Cognitive impairment after traumatic brain injury is associated with reduced long-term depression of excitatory postsynaptic potential in the rat hippocampal dentate gyrus
Bao-Liang Zhang, Yue-Shan Fan, Ji-Wei Wang, Zi-Wei Zhou, Yin-Gang Wu, Meng-Chen Yang, Dong-Dong Sun, Jian-Ning Zhang
Department of Neurosurgery, Tianjin Medical University General Hospital; Tianjin Neurological Institute; Key Laboratory of Post-trauma Neuro-repair and Regeneration in Central Nervous System, Ministry of Education; Tianjin Key Laboratory of Injuries, Variations and Regeneration of Nervous System, Tianjin, China
Funding: This study was supported by the National Natural Science Foundation of China, No. 81330029, 81501057; the Natural Science Foundation of Tianjin of China, No. 17JCQNJC12000; the Tianjin Medical University General Hospital Funding in China, No. ZYYFY2016014.
Abstract Traumatic brain injury can cause loss of neuronal tissue, remote symptomatic epilepsy, and cognitive deficits. However, the mechanisms underlying the effects of traumatic brain injury are not yet clear. Hippocampal excitability is strongly correlated with cognitive dysfunction and remote symptomatic epilepsy. In this study, we examined the relationship between traumatic brain injury-induced neuronal loss and subsequent hippocampal regional excitability. We used hydraulic percussion to generate a rat model of traumatic brain injury. At 7 days after injury, the mean modified neurological severity score was 9.5, suggesting that the neurological function of the rats was remarkably impaired. Electrophysiology and immunocytochemical staining revealed increases in the slope of excitatory postsynaptic potentials and long-term depression (indicating weakened long-term inhibition), and the numbers of cholecystokinin and parvalbumin immunoreactive cells were clearly reduced in the rat hippocampal dentate gyrus. These results indicate that interneuronal loss and changes in excitability occurred in the hippocampal dentate gyrus.Thus, traumatic brain injury-induced loss of interneurons appears to be associated with reduced long-term depression in the hippocampal dentate gyrus.
Key Words: nerve regeneration; long-term depression; traumatic brain injury; hippocampus; interneurons; excitability; dentate gyrus; parvalbumin; cholecystokinin; electrophysiology; quantification; neural regeneration
Introduction
Traumatic brain injury (TBI) can damage the hippocampal formation (Grady et al., 2003; Pedachenko et al., 2015; Mashhadizadeh et al., 2017) resulting in reduced information processing (Mathias et al., 2004; Ashley et al., 2012; Dymowski et al., 2015). This can, in turn, cause impaired physiological function (Witgen et al., 2005; Hoover et al., 2014; Vasudevan et al., 2014; Spiegel et al., 2015; Hosseini-Zare et al., 2017) and frequently leads to impaired cognition and motor skills (Smith et al., 1994; Jourdan et al., 2016; Kamins et al., 2016; Lin et al., 2016; Finch et al., 2017; McGarity et al., 2017; Thomsen et al., 2017). Pathological examinations of post-traumatic human brains have revealed specific damage in the temporal lobe and the limbic hippocampus (Graham et al., 1995; Li et al., 2006).Nonspecific loss of hilar neurons, which usually produce an inhibitory signal, can lead to increased excitability in the dentate gyrus (Santhakumar et al., 2000; Witgen et al., 2005; Aungst et al., 2013; Alwis et al., 2016; Mashhadizadeh et al., 2017). The function of the dentate gyrus is to filter excessive or aberrant activity in the brain. Thus, this brain subregion plays a pivotal role in the consequences of TBI. Several types of GABAergic interneurons regulate cerebral electronic discharges emitted by dentate granule cells (Halasy et al., 1993; Han et al., 1993; Hamidi et al., 2015; Lévesque et al., 2016; Losi et al., 2016; Yamada et al., 2016; Neumann et al., 2017). Intorhino-hippocampal signaling is controlled by the axo-axonic and basket cells in the dentate gyrus (Han et al., 1993). In non-overlapping sub-populations of axo-axonic and basket cells, CCK-immunoreactive and PV-immunoreactive interneurons separately mediate the perisomatic inhibitory control of dentate granule cells (Freund et al., 1996). However, the way in which neuronal loss leads to subsequent alterations in dentate gyrus excitability is unclear.In this study, we sought to define the hippocampal cellular circuit implicated in TBI-induced cognitive impairment in rats.
Materials and Methods
Animals
Sixty adult (two-month-old) male Wistar rats weighing 280–310 g were purchased from the Experimental Animal Laboratories of the Academy of Military Medical Sciences of China[SCXK(Jun)2012-0004]. The rats were individually housed in a temperature-controlled (20 ± 2° C) and humidity-controlled(60%) vivarium with a standard 12-hour light/dark cycle (7:00 a.m. to 7:00 p.m. per cycle) and free access to food and water.The Animal Ethics Committee of Tianjin Medical University of China approved the study protocol. All experimental procedures were conducted according to the principles outlined in the guidance for the Care and Use of Laboratory Animals from the United States National Institute of Health and were in accordance with the Chinese Small Animal Protection Association. The rats were randomly divided into control (n = 30) and TBI (n = 30) groups. Although the rats in both groups underwent surgical procedures, we induced a fluid percussion injury in the rats in the TBI group only.
Establishment of the fluid percussion injury model
After acclimating in the vivarium for 7 days, the rats were anesthetized via an intraperitoneal administration of chloride hydrate (3 mL/kg; Department of General Medical Reagent,Tianjin Medical University, China). The rats were placed in a stereotaxic frame, and their scalp and temporal muscles were reflected to expose the cranium. A craniotomy (3.5 mm × 3.5 mm) was performed over the right parietal bone, 2 mm lateral to the sagittal suture and 3 mm caudal to the coronal suture.Twenty-four hours after surgery, the rats were subjected to an experimental fluid percussion injury at 1.8–2.0 atmosphere(atm), as previously described (Van et al., 2016). Briefly, a male Luer-Lok™ fitting was cemented to the craniotomy site, enabling attachment of the animal to a fluid percussion injury device (model 01-B; New Sun Health Products, Cedar Bluff,VA, USA). A saline bolus from a cylindrical plexiglass reservoir was rapidly introduced into the closed cranial cavity, causing mechanical deformation of the brain. Each group of rats was further subdivided for histological and electrophysiological quantification of hippocampal interneurons.
Modified neurological severity score (MNSS)
We evaluated the neurological function of each experimental rat via the MNSS, which includes tests of sensory, motor,balance, and reflex function (Chen et al., 2001). Lower scores reflect higher function. The test was administrated 24 hours after the fluid percussion injury by an observer who was blinded to the experimental and treatment conditions. Test scores ranged from 3 to 18.
Measurement of long-term depression
On day 7 post-injury, 15 rats from each group were anesthetized with 30% urethane (1.2 g/kg; intraperitoneally) and placed in a stereotaxic instrument (Narishige, Japan). After exposing the surface of the skull, we used a dental drill to create two holes (2 mm × 2 mm) above the left side of the brain, and incised the dura matter. Stimulating electrodes(Advent Co., UK) were then lowered into the perforant path(anteroposterior −8.0 mm, lateral 4.0 mm, dorsoventral 2.8 mm). A recording electrode (Advent Co.) was positioned in the granule cell layer of the dentate gyrus (anteroposterior−4.0 mm, lateral 2.5 mm, dorsoventral 3.0 mm). The stimulating electrodes were adjusted such that stimulation of the perforant path afferents would trigger an optimal excitatory postsynaptic potential in the granule cells. After the responses were stabilized, we recorded 20 minutes of baseline activity under low-frequency stimulation (0.1 Hz), followed by tetanic stimulation (900 pulses, 1 Hz, 4 ms, repeated 5 times) (Su et al., 2009) to induce long-term depression. The excitatory postsynaptic potentials were augmented via a conventional amplifier (AD Instruments Pty Ltd., Australia), and recorded three times per minute after the tetanization. All potentials were monitored on an oscilloscope (AD Instruments Pty Ltd.) and digitized at a sampling interval of 20 µs. We measured the slope for the maximal change in initial excitatory postsynaptic potential and expressed the result as a percentage of the averaged response at baseline: (Slopeaftertetanus/Slopemeanofbaseline) × 100%.
Extracellular input/output curves
We measured long-term depression immediately after assessing the input/output curves. To evaluate synaptic potential, we triggered input/output curves via systematic variation of the stimulus current (0.1–1.0 mA). Stimulant pulses were delivered at 0.1 Hz, and a total of six responses were collected and averaged for each current level. The excitatory postsynaptic potential slopes were augmented via a conventional amplifier(AD Instruments Pty Ltd.).
Immunohistochemical staining

Figure 1 Eあects of TBI on long-term depression and input/output curves in the hippocampal dentate gyrus at 7 days post-injury.

Figure 2 Traumatic brain injury induced PV-IR cell loss in the dentate gyrus.

Figure 3 Traumatic brain injury induced CCK-IR cell loss in the dentate gyrus.
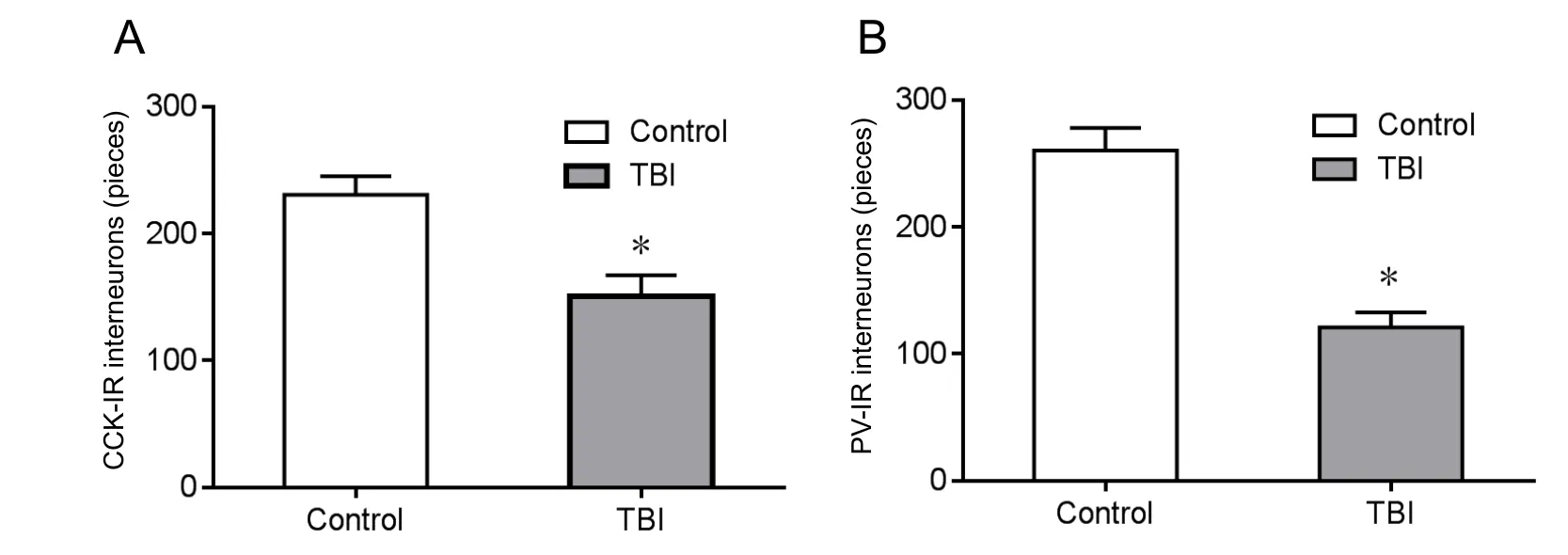
Figure 4 Number of interneurons in the rat dentate gyrus 7 days aたer traumatic brain injury(immunocytochemistry).
On day 7 post-injury, 15 rats from each group were anesthetized with chloride hydrate (30 mL/kg, intraperitoneally) and then sacrificed. The rats were perfused transcardially with a fixative containing either 4% paraformaldehyde (Zsbio, Beijing,China) and 15% picric acid (Zsbio) [for parvalbumin (PV)] or 5% acrolein (Zsbio) [for cholecystokinin (CCK)] (Smith et al.,1994). The brain was removed and hemisected. The ipsilateral hippocampus was dissected and immersed in fixative for an additional 2 days. The hippocampus was then placed on a gelatin-based support block and cut into 50 µm-thick transverse sections on a vibratome. We collected every tenth and eleventh section (i.e., at every 500 µm and 550 µm) in the wells of a tissue culture plate for immunocytochemistry. Sections from the injured rats and control rats were simultaneously processed in the same wells. Sections from each group were labeled accordingly (control and TBI) to prevent confusion. The first and last two sections (i.e., −1.3 mm from the ventral and dorsal tips)were not assessed for immunostained dentate neurons because the granule cell layer and the hilus in these sections were difficult to precisely delineate. The sections were washed in 0.1 M phosphate buffered saline (PBS) with a pH of 7.4. They were incubated with 3% normal goat serum (60 minutes) and then primary antisera against either PV (1:1000; Abcam, Cambridge,MA, USA) or CCK (1:500; Abcam) for 1 or 2 days, respectively.Afterwards, the samples were incubated with a goat-anti-rabbit IgG (for PV, 1:100; for CCK, 1:300; ICN Biochemicals, Costa Mesa, CA, USA) for 6 hours, and then with a peroxidase- antiperoxidase complex (1:100; Dakopatts, Copenhagen, Denmark)overnight. The sections were washed thrice with 0.1 M PBS with a pH of 7.4 and 1% normal goat serum + 0.5% Triton X-100. After immunocytochemical labelling, the sections were removed from the wells and separated into two groups. The sections were mounted on gelatin-coated slides, dried, dehydrated, and covered with a neutral medium and a coverslip. Cell counting was conducted on an IX71 microscope (Olympus,Tokyo, Japan) with the aid of DpController 2.1.1.183 software.
Statistical analysis
Data, expressed as the mean ± SD, were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Interneuron cell counts were compared using an independent samples t-test.Data from the long-term depression recordings and input/output curves in the two groups were compared using a repeated measures analysis of variance. P values < 0.05 were considered statistically significant.
Results
Performance of rat models
A mean MNSS score of 9.5 and mortality rate of 25% was obtained after a controlled impact of 1.8–2.0 atm to the rat brains, indicating significantly impaired brain function.
Changes in input/output curves and long-term depression in TBI rats
The amplitudes of the input/output curves and the long-term depression of the rodent brains are displayed in Figure 1. The input/output curve was higher in the TBI group compared with the control group (Figure 1A). Long-term depression was also higher in the TBI group compared with the control group (Figure 1B; P < 0.05). These data indicate that TBI induced a significant increase in excitability in the dentate gyrus.
TBI-induced hippocampal interneuron loss
Selective vulnerability of particular hippocampal neurons may underlie the cognitive deficits associated with TBI (Mathias et al., 2004). In our intervention, we induced interneuronal loss in the dentate gyrus sub-region of the hippocampus (Figures 2, 3). As per Miki et al. (2000), we defined the different hippocampal sub-regions via immunohistochemical staining.Non-overlapping parvalbumin and cholecystokinin-immunoreactive interneuron (PV-IR or CCK-IR) populations have been found to provide perisomatic inhibitory control of dentate granule cells (Freund et al., 1966). Immunocytochemical analyses (Figure 4) revealed that, compared with the control group, the number of CCK-IR cells in the ipsilateral dentate gyrus was 37.2 ± 4.8% lower than that the TBI group 7 days after fluid percussion injury (Figure 4A). Similarly, the parvalbumin-immunoreactive interneuron population was reduced by 45.6 ± 4.3% in the dentate gyrus (Figure 4B).
Discussion
In this study, we found that rats in the TBI group had conspicuously fewer PV and CCK-immunoreactive cells in the dentate gyrus of the hippocampus, indicating that regional interneurons may have varying degrees of vulnerability according to their location. Non-stereological techniques for inducing TBI have reportedly led to greater neuronal loss(Bussière et al., 2002). Baldwin et al. (1997) used stereological techniques and did not observe a progression of neuronal loss over time. However, Rink and Guo (2004) reported that a significant but small number of injured neurons in the hippocampus and cortex of injured rats underwent apoptosis hours to days following TBI. Several recent studies (Andrews et al.,2017; Andrews and Reisner, 2017; Casaletto et al., 2017; Sood et al., 2017; Spencer et al., 2017) have shown loss of memory and cognitive function, especially auditory memory function associated with the temporal lobe, as well as depression-like disorders and posttraumatic stress disorders (among other features), which are associated with hippocampal cell loss. Van Zomeran et al. (1998) found that the features of electrical and lightning injuries are similar to those seen in TBI. Therefore,cell apoptosis after TBI may play an important role in decreasing interneuron numbers in the hippocampus.
To investigate electrophysiological changes induced by TBI,we employed experimentally controlled fluid percussion injury in rats. We observed a TBI-induced shift in input/output curves, indicating increased excitability in the dentate gyrus after TBI. These alterations were correlated with changes detected in hippocampal dentate gyrus interneurons, including interneuronal loss in the ipsilateral hippocampus dentate gyrus that may contribute to altered dentate gyrus excitability.GABA-mediated inhibition is important in synchronizing,terminating, and initiating both pathological and normal activities in the neuronal network (Yang et al., 2007; Palmer et al., 2014; Faingold et al., 2015; Rombo et al., 2016). Therefore,the observed changes in excitability may be attributable to alterations in neuronal inhibitory circuits. A subtle balance between neuronal inhibition and excitation is required to maintain normal brain function, including hippocampal function. A loss of interneurons in the hippocampal dentate gyrus could disturb this balance. The dentate gyrus may filter excessive or aberrant input to the CA3 area by preventing signal amplification by the CA1 and transduction of the processed information to the rat cortex. Thus, we evaluated excitability at the input (dentate gyrus) region of the hippocampal circuit. Our data revealed increased regional synaptic excitability in the hippocampal dentate gyrus in the TBI rats. Such increased excitability in the hippocampal dentate gyrus may reduce thresholds for developing self-sustained seizure activity (Coulter et al., 1996; Alwis et al., 2016; Hendricks et al., 2016; Magagna-Poveda et al., 2016;Liu et al., 2017; You et al., 2017). TBI-induced disruption of synaptic efficacy in hippocampal circuit conduction may be responsible for the observed cognitive deficits. The complexity of the inhibitory changes observed after TBI indicate that TBI damages synaptic plasticity in the hippocampus. Further studies should be directed towards the mechanism of putative regional excitatory activity alterations in the hippocampus. Additionally,other neurons and glial cells may contribute to the electrophysiological changes observed after TBI. Therefore, examinations of post-TBI changes in multiple cell types may be beneficial.
Acknowledgments: We would like to thank Lei Zhou for his meticulous care of experimental animals, and Wei-Yun Cui for excellent laboratory quality control.
Author contributions: BLZ, JNZ and YSF designed this study. JWW, ZWZ and YGW performed experiments. MCY and BLZ analyzed data. BLZ and DDS wrote the paper. All authors approved the final version of the paper.
Conflicts of interest: There is no conflict of interest.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81330029, 81501057; the Natural Science Foundation of Tianjin of China, No. 17JCQNJC12000; the Tianjin Medical University General Hospital Funding in China, No. ZYYFY2016014. The funders did not participant in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: The study protocol was approved by the Animal Ethics Committee of Tianjin Medical University in China. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer: Christopher J Andrews, University of Queensland,School of Medicine, Australia.
Additional file: Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Validity and reliability of the Ocular Motor Nerve Palsy Scale
- Mitogen-activated protein kinase phosphatase 1 protects PC12 cells from amyloid beta-induced neurotoxicity
- High-frequency (50 Hz) electroacupuncture ameliorates cognitive impairment in rats with amyloid beta 1–42-induced Alzheimer’s disease
- Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia
- Combined VEGF/PDGF improves olfactory regeneration after unilateral bulbectomy in mice
- Comparison of morphological and functional outcomes of mouse sciatic nerve repair with three biodegradable polymer conduits containing poly(lactic acid)