The combination of induced pluripotent stem cells and bioscaffolds holds promise for spinal cord regeneration
Ashley DeBrot, Li Yao*
Department of Biological Sciences, Wichita State University, Wichita, KS, USA
Abstract Spinal cord injuries (SCIs) are debilitating conditions for which no effective treatment currently exists. The damage of neural tissue causes disruption of neural tracts and neuron loss in the spinal cord. Stem cell replacement offers a solution for SCI treatment by providing a source of therapeutic cells for neural function restoration. Induced pluripotent stem cells (iPSCs) have been investigated as a potential type of stem cell for such therapies. Transplantation of iPSCs has been shown to be effective in restoring function after SCIs in animal models while they circumvent ethical and immunological concerns produced by other stem cell types.Another approach for the treatment of SCI involves the graft of a bioscaffold at the site of injury to create a microenvironment that enhances cellular viability and guides the growing axons. Studies suggest that a combination of these two treatment methods could have a synergistic effect on functional recovery post-neural injury. While much progress has been made, more research is needed before clinical trials are possible. This review highlights recent advancements using iPSCs and bioscaffolds for treatment of SCI.
Key Words: induced pluripotent stem cells; bioscaffolds; spinal cord injury; regeneration; transplantation; differentiation; functional recovery; neuron replacement; guidance; microenvironment
Introduction
Spinal cord injuries (SCIs) are highly debilitating and often painful, causing affected individuals physical, financial, and emotional hardship. Vehicle accidents, falls, violent confrontations, degenerative neural conditions, and congenital disorders are known to lead to spinal cord damage. For traumatic injuries, the condition progresses along two phases: primary injury(the initial damaging event) and secondary injury (damage to the spinal cord through inflammation and ischemia). SCIs can be further categorized by the extent of damage to the spinal cord. Incomplete SCIs are those in which motor and/or sensory function is still partially present below the site of injury and in which there is sensation/function at the lowest sacral segment (Maynard et al., 1997). A test for incomplete SCI includes determination of the voluntary control of the anal sphincter. Loss of this function is an indication of a complete SCI. The extent to which the function is lost impacts the overall prognosis and quality of life for affected individuals. Generally, more severe injuries and those located more rostrally are associated with poorer outcomes.
As a result of the injury process, the environment around the site of the injury is not conducive to neural regeneration.The scarring tissue around the lesion site can stunt the growth of axons and innate neural stem cells residing in the spinal column. Cytokines including tumor necrosis factor (TNF)-alpha, endothelin-1, interleukin (IL)-1, IL-6, thrombin, and ciliary neurotrophic factor (CNTF) may trigger the gliosis and thereby contribute to the formation of nonpermissive environment (Fitch and Silver, 2008). Interventions such as surgery, steroids, and blood pressure augmentation can reduce the area of secondary injury and improve the longterm outcome (Ahuja et al., 2017). The rehabilitation and pain management showed improved functional recovery below the site of injury. However, the fully effective treatment yet exists for SCIs. Two particularly promising areas of research are those of stem cell therapy and biomaterial scaffolds to restore the lost neural function. Introducing stem cells at the site of injury can replace neurons and supportive cells lost during the primary injury or subsequent secondary injury. While many different stem cells have been tested, induced pluripotent stem cells (iPSCs) offer several advantages over other cells because they are free of the ethical and immunological considerations often seen with other stem cells. Additionally, biomaterials scaffolds have aided in functional recovery after SCI. Scaffold implantation creates a prolific microenvironment that shields cells from neurotoxic agents and guides growing axons while bridging the transected portions of spinal cord. Numerous materials have been implemented in animal models. The combinatorial treatment of stem cells and biomaterials have shown a synergistic effect in the therapy of SCI. While limited studies of iPSCs with biomaterial scaffolds have been conducted for spinal cord repair in vivo, progress from other stem cells in combination with scaffold suggests that such a treatment regime could prove to be an effective means of treating SCIs.
Transplantation of iPSCs in Spinal Cord Repair
Neural cell loss in SCI
Multiple factors can cause widespread cell death in the spinal cord following injury. Stem cell therapy offers a solution to replace damaged cells. For traumatic SCIs, the damage during both primary and secondary injury leads to loss of neurons and other vital tissues within the spinal column (Oyinbo,2011). During the primary injury, the force from the initial impact and persistent compression or resulting laceration from bone or other foreign material exerts extreme stress on cells,leading to necrotic cell death (Sekhon and Fehlings, 2001;Oyinbo, 2011). Hemorrhaging in the gray matter is a frequent occurrence owing to the rich blood supply in this region(Wolman, 1965). At this point, blood flow along the spinal cord may also be disrupted, leading to ischemia and a hypoxic state. Within minutes of injury, damage from secondary injury begins. Ischemia can cause an increase in free radicals and reactive oxygen species leading to organelle malfunction,DNA damage, and lysing of the cell membrane (Dumont et al., 2001; Sekhon and Fehlings, 2001; Oyinbo, 2011). The immune system contributes to secondary injury by initiating a large inflammatory response, causing cell death and inhibiting axonal growth (Oyinbo, 2011). Collectively these events lead to widespread neuronal death, loss of axonal connections, and reduced myelination in surviving cells. The descending and ascending neural pathways are interrupted by the SCI.
The formation of a glial scar around the site of injury creates a barrier for the establishment of new neural connections. The glial scar, consisting of astrocytes and connective tissue, produces inhibitory molecules that prevent proliferation of endogenous stem cells, growth of new axons, and repair of surviving axons (Fitch and Silver, 2008). Without cellular regeneration, little to no functional recovery is expected.
iPSCs hold promise for SCI therapy
One potential treatment method for SCI is to replace the cells that are lost after the injury. The use of stem cells and their derivatives has gained momentum in recent years. Given their ability to differentiate into multiple types of human cells including neurons, stem cells make an ideal candidate for neural tissue regeneration. Induced pluripotent stem cells, a relatively new type of stem cell, provide a promising new source of replacement cells. The first iPSCs were induced from murine fibroblast cells in 2006 (Takahashi and Yamanaka, 2006).Twenty-four candidate genes selected to test for their ability to induce pluripotency were introduced by retroviral transduction. Of the genes tested, four transcription factors (Oct3/4,Sox2, c-Myc, and Klf4) were found capable of inducing an embryonic-like state by expression of key embryonic stem cell (ESC) markers. While the first iPSCs were created using retroviral transduction, more recent methods have been used to prevent integration of any viral vector sequences into the target cell’s genome (Malik and Rao, 2013). Such methods include use of nonintegrating viruses, certain RNAs, and transposons.
Historically, most studies have used embryonic or mesenchymal stem cells (MSCs) for spinal repair (Xiang and Chen,2012); however, studies utilizing iPSCs are growing in number. iPSCs and ESCs are remarkably similar in their genetic and molecular composition (Goldthwaite, 2016) as well as their differentiation capacity (Yamanaka, 2012). However, it should also be noted that such comparisons of iPSCs to ESCs are reductive in that they assume embryonic stem cells as the standard for comparison while ignoring the inherent capacity of iPSCs (Yamanaka, 2012). While the two stem cell lines are similar in many ways, iPSCs offer several benefits over ESCs because they are free from the ethical concerns associated with ESCs and can circumvent immune system rejection of graphed cells when induced from a patient’s own somatic cells.
iPSCs may also hold select advantages over MSCs in spinal cord regeneration. MSCs cannot be differentiated into functional neurons and glia as iPSCs. Because MSCs must be harvested from a source such as bone marrow or adipose tissue,they risk depleting donor sites of essential stem cells. Additionally, an autologous donor must be used to avoid immune rejection of transplanted cells when taken from a source other than the patient. Some research has suggested that MSCs may be used from allogeneic sources while still avoiding a host immune response; however, results across the board for studies of the supposed immune privilege of MSCs are mixed at best(Griffin et al., 2013). Rejection of transplanted cells remains a viable risk.
Induced pluripotent stem cells may soon become the preferred choice in stem cell replacement therapies, not only for the ethical and immunological problems they avoid but also for their efficiency in neural functional recovery. In fact,promising results have shown that iPSCs promote better recovery after SCI when compared to other stem cell types(Ruzicka et al., 2017). This study measured the effects of three different human-derived stem cell lines (mesenchymal, fetal,and induced pluripotent) in rat SCIs. Animals receiving treatment with iPSCs showed the greatest amount of functional recovery, the most axonal sprouting, and the most preservation of white matter and grey matter at the site of injury.Ultimately, iPSC-based replacement therapies aim to take cells from a patient, reprogramming them to stem cells, and replacing damaged tissue with these immune-compatible cells.More research is needed to improve induction techniques that would generate a rapid, repeatable product while still ensuring safe cell lines free of tumorigenicity before widespread clinical applications are conceivable.
Transplantation of iPSCs improves functional recovery post-SCI
One of the greatest barriers in overcoming spinal injuries is the relatively slow recovery of neurons in the central nervous system. iPSCs offer a solution by providing a reservoir of cells to replenish lost neurons and supportive cells. iPSCs have been shown to survive and recover function in animal models of SCI. They have been shown to aid in myelination, regeneration of axons, and growth of synaptic connections at the site of injury (Tsuji et al., 2010; Nori et al., 2012; Lu et al., 2014)(Table 1). In one study using murine iPSC-derived neural stem cells in a mouse model, functional recovery was noted along with axon regrowth and formation of synaptic connections between transplanted cells and host neurons (Nori et al., 2012).Indeed, transplanted human-iPSCs in a rat model have been shown to generate axons across extended distances, traversing more than 9 cm in length (Lu et al., 2014). In this study,the iPSCs were able to penetrate both white matter and gray matter to successfully form synapses between grafted cells and host cells.
The iPSCs have been differentiated to several cells types in addition to neurons. Astrocytes provide important support functions in the central nervous system and can be damaged in cases of SCI. Replacement of lost astrocytes by iPSC-derived astrocytes overexpressing major glutamate transporter, glial glutamate transporter 1 (GLT1), has been shown to preserve innervation of the diaphragm and aid in respiration in mouse and rat models (Li et al., 2015). Oligodendrocytes are another significant group of supportive cells affected by injury. Transplanted iPSCs expressing the mature oligodendrocyte marker myelin basic protein (MBP) were able to form myelin sheaths around axons in vivo and increased functional recovery (All et al., 2015; Kawabata et al., 2016). The ability of differentiation into a vast number of cells types is suggestive of their therapeutic abilities in SCI as well as a number of other diseases.
As previously noted, one of the earliest interventions after SCI is surgery. During the procedure, tissues around the affected area are removed and often disposed of as waste.One study used human intervertebral disc cells obtained from post-injury surgery to generate iPSCs through retroviral transfection (Oh et al., 2015). Disc cell-derived iPSCs were differentiated into neural precursor cells displaying neural markers such as Nestin, Sox2, Pax6, and Sox1 before transplant into a mouse model of SCI. Treatment mice showed significant improvements in hind limb stepping with weight support and reduced tissue damage at the injury epicenter when compared to a phosphate buffered saline (PBS)-injected control group.The study speculates that epigenetic memory after reprogramming of intervertebral disc cells may aid in functional recovery of the injured spinal cord.
One major concern with stem cell transplantation is the formation of tumors in the host. A study investigating tumorigenicity used the common marmoset as a model for SCI at cervical level 5 (Kobayashi et al., 2012). In a previous study by the same research group (Nori et al., 2011), human iPSCs tested in a mouse host promoted functional recovery without the formation of tumors. Thus, this line was deemed “safe”and selected for use in the common marmoset. The hiPSCs were established by transducing four reprogramming factors(Oct3/4, Sox2, Klf4, and c-Myc) into adult human fibroblasts.After surgical wounding, animals who had received neural stem/progenitor cells (NS/PCs) differentiated from iPSCs showed greater improvement in locomotion, grip strength,and cage climbing when compared to control groups 112 days post-SCI. Immunostaining showed markers for neurons, astrocytes, and oligodendrocyte precursor cells (OPCs) near the lesion epicenter. Transplanted cells showed no signs of tumorigenicity, indicating that tumor prevention is possible with careful induction and appropriate cell screening. Successful functional recovery after SCI in non-human primates marks a milestone in stem cell replacement therapy, bringing human clinical use closer than ever. In a further study (Nori et al.,2015), human iPSCs were established by transducing OCT4,SOX2, and KLF4 into adult human dermal fibroblasts isolated from the same donor in previous study (Nori et al., 2011). After grafted into the wounded mice spinal cord for 47 days, the neurospheres (NSs) derived from human iPSCs differentiated into three neural lineages, improved functional recovery and synapse formation. However, the deteriorated motor function accompanied by tumor formation was detected after long term observation (for up to 103 days). Analysis of tumors from this study showed that the presence of Nestin(+) undifferentiated neural cells and upregulation of OCT4-transgene (Tg) and KLF4-Tg. One previous study evaluated the factors that may influence the teratoma-forming tendency of iPSCs by grafting secondary neurospheres (SNS) induced from iPSCs derived in 11 different ways into the mouse brains (Miura et al., 2009).The outcome of the research revealed that the SNS from adult tail-tip fibroblasts (TTF)-iPSCs produced significantly larger teratomas than did those from the other iPSCs or ES cells.The study suggests the tumor formation may be affected by the methods used for reprogramming and differentiation of iPSCs, the site of transplantation, and the host background(Miura et al., 2009).
Another study investigating iPSC tumorigenicity manipulated the body’s immune response to allogeneic tissue as a “fail-safe” system against tumor formation (Itakura et al., 2015). Immunocompetent mice were separated into two groups, one group receiving immunosuppressant drugs and one control group. Human iPSC-derived NS/PCs known to cause tumors were transplanted into the spinal cords in each group. The grafted cells were completely cleared from the hosts in the control group. Most of the animals in the immunosuppressant group developed tumors along the spinal cord that resulted in the loss of hind limb function. However,discontinuation of the immunosuppressants resulted in destruction of the tumor and subsequent recovery of function.In the event that non-patient-specific stem cells must be used clinically to ensure transplantation before glial scarring occurs,immune rejection would provide for built-in protection against tumor formation.
The Combinatorial Effect of Bioscaffolds and iPSCs on Spinal Cord Regeneration
Scaffolds implanted into injured spinal cords act as a bridge between the severed portions of nerve. Many different types of materials have been suggested for use in spinal cord repair from both natural and synthetic sources. Biodegradable natural materials such as collagen and chitosan offer an added benefit by avoiding the need for a second surgery to remove the scaffold and prevent nerve compression because the material degrades with nerve growth (Straley et al., 2010). Scaffolds impart multiple benefits at the site of injury by guiding axonal growth, providing a microenvironment conductive to growth,and physically blocking scar tissue formation. Additionally,scaffolds can be used to deliver stem cells, prolific molecules,and other drugs to increase regeneration and functional recovery along the spinal cord.
Repair of wounded spinal cord using bioscaffolds
Natural materials have been widely used in scaffold construction, two of the more popular being collagen and chitosan.One of the most abundant materials of the extracellular matrix(ECM), collagen is also a popular choice for creating scaffolds for transplant into SCIs. Its flexible and porous nature allows for cell migration and growth, which is especially important for neuronal processes. Collagen-filament scaffolds were transplanted parallel to the spinal cord in rabbits with SCI(Yoshii et al., 2009). The 5-mm scaffolds bridged a 3-mm transected section of spinal cord. After 24 weeks, rabbits were scored using the Basso-Beattie-Bresnahan (BBB) test, a rating system designed to assess locomotor function. Rabbits with collagen implants scored significantly higher than controls,suggesting axon regeneration along the scaffold. In another study involving rats, linearly ordered collagen scaffolds were used to not only guide axons but also as a means to deliver drugs to the lesion site (Fan et al., 2017). The scaffolds were modified with the collagen-binding EGFR antibody, which is known to neutralize myelin inhibitory molecules. In this way,the antibody was released in a controlled manner as endogenous neural stem cells differentiated into functional neurons.Completely transected SCIs in beagle dogs showed functional recovery after implantation of linear-ordered collagen scaffolds (Han et al., 2015). Groups were tested with a collagen scaffold alone and also a collagen scaffold embedded with a collagen-binding brain-derived neurotrophic factor (BDNF),a protein known to promote neuron survival, stimulate axons, and aid in remyelination. The scaffold-alone groups showed moderate improvement over control groups, while the scaffold-with-protein groups showed the greatest amount of recovered function, with some test animals standing and ambulating without assistance. This study yet again provides evidence for the benefits of slow-release molecules by scaffold delivery.
Chitosan offers another attractive source for naturally derived scaffold material. Originating from the hard exoskeletons of crustaceans, chitosan currently has several medicinal purposes including weight management and high cholesterol.Soon it could very well be used in the treatment of SCI. One study used a chitosan scaffold with glioma extracellular matrix and SB216763 for SCI in rat models (Jian et al., 2015).Extracellular matrix (ECM) is rich in biomolecules important for recruitment and proliferation of cells, while SB216763 is known to inhibit glycogen synthase kinase (GSK)-3beta, an enzyme involved in blocking neurogenesis and differentiation.Results showed that a combination of all three treatments yielded more functional recovery when BBB scored compared to control groups. Another study combined two different biomaterials. A chitosan tube was filled with semifluid collagen for transplant into rats with SCIs (Li et al., 2009). Treatment animals showed greater recovery of function with BBB scoring when compared with control animals (chitosan tube alone and no treatment groups). One year after injury, immunohistochemistry showed large numbers of cells bearing neuron and astrocyte markers in animals with collagen-filled chitosan scaffolds. Such animals also had a reduction in glial scarring and increased vasculature through the site of injury.
Bioscaffolds enhances the therapeutic effect of grafted stem cells in spinal cord repair
In SCI therapy, bioscaffolds have acted as a carrier for stem cell delivery and structural support for cell transplantation.Embryonic stem cells have long been a viable choice for regenerative studies in regenerative medicine. In one study involving rats, embryonic stem cells were differentiated into neural progenitor cells before being loaded into collagen scaffolds and transplanted into the injured spinal cord (Hatami et al., 2009). Rats receiving scaffolds with cells had increased BBB scores and faster response times to heat stimulus of the hind limbs.
Fibrin has been shown to be a suitable material in the construction of bioscaffolds for stem cell seeding. In one such case, fibrin scaffolds embedded with growth factors were successful in the treatment of rat SCI with ESC-derived neural stem cells (Lu et al., 2012b). The cells were able to fill the entire lesion cavity in as little as seven weeks post-transplant.Only three weeks after transplant, cell-treated animals showed increasing BBB scores. However, the risk of tumor formation remains an ongoing issue since bioscaffolds can promote proliferation of undifferentiating stem cells. One investigation used antibiotic selection to create a more purified line of ESC-derived progenitor motor neurons for transplant into a rat model of SCI through use of a fibrin scaffold (McCreedy et al., 2014). Transplanted cells differentiated into the desired mature cell types and showed a reduction of undesired differentiation.
The combinatorial application of bioscaffolds and mesenchymal stem cells enhanced the regeneration of injured spinal cord (Syková et al., 2006; Zeng et al., 2011; Gao et al., 2013;Han et al., 2018). A gelatin sponge encased in a cylindrical poly-(lactide-co-glycolide) (PLGA) film was seeded with MSCs from rat bone marrow before transplant into a rat model of SCI (Zeng et al., 2011). The scaffold was able to bridge the transected segments of spinal cord and encourage growth of blood vessels while decreasing the inflammatory response around the site of injury. Another study of MSCs from rat bone marrow used macroporous hydrogels derived from either 2-hydroxyethyl methacrylate (HEMA) or 2-hydroxypropyl methacrylamide (HPMA) (Syková et al., 2006). Hydrogels were grafted into rat spinal cords either by themselves or after seeding with MSCs. Animals receiving cells showed greater improvements in BBB scoring, more preservation of white matter, and smaller lesion sites when compared with hydrogel only animals. As gels biodegraded in the host, they were replaced with connective tissue elements. MSCs for spinal cord regeneration have also been isolated from human placenta.Such cells were loaded into linear-ordered collagen scaffolds before transplanting into a completely transected canine spi-
nal cord (Han et al., 2018). Dogs receiving scaffolds only and cell-seeded scaffolds showed improved recovery over controls;however, the greatest recovery was seen in the cell-seeded group. Half of the animals in this group were able to stand or walk without assistance by the 16th week of the study. Interestingly, this study reported regeneration of both ascending sensory nerve fibers and descending motor nerve fibers, indicating growth of both sensory and motor neurons.

Table 1 Induced pluripotent stem cell (iPSC) transplantation for spinal cord repair
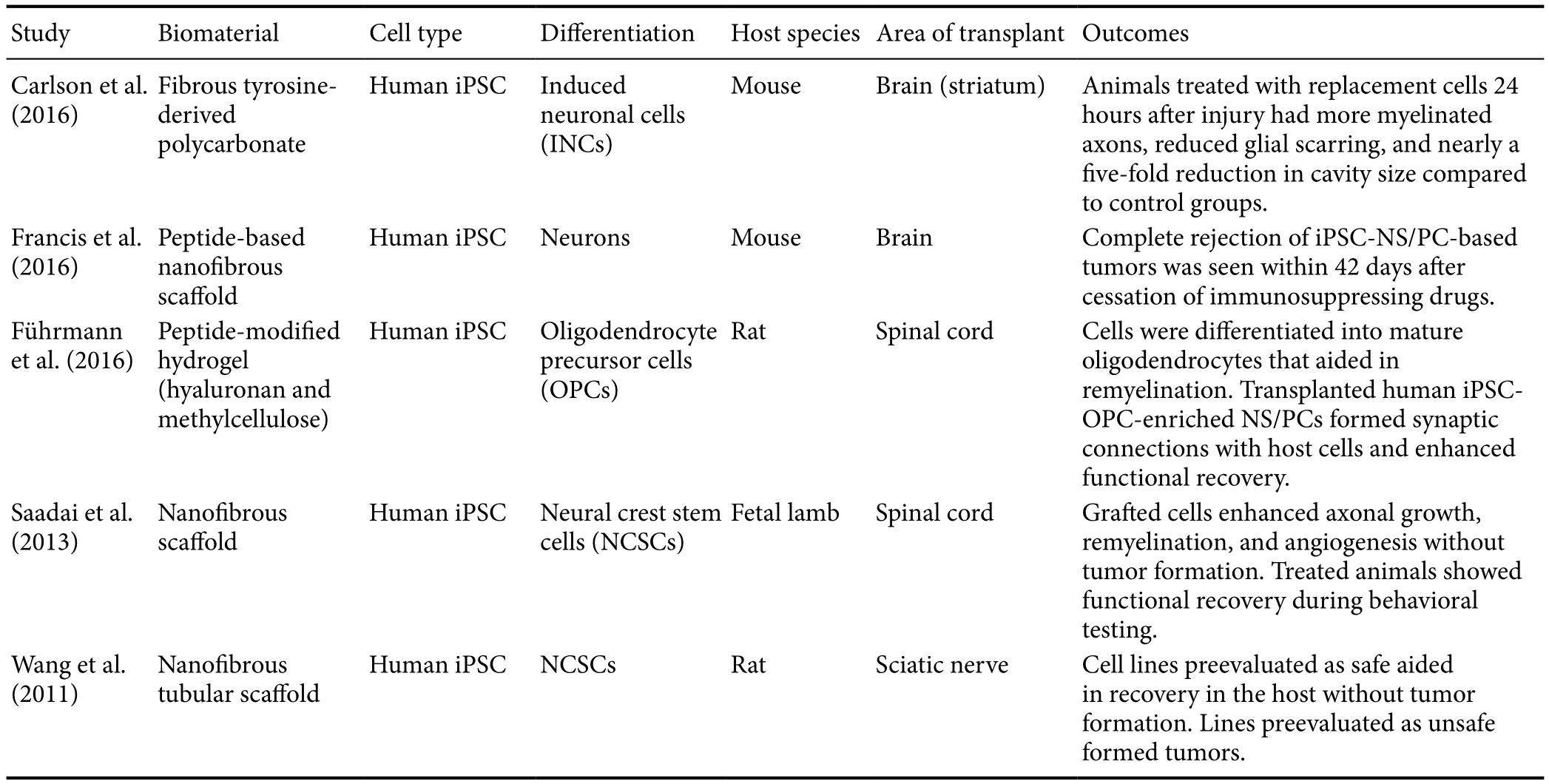
Table 2 Bioscaあolds and induced pluripotent stem cell (iPSC) transplantation for neural regeneration
Co-transplantation of iPSCs and bioscaffolds in the treatment of wounded spinal cord
iPSCs have been used in combination with scaffolds of various materials in the treatment of neurological disorders. These cells seeded into scaffolds have been used in neural regeneration in the peripheral nervous system. The ends of severed rat sciatic nerves were connected using a nanofibrous nerve conduit (Wang et al., 2011) (Table 2). Rats received conduits seeded with neural crest stem cells (NCSCs) differentiated from either iPSCs or ESCs. Transplanted cells terminally differentiated preferentially into Schwann cells and increased the recovery of the peripheral nerve. iPSCs were capable of differentiating to NCSCs with varying efficacy, highlighting the need for careful selection of cells for induction to a pluripotent state. A procedure also involving rat sciatic nerves was performed using mesenchymal stem cells in an erythropoietin(EPO)-loaded chitosan nerve conduit (Zhang et al., 2017).This method allowed for slow-release of the cytokine EPO,a molecule known to have neuroprotective features. Animals receiving both cells and scaffold showed improved motor control and increased numbers of axons with thicker myelination compared to those receiving no or only one treatment method.
This duel therapy has been suggested for regenerating retinal ganglion cells (RGCs) following loss from diseases such as glaucoma or Leber’s hereditary optic neuropathy. An in vitro study of iPSCs observed their ability to successfully grow along a scaffold made from poly(ethylene-co-vinyl acetate)copolymer (Yang et al., 2017). iPSC-derived cells expressing the RGC marker Math5 and neuron marker Tuj1 grown in the scaffold produced longer and more well-organized neurites than those grown on a regular plate. Further studies are needed to understand the integrative capabilities of such cells in vivo.
The brain is another target that has benefited from iPSC-seeded scaffolds where survival of transplanted cells is generally poor. Microscale fibrous scaffolds have been used to improve the survivability of iPSC-derived cells in the murine brain (Carlson et al., 2016). Cells were differentiated into induced neuronal cells before seeding onto polymer microscaffolds which are small enough for injection. The scaffolds were then injected bilaterally into the striatum. Cells on the scaffolds had a 38-fold increase in survival compared with control cells injected without a scaffold. A peptide-based nanofibrous scaffold has also been shown to increase survivability and growth of transplanted iPSC-derived cells (Francis et al.,2016). Using combinations of iPSCs with scaffolds has the potential to treat human patients suffering from traumatic brain injuries or neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases.
Limited research has been done on the effects of iPSCs with biomaterial scaffolds for spinal cord regeneration. Growth of undifferentiated iPSCs in various materials in vitro has been noted to have several beneficial effects on cell culturing. Cells grown in a chitin-alginate three-dimensional (3D) microfibrous scaffold were shown to efficiently differentiate and mature into neurons (Lu et al., 2012a). In fact, more than 90% of neurons grown in this way stained positive for the neuron marker βIII tubulin, indicating a purer line of cells than previous methods. In another study, murine iPSCs were differentiated to neurons in 3D fibrin scaffolds (Montgomery et al., 2015).Growth in these conditions generates a higher portion of neuronal cells and differentiation to occur two days faster than previously established methods. The 3D environment better mimics in vivo conditions when compared with traditional two-dimensional culturing methods, leading to more clinically applicable samples.
iPSCs have also been shown to undergo numerous passages without genetic mutation when grown in specially modified nanofibrous gelatin (Liu et al., 2014). To create a matrix suitable for cell adhesion, electrospinning and cross-linking methods were used. Cells were seeded and grown in feeder- and serum-free conditions. Using this method, cells were passaged more than 20 times with no noted chromosomal abnormalities or loss of pluripotency. Such environments as these offer greater control over cellular conditions while providing a reservoir of cells for future research or clinical applications.
There is a paucity of literature on the use of both iPSCs and bioscaffolds for spinal cord regeneration. One recent study implemented both therapies in a fetal lamb model of myelomeningocele (Saadai et al., 2013). Human iPSCs were differentiated into NCSCs, which were then mixed with hydrogel and seeded onto a nanofibrous scaffold before being transplanted into an animal with SCI. Thirty days after surgery, cells were shown to have survived and integrated with the host’s system while also differentiating into neurons. Another study combining iPSCs with bioscaffolds used a peptide-modified hydrogel composed of hyaluronan and methylcellulose with a rat model of SCI (Führmann et al., 2016). Oligodendrocyte precursor cells injected into the injury were more likely to survive, integrate, and differentiate into glial phenotypes when embedded in the hydrogel than when injected with media. The formation of teratomas was attenuated in the hydrogel, most likely owing to the increase in differentiation. The success of these studies supports the notion that iPSCs with bioscaffolds offer a powerful option when treating damaged spinal cords.
Conclusion
SCIs are highly debilitating conditions that have proven difficult to cure. Recent advances in stem cell therapy offer hope to affected individuals. In the short time since their initial introduction in 2006, iPSCs have already shown a remarkable capacity for regeneration with safer and more efficient techniques continuously being developed. Used alone, these stem cells have been effective in restoring function after SCIs in animal models while avoiding many of the drawbacks of alternative stem cell lines. Another approach in treating SCIs is the application of bioscaffolds in the lesion for the protection and guidance of axonal growth. Materials for the creation of such scaffolds varies widely and includes both natural and synthetic options. While each treatment is effective alone, the combination of stem cells and bioscaffolds has shown a synergistic effect in functional recovery below the site of injury. Though limited studies have been undertaken to repair wounded neural tissue using a combination of iPSCs and bioscaffolds, when attempted, this method yielded effective integration of transplanted cells with host tissue. Given the success of these recent studies, it can be reasonably speculated that the continued use of iPSCs with bioscaffolds will lead to improved outcomes for patients suffering from SCIs.
Author contributions: Concept and design of the paper: LY; manuscript preparation: AD and LY.
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer: Meng-Jen Lee, Chaoyang University of Technology,Taiwan, China.
Additional file: Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Validity and reliability of the Ocular Motor Nerve Palsy Scale
- Mitogen-activated protein kinase phosphatase 1 protects PC12 cells from amyloid beta-induced neurotoxicity
- High-frequency (50 Hz) electroacupuncture ameliorates cognitive impairment in rats with amyloid beta 1–42-induced Alzheimer’s disease
- Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia
- Combined VEGF/PDGF improves olfactory regeneration after unilateral bulbectomy in mice
- Comparison of morphological and functional outcomes of mouse sciatic nerve repair with three biodegradable polymer conduits containing poly(lactic acid)