Release of interleukin-10 and neurotrophic factors in the choroid plexus: possible inductors of neurogenesis following copolymer-1 immunization after cerebral ischemia
Yolanda Cruz , Edna E. García Jessica V. Gálvez Stella V. Arias-Santiago Horacio G. Carvajal Raúl Silva-García, Herlinda Bonilla-Jaime Julio Rojas-Castañeda, Antonio Ibarra
1 Centro de Investigación en Ciencias de la Salud (CICSA), FCS, Universidad Anáhuac México Norte, Huixquilucan, Estado de México, México
2 Lab. De Biología de la reproducción, UAMI. Ciudad de México, México
3 Hospital de Pediatría CMN Siglo XXI. Ciudad de México, México
4 Subdirección de Medicina Experimental, Instituto Nacional de Pediatría.Ciudad de México, México
5 Doctorado en Ciencias Biológicas, División de Ciencias Biológicas y de la Salud, Universidad Autónoma Metropolitana, Unidad Iztapalapa. Ciudad de México, México
Funding: This study was supported by a grant from Universidad Anahuac México Norte (No. 201425).
Abstract Copolymer-1 (Cop-1) is a peptide with immunomodulatory properties, approved by the Food and Drug Administration of United States in the treatment of multiple sclerosis. Cop-1 has been shown to exert neuroprotective effects and induce neurogenesis in cerebral ischemia models. Nevertheless, the mechanism involved in the neurogenic action of this compound remains unknown. The choroid plexus (CP) is a network of cells that constitute the interphase between the immune and central nervous systems, with the ability to mediate neurogenesis through the release of cytokines and growth factors. Therefore, the CP could play a role in Cop-1-induced neurogenesis. In order to determine the participation of the CP in the induction of neurogenesis after Cop-1 immunization, we evaluated the gene expression of various growth factors (brain-derived neurotrophic factor, insulin-like growth factor 1, neurotrophin-3) and cytokines (tumor necrosis factor alpha,interferon-gamma, interleukin-4 (IL-4), IL-10 and IL-17), in the CP at 14 days after ischemia. Furthermore,we analyzed the correlation between the expression of these genes and neurogenesis. Our results showed that Cop-1 was capable of stimulating an upregulation in the expression of the genes encoding for brain-derived neurotrophic factor, insulin-like growth factor 1, neurotrophin-3 and IL-10 in the CP, which correlated with an increase in neurogenesis in the subventricular and subgranular zone. As well, we observed a downregulation of IL-17 gene expression. This study demonstrates the effect of Cop-1 on the expression of growth factors and IL-10 in the CP, in the same way, presents a possible mechanism involved in the neurogenic effect of Cop-1.
Key Words: choroid plexus; growth factors; immunomodulation; protective autoimmunity; Cop-1; Copaxone;stroke; glatiramer acetate; tMCAo; focal cerebral ischemia
Introduction
Inflammation has traditionally been associated with an increase in brain tissue damage after ischemic stroke (Herz et al., 2015). The release of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interferon-γ (INF-γ), affects the proliferation, differentiation,and survival of newly-formed neurons (Musaelyan et al., 2014;Marlier et al., 2015). Consequently, these cytokines modify restorative mechanisms present after stroke, especially neurogenesis (Tobin et al., 2014).
On the other hand, inflammation arising from injury can also evoke a positive immune response through the action of autoreactive T lymphocytes, which possess the ability to reduce neural tissue damage and promote its restoration (Ziv and Schwartz, 2008). Nonetheless, the physiological response of this “protective autoimmunity” (PA) –as it has been called- alone is not enough to convey efficient protection(Yoles et al., 2001). In order to increase its efficiency, this response can be boosted by immunizing with neural-derived peptides (Martiñón et al., 2012).
Copolymer-1 (Cop-1) is a peptide consisting of 40 to 100 amino acid residues randomly constituted by L-alanine, L-lysine, L-glutamic acid, and L-tyrosine in a molar relation of 6.0:1.9:4.7:1.0. Cop-1 is approved by the FDA for the treatment of multiple sclerosis (MS) (Tselis et al., 2007), and has been shown to induce neuroprotection and neurogenesis in experimental models of cerebral ischemia through the stimulation of PA (Ibarra et al., 2007; Cruz et al., 2015). Immunization with Cop-1 alters the pro-inflammatory cytokine profile observed after cerebral ischemia, leading to the aforementioned positive results. Studies have shown that Cop-1 reduces TNF-α and IL-12 production and increases anti-inflammatory cytokines like IL-10(Aharoni et al., 2003). Likewise, Cop-1 increases the production of different growth factors, such as transforming growth factor-beta (TGF-β), brain-derived neurotrophic factor (BDNF),insulin-like growth factor type 1 (IGF-1), and neurotrophin-3(NT-3) (Ibarra et al., 2007; Cruz et al., 2015; Spadaro et al.,2017). These modifications thus induce a microenvironment that favors neuroprotection and neurogenesis (Aharoni, 2014).However, the changes that Cop-1 induces on immunological niches, important sites for immune modulation, are currently unknown. In addition, there is no information regarding whether changes in these sites actually have a direct influence on the effects of Cop-1, and specifically on neurogenesis.
One of the main immunological niches in the brain is the choroid plexus (CP), a network of cells that have been involved in the response to ischemic lesions through the release of growth factors, cytokines, exosomes, or the induction of leukocyte infiltration (Xiang et al., 2017). Therefore, the CP represents the intersection between the peripheral immune system and the central nervous system (CNS), where chemotactic signals from either the brain parenchyma or the periphery are exhibited after any homeostatic alteration (Baruch and Schwartz, 2013).
Cellular and microenvironment modifications at the CP are involved in various mechanisms, such as neural plasticity, neurogenesis, and even the synaptic process in the brain parenchyma;therefore, the CP could possibly be implicated as an important player in the beneficial effects induced by Cop-1-immunization(Raposo et al., 2014; Schwartz and Baruch, 2014).
The purpose of this study was to elucidate whether Cop-1 immunization would modify the expression of genes encoding pro- and anti-inflammatory cytokines or growth factors at the CP, and if so, whether these modifications directly correlate with neurogenesis.
Materials and Methods
Ethical considerations
Experimental use of the animals was performed in adherence to the guidelines for the care and use of laboratory animals provided by the National Institute of Health (NIH) (Council, 2011), as well as the Official Mexican Norm NOM-062-ZOO-1999 (Ochoa Muñóz, 2001) which establishes technical specifications for production care and use of laboratory animals. This work was approved by the Anahuac University Institutional Care and Use of Laboratory Animals Committee under the registration No. 201425.
Experimental design
Our study used 55 Sprague-Dawley (SD) male rats weighing 330–350 g. The animals were obtained from Anahuac University’s Animal Breeding Center and CAMINA Research Project. Rats were housed under controlled conditions regarding temperature and humidity using automated control racks,and were allowed to eat and drink water ad libitum before and after the procedure.
Randomized allocation of animals was performed using GraphPad QuickCalcs (http://www.graphpad.com/quickcalcs/). The experimental procedures and animal allocation were performed in a double-blind setting to avoid bias.
Fifty-five rats were subjected to transient middle cerebral artery occlusion (tMCAo). Of these, three died before the 12-hour post-ischemia mark due to subarachnoid hemorrhage.The rest of the rats did not present any significant variability in lesion displaying signs of ischemia with a minimum score value of three points on the Zea Longa scale (Longa et al., 1989).These animals were randomly allocated into 4 groups: 1) Control (no treatment), 2) Saline Solution plus Complete Freund’s adjuvant (CFA), 3) Saline Solution plus Cop-1 (SS + Cop-1),and 4) CFA plus Cop-1 (CFA + Cop-1). All rats were neurologically evaluated by a double-blinded individual at 1, 2, 3, 7 and 14 days after tMCAo. In order to evaluate gene expression of cytokines (IL-4, IL-10, 1L-1β, IL-17, TNF-α and INF-γ) and growth factors (BDNF, IGF-1 and NT-3) in the CP, 32 randomly selected rats were euthanized at 14 days (32 rats, 8 per group) after ischemia. An additional group of twenty rats (n =five per group) were used to evaluate neurogenesis 14 days after ischemia.
Cerebral ischemia model
Animals were subjected to tMCAo as previously described by Zea Longa in 1989 (Longa et al., 1989). For this procedure, rats were anesthetized by inhalation with 4% isoflurane(Lisorane, Baxter. Guayama, Puerto Rico) until reaching a deep anesthetic state, after which isoflurane was modified to 1.5% for the remainder of the surgery. The left common carotid (CCA), internal carotid (ICA), and external carotid artery (ECA) were identified, while the pterygoid and occipital arteries were cauterized. A 3-0 nylon monofilament with a flame-rounded head was inserted through the ECA towards the ICA, advancing 18 mm until reaching the middle cerebral artery (MCA). This occlusion lasted 90 minutes, after which the filament was withdrawn, allowing reperfusion.
Animals received acetaminophen (200 mg/kg, twice a day,p.o., Tempra, Bristol, Ciudad de México, México) and enrofloxacin (10 mg/kg, once a day, s.c., Baytril, Bayer, Kansas,and USA) for 3 days after surgery.
Immunization
Immunization was performed via subcutaneous injection in the interscapular region 5 minutes after reperfusion. Two hundred micrograms of Cop-1 (Sigma, St. Louis, MO, USA) were diluted in saline solution (SS) or complete Freund’s adjuvant(CFA), containing 5 mg/mL of Mycobacterium tuberculosis H37RA (Sigma). A total volume of 150 μL was administered to each rat in a single injection.
Neurological deficit evaluation
Neurological deficit was evaluated using Longa Scale (Longa et al., 1989) at 1, 2, 3, 7 and 14 days post tMCAo. This scale consists of 5 points: 0, No neurological deficit; 1, failure to fully extend right forepaw; 2, circling to the left; 3, falling to the right; 4, failure to walk spontaneously and diminished level of consciousness.
Immunofluorescence
Starting on day 12 post-ischemia, the rats received one injection of 5-bromo-2-bromodeoxyuridine (BrdU) intraperitoneally every 12 h for five doses. Each dose consisted of 50 mg/kg of BrdU dissolved in 2 ml of PBS. BrdU is a synthetic nucleotide analogue of thymidine incorporated during the S phase of the cell cycle.
On day 14 post-surgery, the animals were euthanized using sodium pentobarbital at a lethal dose (80 mg/kg) and then perfused intracardially with phosphate buffer at a pH of 7.2,followed by paraformaldehyde at 4%. The brains were removed and placed in paraformaldehyde for 24 hours, and later in 30%sucrose for three days. Serials of 40 μm-thick coronal cuts were retrieved at 200 μm from coordinates 10.44 through 1.44 mm according to Bregma and from 6.0 to –3.0 mm from Bregma using a cryostat according to the Paxinos Watson atlas (Paxinos and Watson, 2009), where the subventricular zone (SVZ) and the subgranular zone (SGZ) lie. Nine sections were used for each immunofluorescence test.
The sections were washed twice for ten minutes in phosphate buffer with Triton (PBT) and incubated for 30 minutes in an InmunoRetriever (Bio SB, Santa Bárbara, CA, USA) at 65°C. Sections were then washed twice in a saline phosphate buffer (PBS) and incubated with HCl 1 N at 37°C and subsequently with sodium borate at 0.1 M for 10 minutes. They were later washed again with PBT for ten minutes on three times. The sections were incubated with blocking solution for 30 min at 37°C, and then incubated overnight at room temperature with the primary antibodies anti-BrdU mouse(1:250, Roche, Penzberg, Alemania) and anti-doublecortin(DCX) goat (1:20, Santa Cruz Biotecnology, Santa Cruz, CA,USA). The next day, the sections were washed with PBT three times and incubated for two hours at room temperature with the secondary antibodies donkey IgG anti-mouse (1:500; Invitrogen, Eugene, OR, USA) and rabbit IgG anti-goat (1:500,Invitrogen). Following incubation, they were washed with PB and counter-stained with DAPI. Two more washes were performed for 5 minutes with PBS, after which the sections were then mounted on slides using Vectashield (Vector, Burlingame, CA, USA).
The slides were observed under the confocal microscope(Olympus Fluo View-1000, Olympus, Tokyo, Japan) at a 20×magnification. For each section, images of the lateral ventricles and hippocampus were captured. Neuroblasts were identified by the presence of BrdU+/DCX+at 60× magnification. The images were merged using the FW10-ASW 1.7 Viewer (Olympus) image processor. The BrdU+/DCX+cells were quantified for the SVZ and SGZ using the image processing software Image Pro Plus(Media Cybernetics, Inc., Rockville, MD, USA), and the mean for the nine sections of each region was obtained.
Quantitative polymerase chain reaction
The choroid plexus of the lateral ventricles was obtained from eight rats in each group in order to determine the expression of some genes encoding for growth factors (BDNF, IGF-1 and NT-3) and cytokines (IL-4, IL-10, 1L-1β, IL-17, TNF-α and INF-γ). Total RNA was isolated using the phenol-chloroform extraction method with Trizol (Rio et al., 2010) (Life Technologies, Carlsbad, CA, USA). RNA concentration and purity were evaluated by ultra-violet (UV) spectrophotometry,integrity by electrophoresis, and complementary DNA (cDNA)was obtained by reverse transcription. The cDNA synthesis was performed with oligo (dT) at 55°C for 50 minutes in a final volume of 20 μL from 2 μg of total RNA, following the manufacturer’s instructions for superscript reverse transcriptase-RNase H (Invitrogen, Carlsbad, CA, USA). Real-time RTPCR was performed using a Light Cycler 2.0 instrument (Roche,Mexico City, Mexico). Three independent experiments for each set of RT-PCR analyses were performed. The expression of cDNA was evaluated by quantitative PCR using the selected gene-specific primers pairs listed in Table 1, using 1 μL of each cDNA. For the initial denaturation step, samples were heated up to 95°C for 10 minutes, followed by the first cycle consisting of a denaturation step (95°C, 10 seconds), a primer annealing step (60°C, 10 seconds), an extension step (72°C, 10 seconds), a melting curve (65°C, 1 minute), and a cooling step(40°C, 30 seconds). The reaction was carried out in 40 cycles.All experiments were performed in triplicate. Each reaction was subjected to melting curve and melting temperatures to confirm single amplified products using the Light-Cycler software(build 4.1.1.21). We used two internal controls: Δ1, which was obtained from the constitutive gene hypoxanthine guanine phosphoribosyl transferase (HPRT), and Δ2, which was obtained from healthy, untreated control rats. Using the 2-ΔΔCtmethod,the data are presented as the fold change (arbitrary units) in gene expression normalized to HPRT and relative to the untreated control (Livak and Schmittgen, 2001).
Statistical methods
The data were analyzed using Graph Prism 5.0 (GraphPad Software, Inc. La Jolla, CA, USA). Neurological deficit, neurogenesis, and gene expression data are expressed as mean ±standard deviation (SD) or standard error of the mean (SEM;this is indicated in the legend that correspond to each figure),with a significance level of P < 0.05 considered as statistically significant. Normality was assessed through the D’Agostino and Pearson Omnibus test on every data set. Due to the non-parametric distribution present in all cases, we used theKruskal Wallis test for statistical analysis, corroborating the difference between groups with Dunn’s multiple comparison post hoc test. Additionally, Spearman’s test was used to evaluate correlation.

Table 1 Real-time PCR primers
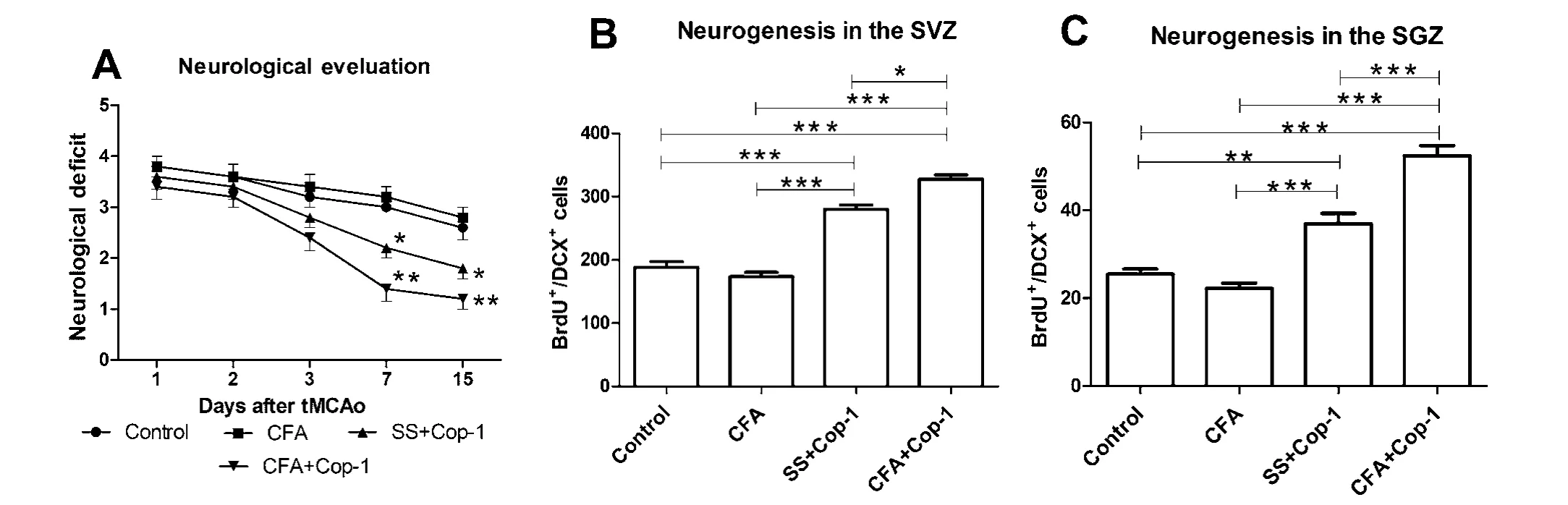
Figure 1 Eあect of Cop-1 on neurological deficit and neurogenesis in tMCAo rats.

Figure 2 Correlation between neurogenesis and neurological deficit.
Results
Cop-1 promotes neurological recovery after tMCAo
All the rats presented a score of three points at this time.During the next 3 days of evaluation, there was no significant difference among the groups. Seven days after tMCAo, evaluations showed a significant reduction in neurological deficit in the CFA + Cop-1 treated group compared with the control and CFA groups (P < 0.05; Figure 1A). Furthermore, animals treated with CFA + Cop-1 presented a significant reduction of neurological deficit 14 days after tMCAo compared with control (P< 0.05) and CFA groups (P < 0.001; Figure 1A).Cop-1 immunization increases neurogenesis in SVZ and SGZ In the adult brain, there are two areas considered neurogenic sites: the lateral ventricle walls, known as the subventricular zone (SVZ) (Lim and Alvarez-Buylla, 2014) and the subgranular hippocampal zone (SVG) (Seri and Alvarez-Buylla, 2002).Neural stem cells have been identified at these areas, and are known to generate new neurons even in injured tissues (Zheng et al., 2013).
To evaluate the effect of Cop-1 on neurogenesis of these sites, a double-labeling immunofluorescence (IF) technique was performed. Subsequently, BrdU+/DCX+cells were counted in the SVZ and SGZ at day 14 post-tMCAo. This point in time was chosen for neurogenesis evaluation due to previous evidence of maximum cell proliferation in the SVZ occurring at 1 to 2 weeks after tMCAo (Arvidsson et al., 2002). Addi-tionally, reported data suggests that studies with follow-up periods of a week (7 days) or shorter are unable to observe the increase in neurogenesis and the resultant functional improvement due to immunization with Cop-1 (Poittevin et al., 2013;Kraft et al., 2014).

Figure 3 Representative microphotographs of the immunolocalization of BrdU+/DCX+cells in the SVZ and SGZ.
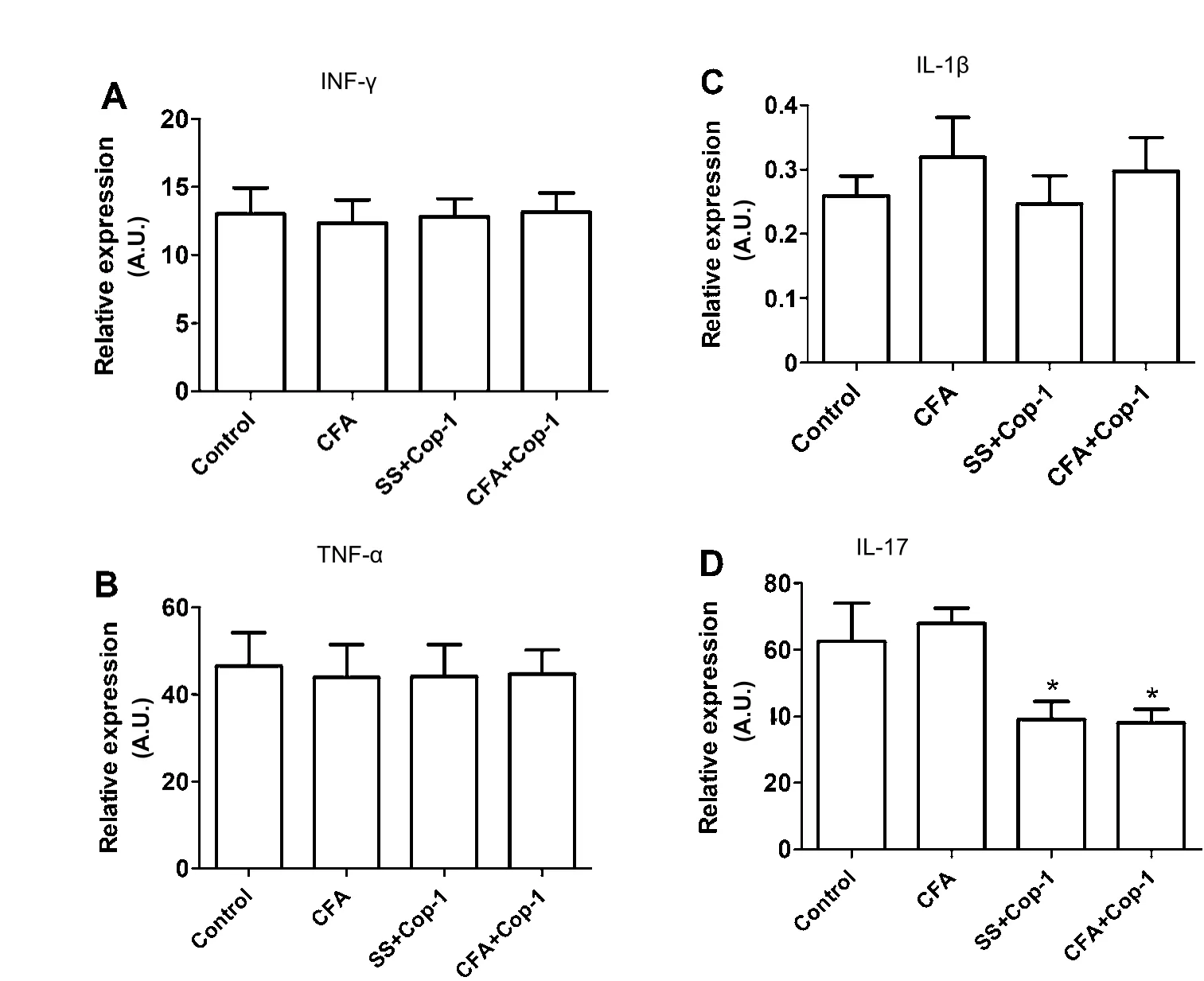
Figure 4 Eあect of Cop-1 on gene expression of pro-inflammatory cytokines in the CP (quantitative PCR).
Groups treated with Cop-1 showed a significant increase in neuroblast counts in the SVZ (Figure 1B) compared with the control and CFA groups (P < 0.0001). Rats treated with CFA+ Cop-1 also exhibited a significant increase in neuroblasts that was statistically different from the one observed in the group treated with SS + Cop-1 (P < 0.05). In the case of the SGZ, the number of neuroblasts was also significantly higher in the groups treated with Cop-1 (Figure 1C) than those in the control and CFA groups (P < 0.01). Likewise, a significantly higher number of neuroblasts were observed in animals immunized with CFA +Cop-1 relative to the SS + Cop-1 group.
Neurogenesis at the SVZ exhibited a negative correlation (r =–0.86, P < 0.05) with neurological deficit in both rats treated with CFA + Cop-1 (Figure 2A) and those with SS + Cop-1 (r= –0.70, P < 0.05; Figure 2B). This was also observed in rats immunized with CFA + Cop-1 (Figure 2C), although to a lesser degree (r = –0.57, P = 0.17), and was even lower in animals treated with SS + Cop-1 (r = –0.35, P = 0.25; Figure 2D).
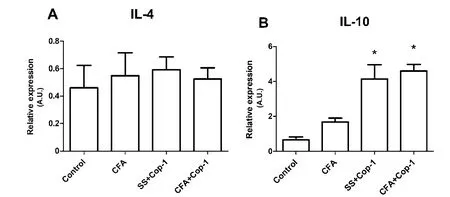
Figure 5 Eあect of Cop-1 on gene expression of anti-inflammatory cytokines in the CP (quantitative PCR).
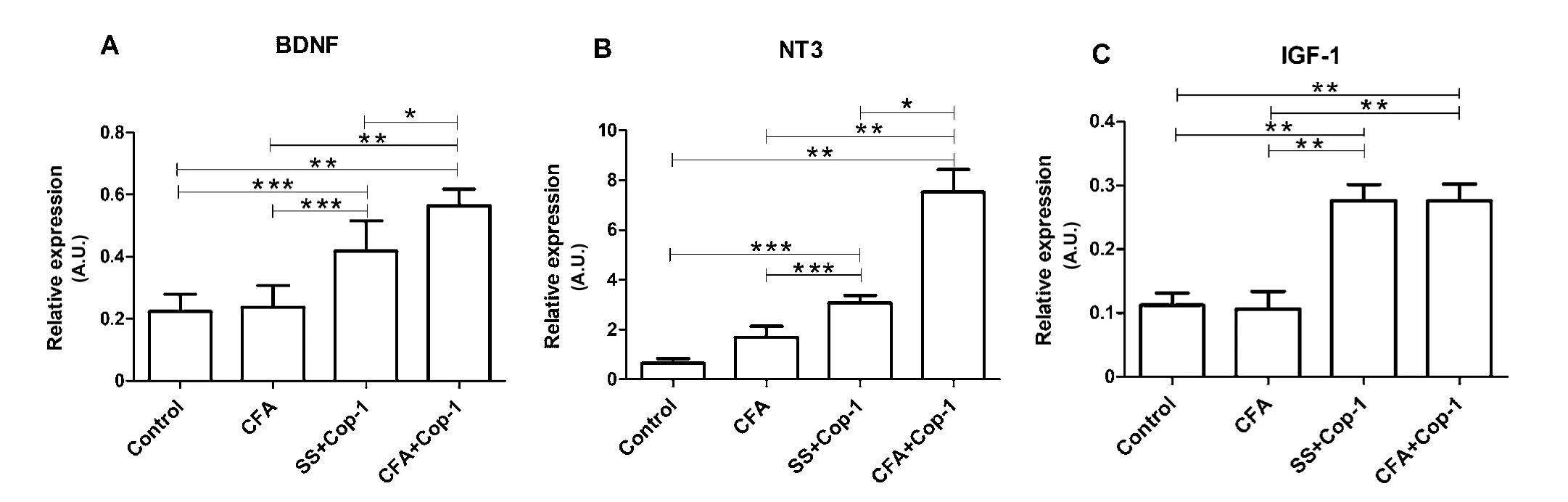
Figure 6 Eあect of Cop-1 on the expression of genes encoding for growth factors in the CP (quantitative PCR).

Figure 7 Correlation of IL-10 or growth factors with neurogenesis in the SVZ of CFA plus Cop-1-treated rats.
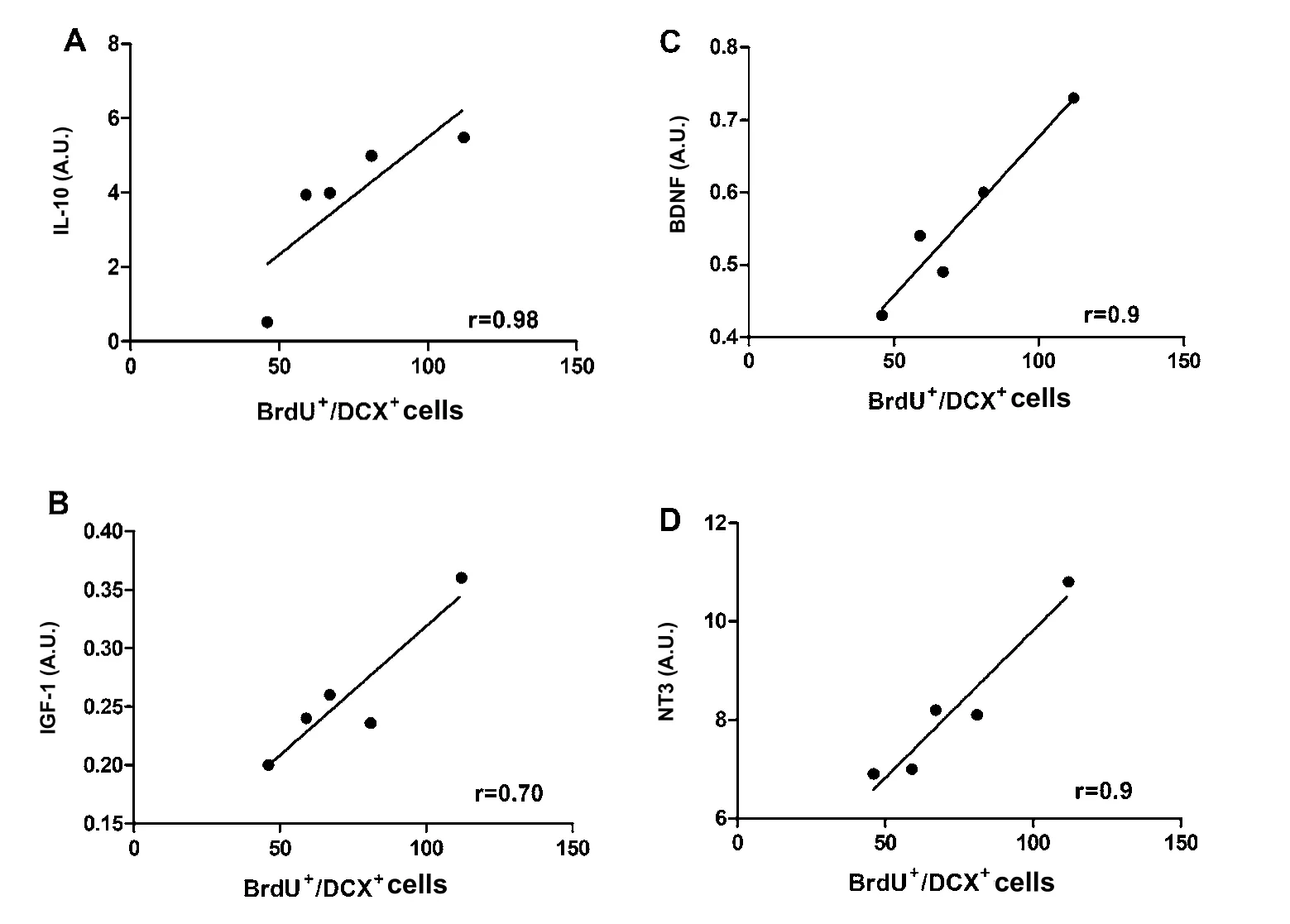
Figure 8 Correlation of IL-10 or growth factors with neurogenesis in the SGZ of CFA plus Cop-1-treated rats.
Figure 3 shows a representative microphotograph of the labeling for BrdU+/DCX+cells at SVZ (Figure 3A) or SGZ(Figure 3B). As can be observed, CFA + Cop-1 and SS +Cop-1 groups presented a higher number of cells labeled BrdU+/DCX+(neuroblasts) in both the SVZ and SGZ.
Cop-1 immunization increases IL-10 and reduces IL-17 gene expression at the choroid plexus
Previous studies have demonstrated the importance of the CP microenvironment in regulating regenerative processes, such as neurogenesis (Lehtinen et al., 2013). In order to evaluate the impact of Cop-1 immunization on the CP microenvironment,the expression of genes encoding for inflammation-related cytokines was analyzed. Genes studied after tMCAo and Cop-1 immunization include: INF-γ, TNF-α, IL-1β, IL-17, IL-4, and IL-10.
Cop-1 immunization showed no effect on INF-γ, TNF-α, IL-1β (Figure 4A–C), and IL-4 (Figure 5A) gene expression. Nevertheless, a marked expression of the gene encoding for IL-10 was observed (P < 0.05; Figure 5B). Similarly, the gene encoding for IL-17 in Cop-1 treated groups was significantly reduced when compared to the control (P < 0.05; Figure 4D).
BDNF, NT-3, and IGF-1 gene expression is increased by Cop-1 immunization after tMCAo
The CP is also considered a key site for neuroimmune communication, due to the production of growth factors directly involved in neurogenesis processes in this area (Baruch and Schwartz, 2013). Therefore, the effect of Cop-1 immunization on the expression of certain growth factors, as well as their correlation with stimulation of neurogenesis, were assessed.For this purpose, levels of BDNF, NT-3, and IGF-1 were evaluated 14 days after tMCAo.
Relative expression of BDNF presented a significant increase in Cop-1-treated groups compared to control and CFA ones (P < 0.05; Figure 6A). Figure 6B shows that Cop-1 treated groups presented a significant increase of the gene encoding for NT-3 in comparison to control and CFA groups (P < 0.05).There was also a significant difference between CFA+Cop-1 and SS + Cop-1 groups (P < 0.05). Regarding IGF-1 gene expression, a significant increase was found in the CFA + Cop-1 and SS + Cop-1 groups compared with the control and CFA groups (P < 0.05; Figure 6C).
IL-10, BDNF, NT-3 and IGF-1 gene expression correlated with neurogenesis
After observing the significant increase in the expression of the genes encoding for IL-10 and growth factors (BDNF, IGF-1 and NT-3), we decided to evaluate whether a direct correlation of these genes with neurogenesis was present. Since the group of CFA + Cop-1 presented the highest neurogenesis and gene expression, we decided to make the correlation analysis only with this group of rats. As shown in Figure 7, a strong correlation between SVZ neurogenesis and the relative expression of genes in CFA+Cop-1 group was observed (IL-10, r = 0.83;BDNF, r = 0.9; NT-3, r =0.9 and IGF-1, r = 0.78). Results for neurogenesis in the SGZ showed very similar values (Figure 8), with the exception of IGF-1 expression, which indicated a moderate correlation (r = 0.70).
Discussion
Copolymer-1 has been proposed as a therapeutic alternative for ischemic stroke. The present results support previous observations regarding the beneficial effects of Cop-1 immunization on neurological recovery and neurogenesis (Cruz et al.,2015). Moreover, the present study offers new insights on the possible mechanisms participating in the induction of these beneficial effects. Our results show that neurological recovery induced by Cop-1 immunization correlated with neurogenesis. Furthermore, our findings suggest that the CP plays an important role in the changes induced by Cop-1, due to the increase in the expression of genes encoding for growth factors and IL-10, and their correlation with neurogenesis in Cop-1-treated rats.
The results from our study are in accordance with the established literature, which has long considered the CP to be a site endowed with neuroimmune and neuroregenerative functions,triggered by lymphoid cells activated by signals from the CNS parenchyma and the peripheral immune system (Borlongan et al., 2006). These cells participate with the CP in the coordination of a molecular language that modulates mechanisms such as neurogenesis in the healthy and damaged brain. In addition, several studies have reported that the CP secrete a vast array of growth factors like BDNF, NT-3, and IGF which regulate neurogenesis (Borlongan et al., 2004; Yan et al., 2006;Schäbitz et al., 2007; Sathyanesan et al., 2012; Delgado et al.,2014). Therefore, the significant increase in the expression of genes encoding for these molecules in the CP of Cop-1-treated groups, might explain, at least in part, the neurogenesis observed at the SVZ and SGZ.
Our results are in line with those observed in models of experimental autoimmune encephalitis (EAE) (Aharoni et al.,2005) and Alzheimer’s disease (Butovsky et al., 2006), where a significant increase of growth factors strongly correlated with neurogenesis.
The growth factors evaluated in the present study have been widely reported as important inductors of neurogenesis. For instance, NT-3 is required for the quiescence and long-term maintenance of stem cells at neurogenic niches (Delgado et al., 2014). This growth factor has also been proven to facilitate plasticity, learning, and memory (Shimazu et al., 2006). Another growth factor studied, IGF-1, promotes stem cell differentiation through the PI3K/AKT or MAP kinases pathways (Yuan et al., 2015), and participates in neuroblast migration (Hurtado-Chong et al., 2009). Likewise, BDNF promotes neuroblast migration and neuronal survival in adult animals (Snapyan et al., 2009).
Regarding BDNF, previous studies failed to demonstrate any positive effect of Cop-1 on BDNF concentrations in the penumbral area seven days after ischemia (Cruz et al., 2015).However, the present study demonstrates that the gene encoding for BDNF increases on day 14 in the CP, providing evidence regarding the selective effect that Cop-1 could exert on specific areas of the brain. Moreover, this finding provides evidence that Cop-1 is capable of inducing important changes in the CP. With this respect, previous studies have also reported the production of BDNF in the CP after Cop-1 therapy in mice with EAE (Aharoni et al., 2003), and an increase of BDNF in the CSF of MS patients treated with Cop-1 (Azoulay et al., 2005). Therefore, the CP could be a key element in the release of BDNF and other neurotrophic factors after Cop-1 immunization, and possibly the cornerstone of the beneficial effects induced by this therapy.
With the aim of evaluating in more detail the effect of Cop-1 on the microenvironment prevalent in the CP, we also analyzed the expression of the genes encoding for some anti- and pro-inflammatory cytokines. In this case, our results indicated that Cop-1 does not present any inhibitory effect on the expression of genes encoding for pro-inflammatory cytokines,like INF-γ, TNF-α, and IL-1β, although it resulted on the inhibition of the IL-17 gene. This observation is consistent with previous studies, in which Cop-1 has shown the ability to reduce IL-17 expression through the increase of regulatory T(Treg) cells (Aharoni et al., 2010; Aharoni, 2014) and the inhibition of Th-17 and Th-1 lymphocytes (Begum-Haque et al.,2008). This inhibition could be of important relevance in the protection from tissue damage; after cerebral ischemia, there is a significant depletion of IL-10 –an important inhibitor of IL-17 which leads to an increase of IL-17 and thus an increment in damage (Li et al., 2001; Gelderblom et al., 2012; Liesz et al., 2013). Consequently, the protective effect triggered by Cop-1 immunization may contribute to its restorative effects.
IL-10 was found significantly increased in the CP of rats immunized with Cop-1. This finding also explains the reduction of IL-17, due to the nature of IL-10 as a strong inhibitor of this cytokine (Gu et al., 2008). Furthermore, this finding provides additional insight about the origin of neurogenesis after Cop-1 immunization. In the present work, we found a significant correlation between IL-10 levels and neurogenesis, which suggests the participation of this cytokine in neurogenic mechanisms observed with Cop-1 therapy. This observation is supported by recent studies, which describe the involvement of IL-10 in neurogenesis and hippocampal synaptic plasticity (Tyrtyshnaia et al., 2017). In the SVZ, IL-10 acts as a growth factor on progenitor cells, stimulating neurogenesis (Perez-Asensio et al., 2013;Pereira et al., 2015). Cop-1 has proven capable of inducing a significant increase of Treg lymphocytes, which in turn produce high amounts of IL-10 (Putheti et al., 2003; Haas et al., 2009;Liesz et al., 2013) and have been implicated in the regulation of neural stem cell proliferation in the SVZ (Wang et al., 2015).Therefore, the increase in IL-10 expression, likely produced by Treg lymphocytes and induced by Cop-1 therapy, provides an additional mechanism for the observed neurogenesis induction.
Neurogenesis is a process highly regulated by intrinsic and extrinsic signals that stimulate stem cells located at neurogenic niches (SVZ and SGZ) (Faigle and Song, 2013). Most of these signals are produced at the CP and travel through apical processes that project into the ventricular space (Lehtinen et al.,2013).
The effect of Cop-1 on neurogenesis has already been studied in other CNS models such as Alzheimer’s disease (Butovsky et al., 2006), EAE (Aharoni et al., 2005), and tMCAo (Cruz et al., 2015). This therapy has also shown significant results in situations where memory and cognitive function are affected, as in the case of liposaccharide-infection (Mohammadi et al., 2016),in rats undergoing cranial irradiation (He et al., 2014), and in the deterioration of cognitive function associated with the aging process (Nieto-Vera et al., 2018).
In this work, we also observed a strong negative correlation between neurogenesis at the SVZ and the neurological deficit presented by Cop-1-immunized rats. This finding supports the idea of a possible migration of neuroblasts from the SVZ to the site of ischemia. Previous studies have reported the ability of SVZ-originated neuroblasts to migrate towards injured areas, allowing them to participate in the replacement of neurons. Thus,they are able to shape new circuits, contributing to the improvement of motor recovery (Zepeda et al., 2009; Xiong et al., 2010;Cruz et al., 2015). These findings are supported by the presence of a significant number of neuroblasts around the infarct zone(Cruz et al., 2015).
Regarding the low correlation observed between neurogenesis at the SGZ and the neurological deficit in Cop-1 immunized rats, it is well known that neuroblasts originated in the SGZ migrate to the dentate gyrus of the hippocampus, where they primarily promote cognitive recovery (Tian et al., 2014).Therefore, neurogenesis originated at this site is not expected to improve neurological function as it is evaluated by the motor performance of animals.
Finally, the neurological recovery observed in this study could also be due to the ability of Cop-1 to modify the infarcted brain parenchyma. Results from a previous study demonstrated that Cop-1 favors an anti-inflammatory milieu, which induces a reduction of free radicals and an increase in growth factor secretion (Ibarra et al., 2007). These mechanisms contribute towards neuroprotection and thus, neurological recovery.
The results of the present study provide evidence on the possible mechanisms induced by Cop-1 for promoting neurogenesis. IL-10 and neurotrophic factors, such as BDNF,IGF-1, and NT-3, released in the CP are some of the elements likely participating in the neurogenic effect of Cop-1. These findings show that Cop-1 is capable of modulating the microenvironment of the CP, a site of neuroimmune communication that warrants further exploration.
Finally, it should be noted that despite these encouraging results, further studies focused on protein expression of the genes analyzed in the present work are necessary in order to clearly evidence the induction of neurogenesis by Cop-1.
Acknowledgments: The authors would like to greatly thank the National Institute of Pediatrics, graduate area at science of UAMI and IMSS for their technical support, which was fundamental to the realization of this work.
Author contributions: Planning, experimental design, definition of intellectual content and literature search: YC, HBJ, JRC and AI. Experimental studies and data acquisition: YC, EEG, JVG, SVAS, and RSG. Data analysis and statistical analysis: YC, HBJ, JRC and AI. Manuscript preparation and Manuscript editing: YC and AI. Manuscript review: YC, HGC and AI. Guarantors: YC and AI.
Conflicts of interest: The authors did not report any conflict of interest.Financial support: This study was supported by a grant from Universidad Anahuac México Norte (No. 201425). The funding body played no role in the study conception design, in the collection, analysis and interpretation of data, in the preparation and writing of paper, and in the decision to submit the paper for publication.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer: Jukka Jolkkonen, University of Eastern Finland,Finland.
Additional file: Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Validity and reliability of the Ocular Motor Nerve Palsy Scale
- Mitogen-activated protein kinase phosphatase 1 protects PC12 cells from amyloid beta-induced neurotoxicity
- High-frequency (50 Hz) electroacupuncture ameliorates cognitive impairment in rats with amyloid beta 1–42-induced Alzheimer’s disease
- Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia
- Combined VEGF/PDGF improves olfactory regeneration after unilateral bulbectomy in mice
- Comparison of morphological and functional outcomes of mouse sciatic nerve repair with three biodegradable polymer conduits containing poly(lactic acid)