Glial cell line-derived neurotrophic factor as a treatment after spinal cord injury
Spinal cord injury (SCI) is a devastating trauma that currently affects 54 people out of every million, which is approximately 270,000 people in the United States (National Spinal Cord Injury Statistical Center, 2013). The effects of such an injury can cause a loss of both motor and sensory function below the injury site, normally leaving the patient unable to care for themselves entirely and relying on family and friends to provide personal care. Currently there are no definitive cures to a SCI; however, several potential treatments are currently being researched. One potential treatment would be the use of glial cell line-derived neurotrophic factor (GDNF), a growth factor that affects both neurons and astrocytes, which are support cells that protect neurons and maintain homeostasis. This has been shown to decrease lesion size, reduce allodynia, and regenerate axons in the central nervous system (CNS) and peripheral nervous system (PNS)(Rosich et al., 2017).
GDNF has been extensively studied as a growth factor that has the potential to promote the survival of dopaminergic neurons. GDNF also has a potent survival effect on other neurons in the CNS and has many other roles in the nervous system (Paratcha and Ledda,2008). GDNF has been characterized as a glycosylated homodimer connected by disulfide bond, and has two forms: a two hundred and eleven amino acid pre-GDNF inactive form and a one hundred and thirty-four amino acid active form. GDNF is the most potent growth factor of the GDNF family ligands (GFLs), which all contain seven conserved cysteine residues (Rosich et al., 2017).
Signaling: GDNF can signal in two different ways, with each pathway producing different effects. Both pathways involve a glycosylphosphatidylinositol-anchored co-receptor GDNF family receptor alpha(GFRα), which specializes in ligand binding and specificity. There are four different GFRαs, with each determining different ligand specificity and affecting different areas of the nervous system (Paratcha and Ledda, 2008). Specifically, GFRα1 and GFRα2 are more prominently expressed in CNS, while GFRα3 and GFRα4 are more prominently expressed in the PNS. GFRα1 can be released by neuronal cells and Schwann cells (SCs) in an injured sciatic nerve (Paratcha et al., 2001).
One pathway, RET-dependent, uses Ret receptor tyrosine kinase as a signaling receptor. GDNF binds specifically to GFRα1 to form a complex that brings two molecules of Ret together, which causes intracellular signaling and transphosphorylation of specific tyrosine residues. This pathway has been shown to lead to neuronal survival,cell differentiation, and cell migration (Airaksinen and Saarma, 2002)(Figure 1). The other pathway, RET-independent, utilizes neural cell adhesion molecule (NCAM). In this pathway, GDNF binds to GFRα1 specifically and to p140NCAM(Paratcha and Ledda, 2008). This pathway has been shown to lead to cytoskeleton remodeling, neurite growth, and cell migration (Figure 1).
Roles pre-SCI: GDNF is an important growth factor in the CNS and PNS as it affects development, neurodifferentiation, and is a chemoattractant for many different cell types, such as neuronal precursor cells, and cells in the spinal lateral motor column. This is accomplished by the activation of Ret on these cells, which increases motility. In mammalian development GDNF is at high concentrations,but eventually becomes undetectable in adults. GDNF is present in high concentrations in the floorplate during development, possibly to aid in axonal development and migration. Moreover, in post-natal motor neurons it has been shown that GDNF’s primary role is to promote terminal axonal branching and synapse formation (Rosich et al., 2017).
Roles and treatment post-SCI: GDNF treatment post-SCI has been shown to significantly increase functional recovery below the vertebral level of injury. One important way GDNF achieves the functional improvement is by reducing secondary damage after SCI. As a neuroprotective agent, GDNF has also been shown to reduce the permeability of the blood-spinal cord barrier (BSCB) and to downregulate nitric oxide synthase (NOS). These effects in combination work to reduce cell injury, reduce cord edema, and have survival-promoting effects on multiple different cell types. Reducing neural apoptosis in the spinal cord can lead to a decrease in secondary damage and an increased recovery (Rosich et al., 2017). Post-SCI treatment with GDNF, in varying types of administration, has been shown to significantly decrease the lesion size. Furthermore, GDNF treatment also increases the amount of spared white matter after SCI. It has also been shown that GDNF promotes axonal sprouting in the spinal cord after a unilateral corticospinal tract (CST) injury (Zhou and Shine, 2003).
Along with decreasing lesion size, GDNF improves axon growth and regeneration post-SCI. With varying degrees of success between different administration techniques, GDNF has been shown to regenerate axons, increase total axonal surface area, increase axon density, and also significantly increase blood vessels at the injury site.Post-SCI, regenerating axons is a major hurdle due to a glial scar that forms at the injury site, along with inhibition by myelin and chemorepellent molecules. Treatment with Schwann cells that express GDNF has been shown to overcome these barriers to some extent (Rosich et al., 2017).
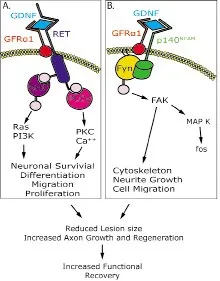
Figure 1 A representation displaying the two diあerent signaling pathways, RET-dependent (A) and RET-independent (B).
There are many ways to treat SCIs with GDNF, and one of the most successful treatments is to combine GDNF with other neurotrophic factors. Different neurotrophic factors have different effects post-SCI and in combination more pronounced effects are observed. For example, a combination of GDNF and neurotrophin-3(NT-3) creates a localized increase in sprouting of the CST neurons post-injury, while a combination of GDNF with nerve growth factor (NGF) improves axon branching and elongation. Combining all three of these in a controlled manner, along with other factors such as brain-derived neurotrophic factors (BDNF), could potentially lead a more pronounced effect on recovery than individually dosed treatments (Rosich et al., 2017). The timing of GDNF treatment post-SCI is also very important. In previous animal studies if the treatment is within one hour of the injury or before the injury occurs, secondary damage was reduced; however, treating after 24 hours failed to promote functional recovery. GDNF has also been shown to reduce the expression of proinflammatory cytokines interleukin-6 and tumor necrosis factor-α. Along with timing, different methods of administration have been tested, and they give rise to differing results. For example, olfactory ensheathing cells (OECs) that were genetically-modified to secrete GDNF provided the best functional recovery post-SCI, while using Schwann cells to secrete GDNF had the largest decrease in lesion size (Rosich et al., 2017).
Different concentrations of GDNF have been tested, and it is most successful at promoting axonal growth and neuronal survival at low doses (2–40 µg/day). Intracortical administration of higher doses failed to promote neuronal survival, which was attributed to desensitization of the receptors. As well as promoting axonal growth,adjusting the concentration in a gradient could help with directional guidance for axons. Due to the chemoattractive properties of GDNF,an increasing gradient towards the distal end of the injury could possibly aid in elongation and an increase in functional connections made.
Future applications: GDNF has a lot of different significant effects on the nervous system, both pre- and post-SCI, which have been studied broadly. At this point it would be beneficial to determine successful strategies to enhance its effectiveness. For example, an enhanced delivery method that not only delivers ample concentrations of GDNF overtime, but also delivers GDNF at a gradient to promote directional guidance would potentially enhance GDNF’s ability to regenerate axons and make functional connections downstream.It is also important to understand if a continued, consistent dosage over a long period of time would be beneficial compared to a single dose. One possible solution to this problem would be to use calcium phosphate minerals to achieve a sustained release of GDNF, and have the ability to bind and release combinations of multiple growth factors (Yu et al., 2014). This technology has been used previously with other growth factors (Hanna et al., 2016). Different gradients could also be possibly tested using this technology.
What cell types are affected by GDNF, and how they are affected,are extremely important questions that still need to be fully answered.It has been shown that GDNF has survival-promoting effects on:noradrenergic, cortical, retinal ganglion, sensory, parasympathetic, peripheral, motor neurons, neonatal motor neurons, microglia, dopaminergic, dopamine-like mesenchymal stem cells, and retinal Müller glial cells (Rosich et al., 2017). However, there are more ways that GDNF can affect cells, and this is not an exhaustive list of cells that can be affected. For example, it has been shown that GDNF and GFRα1 together “promotes the development of hippocampal dendritic arbors and spines via NCAM”, which is independent of Ret (Irala et al., 2016). This could imply that GDNF has a wide array of effects on a diverse amount of cell types in humans, and it is important to increase research into these areas to further our understanding of how GDNF affects the nervous system. More studies including knock-out or conditional knock-out animals may help provide insight into cell expression and function.
Because SCI is such a complex injury and involves multiple different cellular responses, a future treatment will most likely have to be complex as well. Combining different neurotrophic factors together has been shown to be productive, and future research into combinations of neurotrophic factors that produce a robust response to SCI could be potentially beneficial. Along with different combinations of neurotrophic factors and proteins, testing a local sustained release may enhance the therapeutic effect of GDNF.
Conclusion: GDNF is an important neurotrophic factor for development of the CNS and PNS and is an effective post-SCI potential treatment that promotes neuronal survival, axonal regeneration,reduces secondary damage, decreases lesion size, and improves functional recovery. The combination of GDNF with other neurotrophic factors enhances its therapeutic capability. There are many ways to administer GDNF post-SCI, including genetically-modified cells to secrete GDNF and exogenous delivery. Future research should focus on an enhanced delivery method, more knowledge of the cell types that are affected, and a combination of growth factors.
Stephen D. Ortmann, Daniel J. Hellenbrand*
Department of Neurological Surgery, University of Wisconsin, Madison, WI, USA
*Correspondence to:Daniel J. Hellenbrand, MS,hellenbrand@neurosurgery.wisc.edu.
orcid:0000-0003-4730-5752 (Daniel J. Hellenbrand)
Accepted:2018-06-23
doi:10.4103/1673-5374.238610
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Validity and reliability of the Ocular Motor Nerve Palsy Scale
- Mitogen-activated protein kinase phosphatase 1 protects PC12 cells from amyloid beta-induced neurotoxicity
- High-frequency (50 Hz) electroacupuncture ameliorates cognitive impairment in rats with amyloid beta 1–42-induced Alzheimer’s disease
- Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia
- Combined VEGF/PDGF improves olfactory regeneration after unilateral bulbectomy in mice
- Comparison of morphological and functional outcomes of mouse sciatic nerve repair with three biodegradable polymer conduits containing poly(lactic acid)