Exploring the use of transcranial photobiomodulation in Parkinson’s disease patients
Parkinson’s disease is a neurological disorder with distinct motor signs of resting tremor, akinesia and/or lead-pipe rigidity, together with non-motor symptoms of impaired smell,cognition and autonomic function. These manifest after a major degeneration of neurones mainly within the brainstem,particularly among the dopaminergic neurones in the substantia nigra pars compacta, together with their terminations in the striatum (Johnstone et al., 2016; Mitrofanis, 2017). A number of recent studies have shown that photobiomodulation, the use of red to infrared light (λ = 600–1070 nm) on body tissues, has beneficial effects in many animal models of Parkinson’s disease, from flies to monkeys (Hamblin, 2016; Johnstone et al., 2016; Mitrofanis, 2017). These benefits include, a restoration of the abnormal neuronal activity in the basal ganglia, an improvement in locomotive behaviour and reduction in clinical signs, as well as an increase in the survival patterns of neurones damaged by either the parkinsonian toxin or the genetic mutation of the model used. This latter neuroprotective disease-modifying effect is particularly relevant because it is the key process in Parkinson’s disease and is currently not addressed by drug and surgical therapies (Johnstone et al.,2016; Mitrofanis, 2017). In this perspective, we will explore several issues associated with the use of photobiomodulation in patients. First, we will consider the early evidence indicating that this therapy is effective in improving the signs and symptoms of the disease, as well as the mechanisms that may underpin these improvements. Second, we discuss how these preliminary reports (and experimental findings) can be developed into a viable treatment option for patients, together with some of the potential issues and/or problems associated with this process.
As it stands, there are early indications that photobiomodulation has beneficial effects on Parkinson’s disease patients. Several clinical case reports have used a transcranial approach, with either a hand-held laser or light emitting device (LED) or a helmet lined with many LED strips covering the bulk of the head. From a Quietmind Foundation trial,there is a linked YouTube video (http://www.youtube.com/watch?v=9X-hjgay7pg) of a Parkinson’s disease patient showing improved movement and reduced tremor after application of 1072 nm across the head from a hand-held laser device. There is a clinical report indicating improved speech,cognition, freezing episodes and gait in eight parkinsonian patients after a two week application of photobiomodulation across the head from a laser device (Maloney et al., 2010). In addition, from a study of thirty six patients, photobiomodulation from an intranasal device resulted in improvements in the majority of parkinsonian signs (~90%) after treatment for thirty minutes per day for ten days (Zhao et al., 2003). There is also an incidental finding of a reduction in clinical signs in one patient after photobiomodulation with a 660 nm laser device for a dental problem; the device was directed at the back of the head and the photobiomodulation was applied for two to three minutes (Burchman, 2011). Finally, there are some encouraging observations from four patients (Hamilton et al.,2018), one with progressive supranuclear palsy and three with Parkinson’s disease, using a photobiomodulation helmet(http://redlightsonthebrain.blog/), lined with LED strips of various wavelengths across the red to near infrared light range(i.e., 670 nm, 810 nm, 850 nm, 940 nm). For each of these patients, photobiomodulation generated subtle but distinct improvements, enough to make a difference to their day-today lives. Of the initial signs and symptoms, including tremor,akinesia, gait, difficulty in swallowing and speech, less facial animation and reduced fine motor skills, smell and social confidence, ~75% showed overall improvement, ~25% stayed the same and none got worse. For the majority of these signs and symptoms, changes were assessed by either the patient themselves, their carers or their attending medical practitioners.For changes in fine motor skills, this was assessed by a more objective analysis, with each patient writing the same sentence before and during the course of treatment. The area and perimeter of distance of each word was measured and the data collated at each time point during the course of the treatment,ranging from four to fourteen months. For this analysis, two patients showed no deterioration in the area and distance of the words, while two patients, quite remarkably, showed significant increases in the area and distance of the words (using one way analysis of variance test; Hamilton et al., 2018). Taken together, all these changes after photobiomodulation were not typical of the placebo phenomenon, in that they were slow in onset and sustained. Further, they were often observed by the carer or medical practitioner rather than the patient themselves. Finally, it should be noted that none of the patients developed any long-term side-effects with photobiomodulation.This feature is particularly pertinent because in two of the patients, treatment was over an extended period of twelve to fourteen months. This aligns well with many previous findings of no safety concerns regarding this photobiomodulation therapy (Hamblin, 2016; Johnstone et al., 2016; Mitrofanis, 2017).Further, each of the patients continued with their dopamine replacement medication without complication while using photobiomodulation suggesting that this treatment is compatible with drug therapy. It remains to be determined whether photobiomodulation can be used in patients receiving deep brain stimulation.
The mechanism that underlies the observed beneficial outcomes of the transcranial photobiomodulation in the patients is not known, although there are - as we see it - three possibilities. First, by direct stimulation, where photobiomodulation is applied directly on the distressed neurones themselves, activating mitochondrial function that then increases both adenosine triphosphate energy and the expression of stimulatory and/or protective genes (Hamblin, 2016; Johnstone et al., 2016;Mitrofanis, 2017). Second, by indirect stimulation, where photobiomodulation triggers recruitment of a “middle man”,such as cells of the immune and/or stem cell systems (Figure 1; Johnstone et al., 2016; Mitrofanis, 2017). These activated cells may swarm to the region of distressed neurones and helps them survive and function, by potentially increasing the expression of anti-inflammatory cytokines while decreasing the pro-inflammatory ones (Figure 1; Johnstone et al., 2016;Mitrofanis, 2017). Third, rather than acting on the distressed neurones through either a direct or indirect stimulation as described above, photobiomodulation may act on other brain regions, for example motor cortex, that then stimulate the neural networks that underpin the behavioural improvements(Figure 1; Reinhart et al., 2016). In the context of the patient improvements seen after transcranial photobiomodulation described here, it is unlikely that direct stimulation is the main mechanism involved. Previous studies have reported that transcranial photobiomodulation in humans cannot penetrate to the very deep lying brainstem, the main area of lesion in Parkinson’s disease; light has been shown to penetrate, at best,20–30 mm through body tissues, and the brainstem lies some 80–100 mm below the cranial surface (Hamblin, 2016; Johnstone et al., 2016; Mitrofanis, 2017). However, transcranial photobiomodulation can reach the rich network of blood vessels in the skin over the cranium and within the meninges to activate immune and/or other cells (Figure 1); indeed, photobiomodulation has been shown to influence immune and stem cell function in experimental animals (Muili et al., 2012; Oron and Oron, 2016). Further, the transcranial photobiomodulation can also reach the motor cortex, which lies superficially in the brain, just underneath the cranial surface (~10 mm; Figure 1). It is clear that further investigation is required as to which of these mechanisms are involved in generating the benefits of transcranial photobiomodulation in humans, in particular the identity of any circulatory cells that are activated and/or the neural circuits within the motor cortex that are stimulated.
Although these photobiomodulation-induced improvements in the patients described above are encouraging, they need to be expanded to a large scale clinical trial, with appropriate controls and placebo devices. In addition, such a trial could include strategies to determine whether the photobiomodulation is indeed disease-modifying or neuroprotective, by employing various measures, such as fluorodopa positron emission tomography, monitoring closely the clinical progression and spread of the disease across individual patients, particularly during an extended “washout” period, after the treatment has stopped.
A final issue we would like to consider is the problem associated with the development of this evidence to mainstream clinical use. Despite the large body of experimental and early clinical evidence (as described above), there is still much resistance and scepticism as to the potential use of photobiomodulation by patients and health professionals. From our experience, the main “issues” or “problems” involve the following. First, the concept of light inducing a chemical and metabolic change in neurones appears difficult to accept by some colleagues, although they have no trouble accepting the concept that a drug can induce a change through a series of receptors. Following along these lines, one should consider that photobiomodulation works on a similar principle, that it has receptors (i.e., chromophores) that, when activated, prompt a series of intrinsic cellular activities beneficial to the cell’s survival and function (Hamblin, 2016). The “hard” scientific evidence that photobiomodulation does indeed change neuronal activity and influence neuronal survival against insult has become irrefutable (Hamblin, 2016). Second, it is often the case that any result on an animal model of Parkinson’s disease is met with suspicion, with the argument that no model mimics faithfully the human condition. In particular, that no model reflects exactly the neuropathology (e.g., Lewy bodies),the chronic progressive nature (e.g., slow and relentless degeneration of neurones), the topography of degeneration (e.g.,across different transmitter cell groups in brainstem) and the clinical signs and symptoms (e.g., no model reproduces effectively the resting tremor) of the disease in humans. Further,most cases in humans are idiopathic (no known cause), while the animal models are all “induced” whether by either toxin or genetic mutation (Torres et al., 2017). Notwithstanding these suspicions, many of the animal models do, in fact, get it “right” in many respects. For example, they can generate a loss of neurones in the main zone of pathology (i.e.,substantia nigra pars compacta), create comparable patterns of abnormal activity in basal ganglia nuclei (e.g., subthalamic nucleus), reproduce many of the major clinical signs, particularly in the non-human primates (e.g., rigidity, akinesia),and finally, replicate closely the chronic progressive nature of degeneration. If one trials agents and gathers data from several models (i.e., toxin-induced and transgenic), then one can create a more than worthwhile overall picture of the human condition (Torres et al., 2017). Such experimental data is, at the very least, worthy of consideration as a template for translation to humans. Photobiomodulation has been tested in a wide range of animal models of Parkinson’s disease, from toxin-induced mouse, rat and monkey to transgenic fly, mouse and rat models (Hamblin, 2016; Johnstone et al., 2016; Mitrofanis, 2017). In all models, of which include both acute and chronic varieties, photobiomodulation offers neuroprotection,restores functional activity, improves motor behaviours and reduces clinical signs, reduces gliosis and induces expression of trophic growth factors. Third, if one considers the transcranial approach, then the issue of penetration is raised, namely,how can external light reach the main zone of pathology in the very deep lying brainstem, covered by hair, skin, bone, thick meninges and a mass of brain tissue? This is a valid issue, because - as discussed above - most studies have noted that light can not penetrate down to the brainstem. The simple response to this issue is that it does not have to penetrate all that far.Photobiomodulation may influence the neuronal function and survival in the brainstem through an indirect stimulation, using a “middle-man” in the circulation (e.g., immune cells), and/or by activating motor cortex and other neural circuits which have components closer to the brain surface (see above). It should be noted that if one considers application by the intracranial method, where an optical fibre is implanted within the brainstem near the distressed neurones, then there would be no penetration issues, because the photobiomodulation would be delivered directly onto the neurones themselves (i.e., direct stimulation; Darlot et al., 2016).
In conclusion, there are solid arguments to challenge the bulk of the suspicion and scepticism with regard to the potential development of transcranial photobiomodulation in Parkinson’s disease patients. The translation of the experimental evidence in the laboratory to use by patients in the clinic is well-founded and certainly worthy of consideration. Indeed,the treatment is safe to use, with no side effects and there are early and encouraging indications of photobiomodulation-induced improvements in a number of patients. The next step would be to replicate these early clinical findings in controlled and blinded larger clinical studies and, upon replication, then proceed to a double-blind clinical trial on a large cohort of patients. Such trials could include strategies to determine whether the photobiomodulation is indeed neuroprotective, as has been reported in animal models of Parkinson’s disease(Hamblin, 2016; Johnstone et al., 2016; Mitrofanis, 2017).
Catherine Hamilton, David Hamilton, Frank Nicklason,
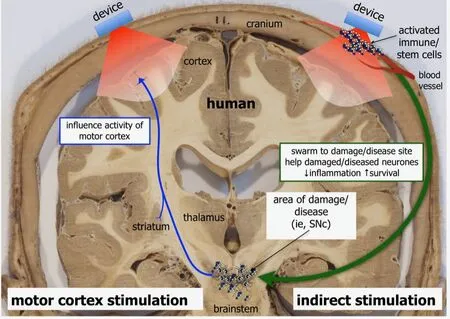
Figure 1 Possible mechanisms involved in generating beneficial outcomes by photobiomodulation in human patients.
Nabil El Massri, John Mitrofanis*
Department of Anatomy, University of Sydney, Sydney,Australia (Hamilton C, Hamilton D, Nicklason F, El Massri N,Mitrofanis J)
Department of Geriatric Medicine, Royal Hobart Hospital,Hobart, Australia (Nicklason F)
*Correspondence to:John Mitrofanis, PhD,
john.mitrofanis@sydney.edu.au.
Accepted:2018-06-26
doi:10.4103/1673-5374.238613
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer: Maria Antonietta Panaro, University of Bari, Italy.
Additional file: Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Validity and reliability of the Ocular Motor Nerve Palsy Scale
- Mitogen-activated protein kinase phosphatase 1 protects PC12 cells from amyloid beta-induced neurotoxicity
- High-frequency (50 Hz) electroacupuncture ameliorates cognitive impairment in rats with amyloid beta 1–42-induced Alzheimer’s disease
- Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia
- Combined VEGF/PDGF improves olfactory regeneration after unilateral bulbectomy in mice
- Comparison of morphological and functional outcomes of mouse sciatic nerve repair with three biodegradable polymer conduits containing poly(lactic acid)