke patients
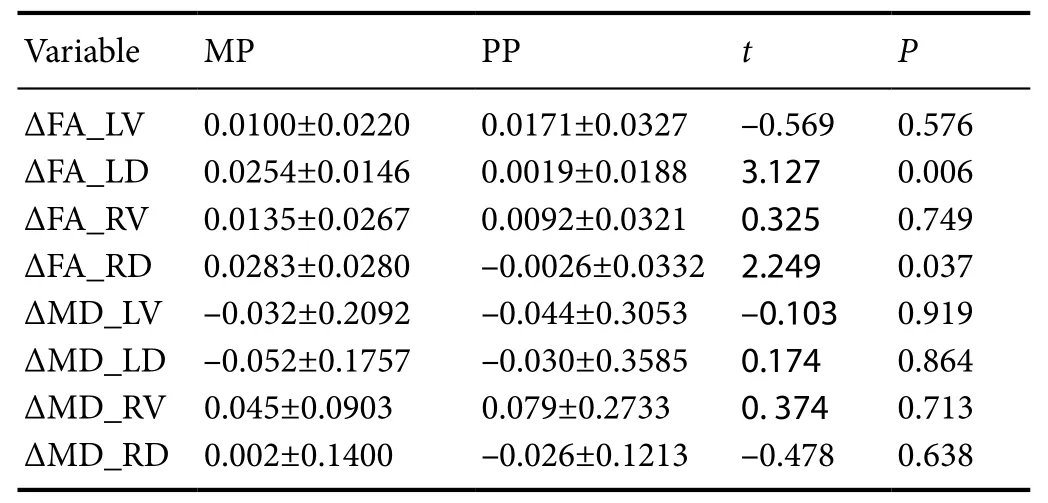
Table 5 Eあect of motor imagery training combined with traditional rehabilitation treatment on FA and MD (× 104) in stroke patients
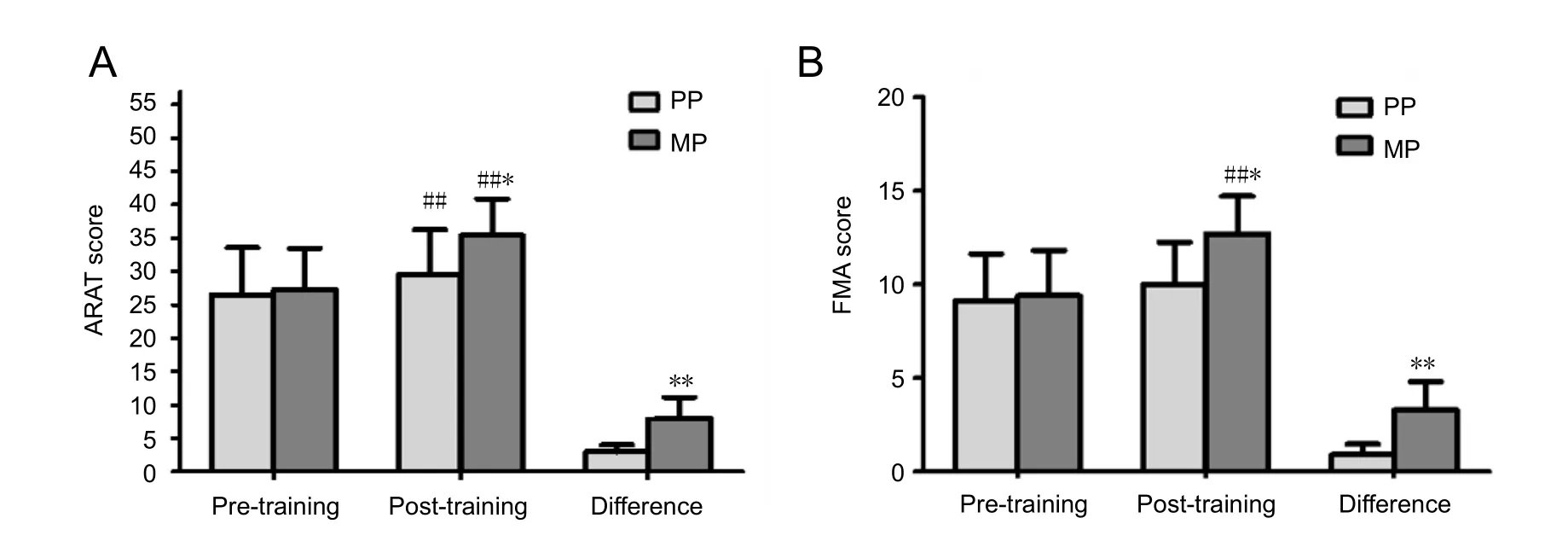
Figure 1 Eあect of motor imagery training combined with traditional rehabilitation on hand function in stroke patients.
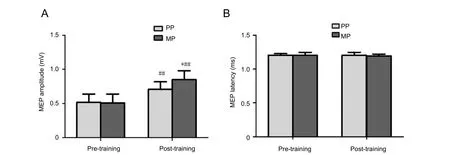
Figure 2 Eあect of motor imagery training combined with traditional rehabilitation treatment on MEPs in the abductor pollicis brevis in stroke patients.
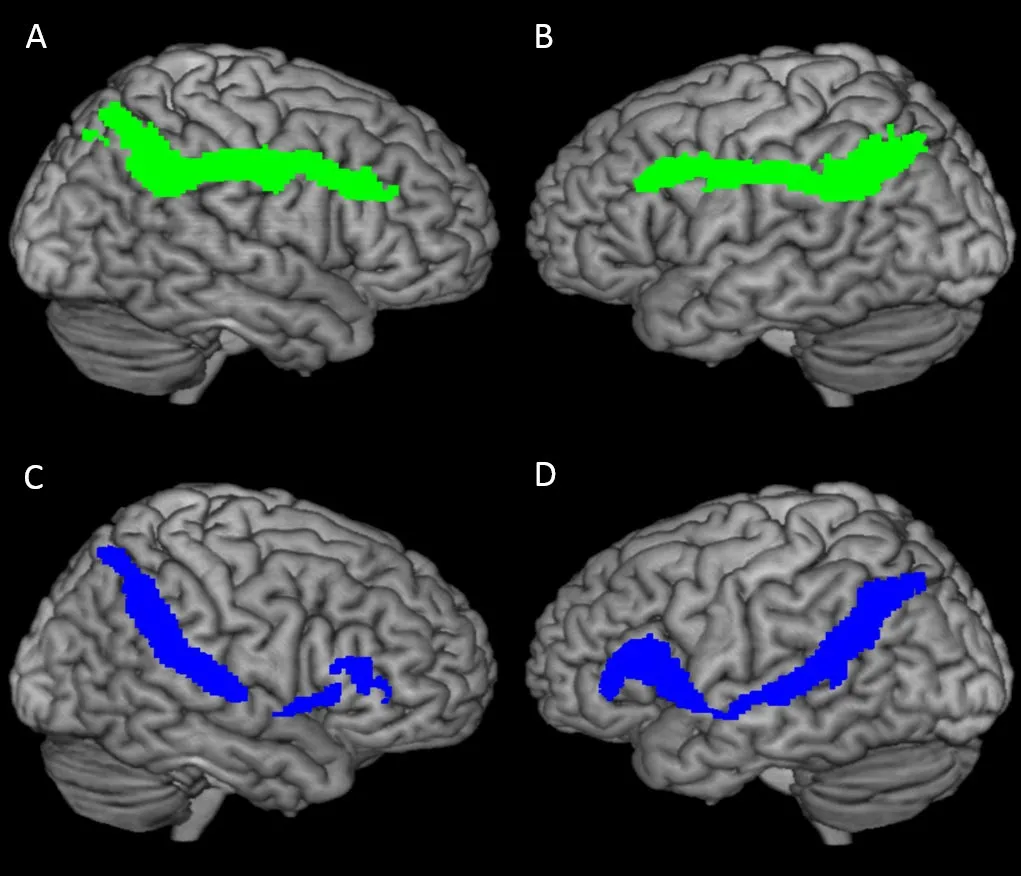
Figure 3 Leた/right and dorsal/ventral pathway tracked by the fiber probability tracking method.
Tracking the left/right and dorsal/ventral pathways by probabilistic fiber tracking
Using the probabilistic fiber tracking method, we tracked the left/right and dorsal/ventral pathways (Figure 3).
Effect of motor imagery training combined with traditional rehabilitation treatment on the bilateral dorsal/ventral pathway in stroke patients
There were no significant differences in the FA or MD of the bilateral dorsal and ventral pathways between the two groups pre-training. FA_RD was significantly higher in the MP group than in the PP group post-training (P = 0.008), but there were no significant differences in FA_LV, FA_LD or FA_RV.
Changes in FA: In the MP group, FA_LD and FA_LD were significantly higher compared with pre-training (P < 0.05).Although FA_LV and FA_RV in the MP group increased post-training, the differences were not significant. FA in the PP group did not change substantially pre- and post-training,and there was no statistically significant difference (Table 3).
Changes in MD: The MD_LV and MD_LD decreased in the MP and PP groups post-training (P > 0.05). In the two groups, MD_RV and MD_RD increased slightly, but not significantly, after training (Table 4).
The ΔFA_LD (P = 0.006) and ΔFA_RD (P = 0.037) in the MP group were significantly higher than those in the PP group(Table 5).
ΔMD_LV and ΔMD_LD in the two groups were negative values, but there was no significant difference between the two groups. There was also no significant difference between the ΔMD_RV and ΔMD_RD in either group (Table 5).
Discussion
Effects of motor imagery training and traditional rehabilitation training on hand motor function
In this study, hand function improved in both the MP and PP groups after 4 weeks of training. Furthermore, Action Research Arm Test and Fugl-Meyer Assessment results showed that functional improvement in the MP group was substantially better than that in the PP group, indicating that motor imagery training combined with traditional rehabilitation training is more effective than traditional rehabilitation training alone. This finding is consistent with our previous study (Liu et al., 2014a). Moreover, the amplitude of MEPs in the MP group was significantly higher after training. Previous studies(Cincotta et al., 1999; Kaneko et al., 2003, 2014; Leonard and Tremblay, 2007; Kang et al., 2011; Xu et al., 2014) demonstrated that neural activity in the motor areas increases during motor imagery, suggesting that the increase in the MEPs induced by motor imagery likely results from cerebral activation.Furthermore, a shortened latency was noted. However, inconsistent with our previous results, we found here that the MEP latency in the musculus abductor pollicis brevis under resting conditions after motor imagery training did not substantially decrease. The discordance might be attributable to differences in the patient sample. Furthermore, the small sample size and procedural differences might also contribute to the discrepancy.
Braun et al. (2013) conducted a meta-analysis on the effects of motor imagery in patients with neurological diseases.They showed that motor imagery markedly improved poststroke hand function (Action Research Arm Test: SMD 0.62;95% CI: 0.05–1.19). However, other studies show that the efficiency of motor imagery training is not consistent. According to a recent systematic review (Guerra et al., 2017), if the quality of the studies is not taken into consideration, it can be concluded that motor imagery training substantially promotes the recovery of motor function following stroke. However, if only high-quality studies are analyzed, it can be concluded that motor imagery training does not improve outcome. Harris and Hebert (2015) used the PETTLEP (Physical, Environment,Task, Timing, Learning, Emotion and Perspective) model to systematically review studies on motor imagery for upper extremity function published between 1987 and 2014. They found that motor imagery training was performed differently in the various studies. There is a high heterogeneity in methodological quality of the studies and conflicting results on the effects of motor imagery. More high-quality studies and greater standardization of interventions are needed to better assess the effectiveness of motor imagery for persons with stroke.
Although it is unknown whether all stroke patients could benefit from motor imagery, our studies suggest that the effectiveness of motor imagery varies according to age. Relatively young patients with normal cognitive function exhibit greater motor functional improvements after motor imagery training(Zhang and Zhang, 2013; Liu et al., 2014c). This prompted us to evaluate the cognitive and executive abilities of patients before motor imagery training. Subjects recruited in this study were assessed for implicit motor imagery ability, and we excluded patients with poor motor imagery ability. Our study shows that only patients with good motor imagery ability can benefit from motor imagery training. The majority of eligible subjects were between 40 and 50 years of age, suggesting that motor imagery training is more effective in stroke patients aged 40–50 years.
Selection of seed points for tracking the ventral/dorsal pathway
In this study, we focused on the recovery of hand function in stroke patients. Brain motor execution and motor imagery-activated brain regions were selected as seed points, and changes in fiber connectivity among seed points were observed by DTI. According to previous studies, the rostral side of the bilateral motor area, inferior frontal gyrus pars opercularis, left inferior parietal cortex, adjacent intraparietal sulcus, bilateral secondary sensory cortex, left anterior and medial parts of the intraparietal sulcus, basal ganglia and cerebellum are markedly activated by both the motor execution and motor imagery processes of wrist motion (Vry et al., 2012). The left ventrolateral prefrontal cortex and dorsolateral prefrontal cortex, other frontal lobe regions and the left medial intraparietal sulcus, the caudal, ventral and tegmental sections of the inferior frontal gyrus corresponding to the Broca region, and the inferior frontal junction are also activated during motor imagery training in comparison with motor execution (Vry et al., 2012).The rostral dorsal premotor cortex in the left motor area, the rostral supplementary motor area and the cingulate cortex are also activated (Vry et al., 2012). In the parietal lobe, the medial intraparietal sulcus is specifically activated only during motor imagery training (Choi et al., 2006). These observations suggest that the main brain area activated during wrist motor execution and motor imagery training is the parietal lobe.Therefore, we selected the parietal lobe as the seed point and tracked the ventral dorsal pathway to investigate how motor imagery affects hand function.
Changes in the dorsal and ventral pathways pre- and post-training
There are numerous studies on functional connectivity in the brain (Saiote et al., 2016; Wang et al., 2016; Makary et al.,2017; Rathee et al., 2017; Stefano Filho et al., 2017). Schulz et al. (2015, 2016) investigated the M1–ventral premotor cortex and anterior intraparietal sulcus–ventral premotor cortex pathways and found a strong association with basic residual motor function of the affected hand after stroke. Furthermore,the ventral premotor cortex and the M1 communicate with secondary parietal and frontal motor areas (anterior intraparietal sulcus and ventral premotor cortex) (Schulz et al., 2015).The anterior intraparietal sulcus–premotor cortex pathway is involved in hand dysfunction caused by stoke (Rehme et al.,2012). The functional connectivity between the ipsilesional M1 and ipsilesional inferior parietal lobe is associated with the recovery of motor function (Yin et al., 2012). The M1, ventral premotor cortex and anterior intraparietal sulcus-related brain activity and interactions are considered related to skilled hand function (Davare et al., 2009, 2010). The association between the M1 and ipsilesional posterior parietal regions is enhanced,whereas M1 function connectivity with the contralesional posterior parietal cortex is reduced by subacute stroke. The anterior intraparietal sulcus may provide the visual information involved in motor execution (Reichenbach et al., 2011;Karabanov et al., 2013).
Our knowledge of the functional connectivity changes induced by motor imagery after stroke is still limited. The present study provides the first DTI data on dorsal and ventral pathway changes in stroke patients given motor imagery training. Our findings show that motor imagery improves hand functional recovery by enhancing the plasticity of brain neural networks. After rehabilitation training, MEP amplitude was higher in the MP group than in the PP group, suggesting that the amplitude increase may be associated with the integrity of white matter fibers. Mizuguchi et al. (2009) showed that during motor imagery of hand muscle contraction, corticospinal excitability of the muscle changed depending on the force level of the imagined contraction. In agreement with this observation, another study (Kato and Kanosue, 2017) showed that during motor imagery, the amplitudes of the MEPs of the extensor carpi radialis and flexor carpi radialis increased.Furthermore, motor imagery robustly increases corticospinal excitability, which persists for at least 30 minutes after the end of the training session (Mrachacz-Kersting et al., 2017).During hand laterality judgments and implicit motor imagery,subjects often employ visual strategies, which might underlie the lack of corticospinal facilitation, as evaluated by MEPs measurement, in some studies (Ferron and Tremblay, 2017).This suggests that only first-person perspective motor imagery results in corticospinal facilitation. However, data on MEP latency changes induced by motor imagery are limited.
The magnitude of the MEP may reflect the integrity of white matter fibers. The DTI findings show that dorsal pathway integrity in the MP group was greater than that in the PP group. Together, our results suggest that motor imagery combined with traditional rehabilitation rapidly improves dorsal pathway integrity, helping to restore hand function.
The FA value is associated with the integrity of white matter myelination, fiber density and organization. The larger the value, the stronger the nerve conduction ability (Song et al.,2012). Our study showed that the FA values in the bilateral dorsal pathways in the MP group were markedly higher after training than before training, suggesting that motor imagery combined with traditional rehabilitation improves dorsal pathway integrity. There was no significant difference in the FA values of the bilateral dorsal pathway between pre- and post-training in the PP group, indicating that traditional rehabilitation training does not significantly improve the integrity of the dorsal pathway. Taken together, these findings suggest that motor imagery facilitates the restoration of the integrity of the bilateral dorsal pathways, while traditional rehabilitation training improves upper extremity function independently of the bilateral dorsal pathways. After training, the FA value in the right dorsal pathway was significantly higher in the MP group than in the PP group, indicating that white matter fiber transport ability in the right dorsal pathway was greater in the MP group than in the PP group. The ΔFA_LD and ΔFA_RD values in the MP group were greater than those in the PP group, suggesting that dorsal pathway integrity improved to a greater degree in the MP group compared with the PP group.However, there was no distinct change in the ventral pathway in either group, indicating that motor imagery has no effect on ventral pathway integrity.
The ventral and dorsal pathways are involved in motor cognition. Some researchers have suggested that the ventral pathway may only be involved in time-independent cognitive processes (such as semantics and meaning) and that time-dependent processes (such as online sensory and motor information integration) may involve the dorsal pathway (Weiller et al., 2011; Rijntjes et al., 2012). In the present study, we investigated the effect of motor imagery on hand function in patients with stroke. Motor imagery is a complete cognitive process.Accordingly, we speculated that motor imagery training may affect the integrity of the ventral dorsal pathway. A recent study on the ventral and dorsal pathways mainly focused on language and vision and less on motion (Vry et al., 2015). Only a few studies have demonstrated that the dorsal or ventral pathway participates in motor imagery/exercise and motor hallucinations (Amemiya and Naito, 2016). The impact of motor imagery on the dorsal and ventral pathways had not yet been investigated. Therefore, we performed DTI scans preand post-training to investigate the effect of motor imagery training on the dorsal and ventral pathways.
Motor execution and motor imagery share the dorsal pathway, but the cognitive mechanisms involved in imagery require further involvement of the ventral pathway (Vry et al., 2012).Here, we found no significant changes in the FA or MD values of the ventral pathway between the two groups pre- and post-training, indicating no significant change in the integrity of the ventral pathway. As mentioned earlier, motor imagery cognitive function is associated with the ventral pathway, and the cognitive functions of all subjects were normal. Before and after training, cognitive function did not change, indicating that the brain regions associated with cognitive activation remain unchanged, and the efferent fibers also remained unchanged.
Previous studies showed that the brain networks activated by motor imagery are similar to those activated by motor execution, and that both activate the frontal cortex (Makris et al., 2005; Vry et al., 2012). In this study, the seed points for tracking the ventral and dorsal pathways were located in the frontal and parietal lobes, as in previous studies. Therefore, we surmise that the motor imagery process may be similar to the execution process (Liu et al., 2014b).
The improvement in the FA values in the bilateral dorsal pathway after training in the MP group suggests that a change in the excitability of the cortex enhances the integrity of the bilateral dorsal pathway. In an electroencephalogram-based experiment of hand motor imagery in patients with unilateral stroke, reduced small-worldness and improved local efficiency was induced by motor imagery of the affected hand, compared with the unaffected hand, in the beta (13–30 Hz) frequency band (De Vico Fallani et al., 2013). Moreover, the abnormal reduction in local efficiency was associated with an increase in interhemispheric connectivity. These observations advance our understanding of motor imagery-induced alterations in functional brain networks. In summary, motor imagery training may change the integrity of the white matter, thereby rapidly improving the plasticity of the brain neural network.
Limitations
(1) Because of the short duration of our study, we did not assess long-term effects of motor imagery training on hand function and brain plasticity in stroke patients. In the future,we will follow-up these patients to further observe the longterm effects of motor imagery training on the ventral/dorsal pathway and to explore the long-term impact of motor imagery training on brain neural network plasticity.
(2) We will further increase sample size and perform randomized controlled clinical trials to further verify the clinical effect of motor imagery training.
Conclusion
Four weeks of motor imagery training combined with traditional rehabilitation treatment improved hand function in stroke patients by enhancing their dorsal pathway. Our novel findings strongly suggest that the dorsal pathway plays a critical role in the recovery of upper extremity function after stroke.
Author contributions: LF and ZT conceived and designed the study,and wrote the paper. LF, LBJ and ZW performed experiments. LF, SLP and ZJ analyzed the data. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Financial support: This study was supported by the National Natural Science Foundation of China, No. U1613228; a grant from the Sub-Project under National “Twelfth Five-Year” Plan for Science& Technology Support Project in China, No. 2011BAI08B11; a grant from the Beijing Municipal Science & Technology Commission in China, No. Z161100002616018; the Special Fund for Basic Scientific Research Business of Central Public Scientific Research Institutes in China, No. 2014CZ-5, 2015CZ-30. The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement: The study protocol was approved by the Ethics Committee of China Rehabilitation Research Center (approval number: 2012-045-1).
Declaration of patient consent: The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients have understood that their names and initials would not be published and due efforts would be made to conceal their identity, but anonymity cannot be guaranteed.
Reporting statement: This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of China Rehabilitation Research Center at the Beijing Boai Hospital, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Individual participant data after deidentification (text, tables, figures, and appendices) will be in particular shared.The study protocol and clinical study report will be made public within 6 months after completion of the trial. Anonymized trial data will be available indefinitely at www. figshare.com.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Additional file 1: Sample size calculation.
- 中国神经再生研究(英文版)的其它文章
- Validity and reliability of the Ocular Motor Nerve Palsy Scale
- Mitogen-activated protein kinase phosphatase 1 protects PC12 cells from amyloid beta-induced neurotoxicity
- High-frequency (50 Hz) electroacupuncture ameliorates cognitive impairment in rats with amyloid beta 1–42-induced Alzheimer’s disease
- Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia
- Combined VEGF/PDGF improves olfactory regeneration after unilateral bulbectomy in mice
- Comparison of morphological and functional outcomes of mouse sciatic nerve repair with three biodegradable polymer conduits containing poly(lactic acid)