Characterization of Cnidoscolus quercifolius Pohl bark root extract and evaluation of cytotoxic effect on human tumor cell lines
Paulo Fernando Machado Paredes, Selene Maia de Morais, Fernando César Rodrigues Brito, Luiz Francisco Wemmenson Gonçalves Moura, Patrícia de Araújo Rodrigues, Stephen Rathinaraj Benjamin✉, Francisco Ernani Alves Magalhães, Eridan Orlando Pereira Tramontina Florean, Maria Izabel Florindo Guedes
1Northeast Biotechnology Network, Graduate Program of Biotechnology, State University of Ceará, Itaperi campus, 60714-903 Fortaleza, CE, Brazil
2Department of Chemistry, State University of Ceará, Itaperi campus, 60714-903 Fortaleza, CE, Brazil
3Laboratory of Bioprospecting of Natural Products and Biotechnology, State University of Ceará, CECITEC, 6366000 Tauá, CE, Brazil
Keywords:Cnidoscolus quercifolius Pohl. methylfaveline Deoxofaveline Neofavelanone Faveline Cytotoxicity
ABSTRACT Objective: To evaluate the chemical components of active extract from Cnidoscolus quercifolius root bark and its cytotoxic potential against several tumor strains. Methods: The highperformance liquid chromatography with diode-array detection and 1H and 13C nuclear magnetic resonance spectroscopy of the extract were used to distinguish the existence of possible functional groups in the root bark extract. The in vitro cytotoxic activity of methanol extract on human colon cancer cell lines was evaluated using OVCAR-8, SF-295, HCT-116, HL-60 strains and the samples were assessed by 3-(4,5-dimethylthiazol2-yl)-2,5-diphenyltetrazolium bromide method. Results: The analysis of nuclear magnetic spectra of the active chloroform fraction revealed the presence of absorptions bands correspondent to a mixture of favelines such as neofavelanone, deoxofaveline or methyl-faveline, which structures were confirmed by ultraviolet spectra upon high-performance liquid chromatography with diode-array detection analysis. The active fraction showed cytotoxic effects in the tested strains, HCT-116, SF-295, OVCAR-8 and HL-60 cells with IC50 of 72 hours ranging from 4.95 to 15.23 µg/mL. Conclusions: The results suggest that the substances present in faveleira (Cnidoscolus quercifolius) root bark extract have a cytotoxic potential against several tumor lines, showing a broader antitumour potential, and in addition no adverse to healthy cells. Therefore, the root bark extract of Cnidoscolus quercifolius has a possibility of use for anticarcinogenic therapies.
1. Introduction
Cancer is one of the main sources of death globally and is present in all countries regardless of their socioeconomic status.Unfortunately, the number of cases of this disease accompanies the growth of populations. Increased control of risk factors (smoking,weight control, physical activity and changes in reproductive patterns) has reduced the incidence of some types of cancer.However, a large part of the world population still suffers from the repercussions and treatment of this disease[1]. Although some limitations in the treatment of cancer with chemotherapy, such as drug resistance, liability to reinfection, need for regular monitoring and damage to healthy cells, the extending of research involving traditional herbal medicines, in addition offering a possibility of a less toxic treatment, approximates the popular scientific knowledge[2].Several studies have demonstrated the potential of extracts present in Brazilian flora with antitumor effect[3]. According to WHO statistics,nearly 70%-80% of the global population all over the world use traditional treatment. Sixty-five new drugs confirmed for cancer treatment by FDA from 1981 to 2002 were segregated from regular sources of plants specially, 48 of which are derived from natural products[4,5].
In the semi-arid Northeastern Brazil region, the potential of several native species has long been known, which however is not properly explored. The benefits of the scientific exploration of these species have an influence not only on the treatment of diseases but also on the socioeconomic potential of the region[6].Cnidoscolus quercifolius(C. quercifolius) Pohl or favela orfaveleirais a plant that adapts very well to the climate type in semi-arid region, and provides excellent food source for livestock[7]. One of the characteristics of plants from Euphorbiaceae family, whichfaveleirabelongs to, is the existence of secondary metabolites, for example, alkaloids, flavonoids, tannins,terpenoids and coumarins. These compounds are notable for their biological and pharmacological activity against different diseases such as cancer and cardiovascular and gastrointestinal disorders. In this direction, the presence of alkaloids and terpenoids is common to the genusCnidoscolus[8]. Generally, the various known species from genusCnidoscolushave been accounted for their pharmacological activities such as antiseptic, antitumoral, to treat arthritis, liver and urinary disorders, and ulcers[9-11].
More recently, a review demonstrated the pharmacological and chemical features of the genusCnidoscolus, revealing new bioactive molecules in thefaveleiraseed oil as a bioactive product[12]. In our previous study, it was possible to investigate its antioxidant,antimicrobial and inhibitory activity of the acetylcholinesterase enzyme, as well as the cytotoxicity of the methanolic root bark extract ofC. quercifolius[13]. Recently, Liraet al.[14] evaluated the hypoglycemic potential and oral toxicity of the leaves of thefaveleirawith promising results in the treatment of diabetes. Other studies reported that flowers and stem of thefaveleirahave antioxidant,antimicrobial and therapeutic indications for liver problems, tumors and uterine inflammation[8]. Santoset al.[15] detected significant amounts of tocopherol, phytosterol, total phenolic content, as well as the antioxidant potential and its thermal and oxidative capacity offaveleriaseed oil.
From the point of view of previous studies, thefaveleriapresents an economic potential as a therapeutic hotspot for the pharmaceutical industry. Despite this importance, the presence of chemical constituents and biological activities ofC. quercifoliusaffirmed cytotoxic action against some tumor cell lines. The objective of present investigation was to determine the cytotoxic activity ofC. quercifolius, identifying molecules which can be used in the antitumoral treatment.
2. Materials and methods
2.1. Plant materials
The roots offaveleira(C. quercifoliusPohl) were collected in the city of Fortaleza (040° 18 ‘05,4 “W, 06° 01’ 03,6” S) and then transported in plastic bags to the Laboratory of Biotechnology and Molecular Biology of the State University of Ceará where they were washed, then the bark was removed and dryed in a oven at 60 ℃. The identification of the botanical material was deposited in the Herbarium Prisco Bezerra of the Federal University of Ceará, under registration number 56043.
2.2. Chemicals
All chemicals utilized as a part of this investigation were obtained from SigmaAldrich®and used without further purification. Silica gel 60 UV 254 (Macherey-Nagel), Silica gel 60 (70-230 mesh ASTM)from Merck, and silica octadecyl-functionalized (C18) obtained from Aldrich were utilized for the chromatographic fractions. Deuterated methanol and chloroform were attained from TEDIA (São Paulo,Brazil) and used to analyze solvent effect on the structural properties.
2.3. Preparation of extracts and fractions
The dried roots ofC. quercifoliuswere ground and macerated with methanol for 7 d. The crude methanol extract was filtered and dispersed to dryness under vacuum at approximately 40 ℃. The resulting material was washed with hexane (1 L), chloroform (1 L) and ethanol (1 L). After removing the solvents, the choroform extract was subjected to silica gel column chromatography and was eluted with hexane, chloroform, ethyl acetate, methanol and water in mixtures of accelerating polarity. The fractions were collected and compared by thin layer chromoatography, then the fractions which present blue spot under UV light were joined for chemical analysis. These three fractions were analyzed for cytotoxicity against various cell lines. This chloroform fraction was also chromatographed in silica gel column and several sub-fractions were analyzed under UV light (250 nm) and some of them displayed blue light spots. The1H and13C-NMR spectra of active products showed chracteristics of favelines, common compounds detected in this species in previous works[16,17]. The chemical investigation was done and isolated compounds that were analyzed by Nuclear magnetic resonance (NMR) spectra were recorded with Bruker Avance DRX-500 spectrometer, in CDCl3, and by UV spectroscopy of high performance liquid chromatography (HPLC) analysis. The chloroform fraction was named CEF (Chlororoform rich fraction of favelines from the root bark offaveleira)
2.4. Characterization of CEF through HPLC
The HPLC analysis was carried out on a Shimadzu liquid chromatograph [Kyoto, Japan equipped with on-line degasser(DGU-14A), LC-10AD VP pump and an autosampler (model ALS,DS11116519)]. The output signal was detected by Ultraviolet-Visible spectrophotometry detection system with spectral sweep of 190 to 600 nm, and incorporated using Excel 2010 (Microsoft,USA). The chromatographic separation was performed pre-column C18Polymer Aphera 5 µm (1 cm × 4.6 mm) and Hibar LiChrospher®Merck 5 µm column. The whole HPLC system was composed by Class-VP software. All chromatographic experiments were performed at room temperature. Firstly, the mobile phase consisted of acetonitrile and water, 0.5% of acetic acid with ratio of 25:75 (v/v) which was delivered at a 1 mL/ min, 20 µL of injection volume.The chromatographic peaks were then detected and wavelength was monitored at 280 nm, respectively. All solvents and solutions were filtered through filtration unit (Millipore, 0.45 µm pore size) and degassed before use to carried out HPLC analysis. The identification of compounds was accomplished by the correlation of retention times and UV spectra of isolated compounds with the standard.
2.5. Structural determination of isolated compounds
The CEF was subjected to spectroscopic analysis by nuclear magnetic resonance of hydrogen and carbon-13 for the determination of the chemical structure. The experiments were carried out in a Bruker Avance DRX-300 spectrometer running at 300 MHz (1H) and 75 MHz (13C), at room temperature, with the acquisition parameters(45° pulse width and 1.0 s relaxation delay).1H (300 MHz) and13C NMR (75 MHz) spectra were expressed in chemical shifts (ppm)using CDCl3(deuterochloroform) as solvent, and referenced to standard absorptions at δ 77.0.
2.6. Cytotoxic assay in human cancer cell lines and normal cells
The cytotoxicity was assayed against HL-60 (human leukemia),HCT-116 (human colon), SF-295 (human central nervous system)and OVCAR-8 (human ovarian) cancer cell lines as well as normal Vero cell lines (monkey kidney normal cells), attained from the National Cancer Institute, USA. Cells were sub-cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 1%antibiotics and incubated at 37 ℃ under a 5% CO2atmosphere.The cytotoxicity of the samples was evaluated by the 3-(4,5-dimethylthiazol2-yl)-2,5-diphenyltetrazolium bromide (MTT)technique. For experiments, cells were seeded in 96-well plates. The compounds (0.05-25 µg/mL) were dissolved in DMSO 5%, added to each well and incubated for 3 d (72 h). After 69 hours of incubation,the supernatant was substituted by recent medium comprising MTT(0.5 mg/mL)[18]. After three hours, the MTT formazan product was dissolved in 150 µL of DMSO, and absorbance was measured at 595 nm. Doxorubicin (0.009-5 µg/mL) was used as positive control.Control groups received the same amount of DMSO.
2.7. Statistical analysis
Data were presented as mean±SEM/SD or inhibition concentration of 50% (IC50) values and their 95% confidence intervals (CI 95%)obtained by nonlinear regression and analyzing concentrationdependent effects on differentiation and cell number. All statistical analyses were performed using GraphPad (Intuitive Software for Science, San Diego, CA, USA). All studies were carried out in duplicate and characterized by independent biological evaluations.
3. Results
The1H-NMR spectrum (Figure 1) of CEF showed the peaks corresponding to faveline: 3 peaks due to the Csp2 hydrogens of the aromatic ring at 7.61 ppm (H-1), 6.62 ppm (H-4) and the third at 6.16 ppm (H-3). In faveline the hydrogens attached to the other rings were H-8 (2H, 3.01 ppm, d,J= 9.6 Hz) next to carbonyl,H-12 (2H, 2.31 ppm, t,J= 9.35 Hz), as well as the methyls groups at C-16, C-17 and C-18 at 0.75, 1.2 and 2.2 respectively. In13C NMR spectrum (Figure 2) of favelines-rich fraction, the peak at 202.03 ppm corresponded to carbonyl at carbon 7 as well as the absorptions at 155.45 (C-3), 147.58 (C-10), 136.69 (C-5), 132.18(C-1), 129.89 (C-6), 125.26 (C-11), 122.56 (C-2) and 118.28 (C-4)of the aromatic ring carbons and the double bond between the C-10 and C-11 carbons complemented the faveline data as well as the other absorptions of the sp3 carbons as shown in Table 1. In the1H NMR spectrum of the active fraction offaveleira(CEF) in addition to the faveline signals, a multiplet at 3.9 ppm corresponded to the absorptions of faveline aromatic methoxyls such as those present in neofavelanone, deoxofaveline or even methyl faveline.13C NMR spectrum signals confirmed the presence of these compounds as shown in Table 2. The structure of favelines present in CEF was shown in Figure 3.

Figure 1. 1H NMR spectrum (CDCl3, 300 MHz) of faveline isolated from a chloroform fraction of root barks of faveleira.
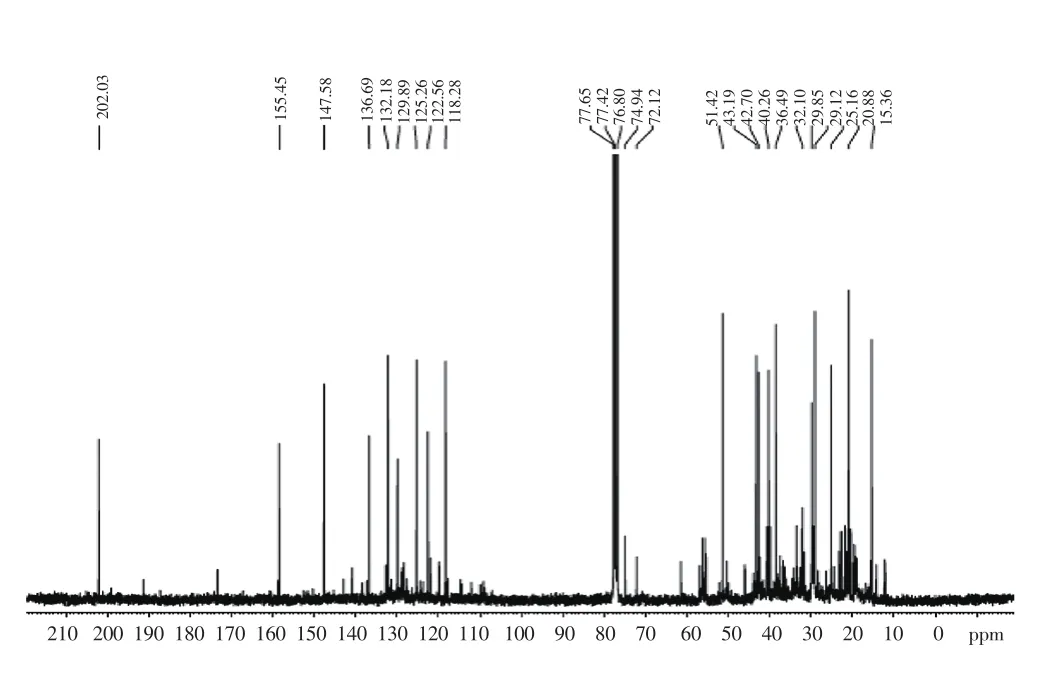
Figure 2. 13C NMR spectrum (CDCl3, 75 MHz) of faveline isolated from a chloroform fraction of root barks of faveleira.

Table 1 Spectral data of 13C and 1H NMR in CDCl3 of faveline in cytotoxic fraction of bark extract from C. phyllacanthus.

Table 2 13C NMR spectral data of methyl faveline, neofavelanone and deoxofaveline obtained in active fraction spectrum (solvent: CDCl3).

Figure 3. Structural representation of favelines present in the active fraction of faveleira.
With the proposition of structures for the compounds present in the active fraction CEF by NMR spectra analysis, the absorption bands in the ultraviolet spectra confirmed the presence of favelines by HPLC-DAD analysis as follows: The HPLC chromatogram of CEF(Figure 4) showed the presence mainly of four peaks with ultraviolet spectra in the range 204-340 nm and retention times of 6.75; 7.38;11.92 and 12.44 min, with different concentrations. The HPLC-DAD analysis showed the presence of four favelines, which displayed antitumour activities. The ultraviolet spectrum of favelines showed peaks with absorption maxima in 316, 308, 293, 284, 259, 246,231 and 209 (Figure 5). Compounds having absorption maxima at approximately 240, 280 and 310 nm were due to an α, β-unsaturated carbonyl system and a benzene ring with a ketone conjugated.
HPLC-DAD showed the UV absorption bands of each compound in the active mixture CEF (Table 3) which in comparison with previously reported data indicated the representative data of chemical structures proposed by NMR analysis. The cytotoxic activity of the extract obtained from the root bark ofC. quercifoliusPohl was tested against human cancer cell lines. In this work, the cytotoxic activity was assessed in various cell lines, demonstrating a broader antitumour potential for these substances. Among the five extracts tested, only the chloroform fraction ofC. quercifoliusPohl showed considerable cytotoxic activity at tested concentrations (data not shown). In this context, we found that the chloroform fraction showed a notable antitumoral activity at single concentration of 25 µg/mL in three different cell lines: colon carcinoma (HCT-116), ovarian carcinoma(OVCAR-8) and human glioblastoma (SF-295). Interestingly, our best results were for HCT-116 and OVCAR-8 cell lines, which indicated that this fraction could be a more promising tool to treatment of neoplasia conditions.

Figure 4. High efficiency liquid chromatogram of CHCl3 fraction from the root bark extract of faveleira.
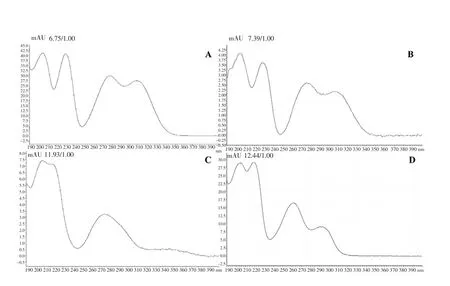
Figure 5. UV/VIS spectrum for each chromatogram peak (HPLC–DAD)from faveleira (A) Faveline, (B) Faveline methyl ether (C) Deoxofaveline,(D) Neofavelanone.
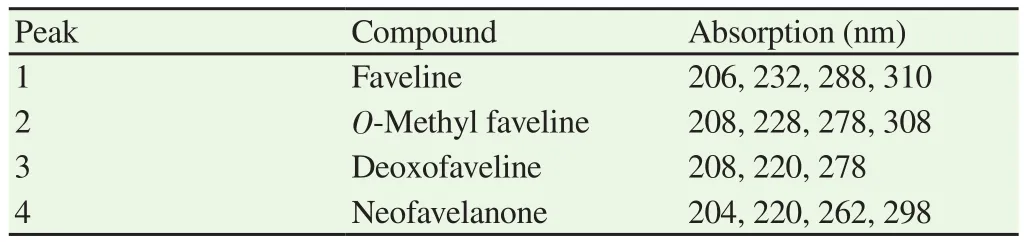
Table 3 Absorption in UV/VIS spectrum of compounds in active sample of faveleira.
The chloroform fraction of the extract showed high cytotoxicity with IC50values of OVCAR-8, HCT-116 and HL-60 cell lines and their respective positive controls (Dox): (15.23±2.0; 0.34±0.05),(7.07±0.59; 0.020±0.002) and (4.95±0.19; 0.010±0.001) µg/mL,respectively. Results of their growth inhibition percentages were demonstrated in Table 4. The extract showed a notable antitumoral activity with inhibition values ≥ 75% in two HCT-116 and OVCAR-8 cell lines, thus showing a high cytotoxic potential, which indicated the importance of fractionation of the extract.

Table 4 Growth inhibition percentage (GI%) for three tumor cell lines of extracts and fractions of C. quercifolius Pohl measured by MTT assay.
4. Discussion
According to studies of Paredeset al.[13], different plant tissues of theC. quercifoliusPohl, revealed the presence of the bioactive molecules, such as tannins, phenols, flavonoids, saponins, steroids and glycosides, besides favelines.Various studies on phytochemical composition resulted in the separation of benzocycloheptenes diterpenes fromC. quercifolius(faveleira). Endoet al.[17] and Lemoset al.[16], reported the occurrence of faveline, faveline methyl ether and deoxofaveline from the stem bark extract ofCnidoscolusphyllacanthus.Similarly, Ohtaet al.[18] carried out the phytochemical assessment of methanol extract of the dried bark extract ofCnidoscolus phyllacanthusand isolated the cytosidal compounds favelol, isofavelol and favelone[19]. Endoet al.[20] reported the presence of a novel tetracyclic cyclopropane derivative, favelanone (cyclopropane derivative), and a new tetracyclic cyclobutene derivative, neofavelanone (cyclobutene derivative), isolated from the stem bark of thefaveleria.
In the13C NMR spectrum, the lower intensity signals, besides the faveline peaks, were those correspondent to neofavelanone: two carbonyls at 199 (C-7) and 192 ppm (C-12) ppm, as well as sp2 carbons at 174 (C-9), 162 (C-13), 153 (C-5), 143 (C-10), 129 (C-6),128 (C-2) and 108.9 (C-4). Concerning the deoxofaveline ring (C ring) of both C-7 and C-9 are clearly absorbed with non-aromatic and aromatic ring at the values of 32.7 ppm and 54.9 ppm. The absorptions correspondent toO-methyl faveline are already reported by Endoet al.[17].
By analyzing the nuclear magnetic resonance spectral data, the active sample contains a major proportion of faveline (1) and others are present in a much smaller proportion such as the faveline methyl ether (2), neofavelanone (3), and favelol (4) already reported in the plant[17,20].
Liraet al.[14] studied the toxicity and hypoglycemic effect of aqueous extracts ofC. quercifoliusPohl leaves in diabetic mice. It is well documented fact that the presence of these compounds is related with various medicinal properties[21], encompassing the ability to reducing inflammation, protecting the body against cancer, and producing hypoglycemic effects due to its polyphenols, especially anthocyanins and anthocyanidins[22,23].
A few chemotherapeutic agents are being utilized for treatment of cancer, however the issue of specific toxicity and serious side effects still persists. The principal objective of chemotherapy is to explicitly target cancer cells without causing any cytotoxic effects towards normal cells. Subsequently, the fraction separated from thefaveleirawas tested for assessment ofin vitrocytotoxicity in non-tumoral Vero cells, no cytotoxic effect was observed up to the concentration of 2.0 mg/mL (data not shown). Compiling the cytotoxic data, it is suggested that the extract is more toxic to cancer cell lines than normal cell lines.
Among the medical properties attributed to plants and varieties encountered in tropical countries, including in Brazil, there will be countless exploration for the target for distinguishing productive activities against cancer, from which a few investigations are being made to discover novel effective substances for anti-cancer action[24].Plants of the Euphorbiaceae family are normally utilized in folk medicine in the treatment of inflammatory and infectious diseases.Moreover, Kanunfre and co-workers showed the antineoplastic agents from Euphorbiaceae family[25].
A second protocol was conducted to determine the concentrations that induced IC50of the chloroform fraction. The fraction was tested in the two aforementioned more promising cell lines, OVCAR-8 and HCT-116; we also included a lineage of promyelocytic leukemia HL-60, using the MTT assay[26]. In the preclinical cytotoxic drug screening program used in this work, which is based in the United States National Cancer Institute program, extracts/oils with available IC50values below 30 µg/mL are considered to be capable for anticancer drug improvement[27,28]. Therefore, the chloroform fraction ofC. quercifoliusPohl demonstrated a promising cytotoxic activity based on our tested results.
The results indicate that the chloroform fraction exhibited a good cytotoxic activity against the three cancer cell lines, although the best cytotoxic effect was against HL-60 cell line with an IC50value almost three times lower than that in the OVCAR-8 cell line.Comparing the IC50values, chloroform fraction was approximately one hundred and thirty times more toxic to the cancer cell lines compared to normal cells (Vero).
Paulaet al.[29] demonstrated that the hexane extract fromCnidoscolus phyllacanthusstem bark showed cytotoxic activity against human tumor lines, and that the isolated compound deoxofaveline was selective for leukemia cells (HL-60, IC50: 1.6µg/mL). Similarly, in our results, the chloroform fraction showed a significant effect on the HL-60 lineage, as demonstrated by the low concentration required to cause 50% of cell death (4.95 µg/mL). The results of these tests corroborated the results obtained previously by Endoet al.[17] who reported the cytotoxic activity of deoxofaveline and methyl favelin in murine leukemia cell lines (P-388). Corroborating our results, the previous study has demonstrated that terpenoids inEuphorbia kansui, a Euphorbiacea possess anti-tumor activity in five human cancer cell lines[30]. This finding suggests a possible effect of this extract fraction in drug resistance mechanisms, such as drugtransporting proteins. However, it needs to be confirmed in future studies.
The present study revealed the chemical characterization of the root bark extract of theC. quercifolius. The following majority substances were identified in the chloroform fraction: faveline,O-methyl faveline, deoxofaveline and neofavelanone. In addition,this fraction showed promising inhibitory activity of tumoral cell growth, particularly in tumor lineages. Therefore, our results suggest the potential application of this extract fraction and its components for the development of new anti-cancer drugs. Despite this, further investigation is required to verify the mechanism of action of this extract and its components, as well as its possible deleterious effects.
Conflict of interest statement
The authors declare there is no conflict of interest.
Acknowledgements
The authors wish to thank the CENAUREMN (Northeastern Center for the Application and Use of Nuclear Magnetic Resonance) of the Federal University of Ceará for the NMR spectra of the compounds.
 Asian Pacific Journal of Tropical Biomedicine2018年7期
Asian Pacific Journal of Tropical Biomedicine2018年7期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Role of toll like-receptor 2 in inflammatory activity of macrophage infected with a recombinant BCG expressing the C-terminus of merozoite surface protein-1 of Plasmodium falciparum
- Moderating effect of synthesized docosahexaenoic acid-enriched phosphatidylcholine on production of Th1 and Th2 cytokine in lipopolysaccharide-induced inflammation
- Anti-epileptic effect of morin against experimental pentylenetetrazol-induced seizures via modulating brain monoamines and oxidative stress
- Expression of fluorescent tagged recombinant erythroferrone protein
- Identification of a toxin coding fragment in pBSSB1, a linear plasmid from Salmonella enterica serovar Typhi that can stabilize a multicopy plasmid
- Influence of different cultivars of Phoenix dactylifera L-date fruits on blood clotting and wound healing
