Gualou Guizhi decoction promotes neurological functional recovery and neurogenesis following focal cerebral ischemia/reperfusion
Jing Han, Ji-Zhou Zhang, Zhi-Feng Zhong, Zuan-Fang Li, Wen-Sheng Pang, , Juan Hu, , , Li-Dian Chen,
1 Institute of Materia Medica, Fujian Academy of Traditional Chinese Medicine, Fuzhou, Fujian Province, China
2 Fujian University of Traditional Chinese Medicine, Fuzhou, Fujian Province, China
3 The Second People’s Hospital of Fujian Province, Fuzhou, Fujian Province, China
Abstract Recovery following stroke involves neurogenesis and axonal remodeling within the ischemic brain. Gualou Guizhi decoction (GLGZD) is a Chinese traditional medicine used for the treatment of post‐stroke limb spasm. GLGZD has been reported to have neuroprotective effects in cerebral ischemic injury. However, the effects of GLGZD on neurogenesis and axonal remodeling following cerebral ischemia remain unknown. In this study, a rat model of focal cerebral ischemia/reperfusion was established by middle cerebral artery occlusion. Neurologi‐cal function was assessed immediately after reperfusion using Longa’s 5‐point scoring system. The rats were randomly divided into vehicle and GLGZD groups. Rats in the sham group were given sham operation. The rats in the GLGZD group were intragastrically administered GLGZD, once daily, for 14 consecutive days. The rats in the vehicle and sham groups were intragastrically administered distilled water.Modi fied neurological severity score test, balance beam test and foot fault test were used to assess motor functional changes. Nissl staining was performed to evaluate histopathological changes in the brain. Immuno fluorescence staining was used to examine cell proliferation using the marker 5‐bromo‐2′‐deoxyuridine (BrdU) as well as expression of the neural precursor marker doublecortin (DCX), the astrocyte marker glial fibrillary acidic protein (GFAP) and the axon regeneration marker growth associated protein‐43 (GAP‐43). GLGZD substan‐tially mitigated pathological injury, increased the number of BrdU, DCX and GFAP‐immunoreactive cells in the subventricular zone of the ischemic hemisphere, increased GAP‐43 expression in the cortical peri‐infarct region, and improved motor function. These findings suggest that GLGZD promotes neurological functional recovery by increasing cell proliferation, enhancing axonal regeneration, and in‐creasing the numbers of neuronal precursors and astrocytes in the peri‐infarct area.
Key Words: nerve regeneration; Gualou Guizhi decoction; cell proliferation; neurogenesis; neuroblast; astrocyte; axon remodeling; ischemic stroke;Chinese medicine compound; neural regeneration
Introduction
Ischemic stroke results from the occlusion of a cerebral artery, leading to a reduction in cerebral blood flow, tran‐siently or permanently (Dirnagl et al., 1999). It is the leading cause of death and disability in adults in China and the sec‐ond most common cause of death and disability worldwide(Haque and Nasreen, 2008; Sun et al., 2013). Ischemic stroke causes severe brain damage and induces neurological dys‐functions, including hemiplegic paralysis, limb spasm and vascular cognitive impairment, which lower the quality of life of affected patients. Because of a lack of effective treat‐ments, the disability rate resulting from stroke remains high.As a consequence, the socioeconomic burden imparted by the illness continues to rise (Schmidt and Minnerup, 2016).
Spastic limb paralysis is one of the main symptoms of motor impairment in post‐stroke patients, appearing during rehabilitation therapy after stroke. Because of the lack of effective therapies, spasticity remains an important clinical problem. Limb paralysis is the outcome of stroke‐induced neuronal cell death, and therefore, the ideal therapeutic strategy is to prevent neuronal cell death to improve neural recovery. Post‐stroke neural recovery begins at the subacute stage of stroke (Yenari and Han, 2012), and involves neuro‐genesis, axonal remodeling and angiogenesis. Neurogenesis occurs following stroke or brain trauma. Stroke induces a signi ficant increase in cell proliferation in the subventricular zone (SVZ) of the lateral ventricle in the ischemic cerebral hemisphere, and the newborn cells differentiate into many types of neural cells (Arvidsson et al., 2002). Axonal re‐modeling is also induced by cerebral ischemia (O’Donovan,2016). However, the intrinsic neuroregenerative and axonal remodeling abilities are generally not sufficient to improve functional recovery (Rueger and Schroeter, 2015). Thus, new therapies are needed to enhance endogenous neurogenesis and axonal remodeling in the ischemic brain.
A number of pharmacological agents have been used to improve neural repair and ameliorate motor recovery; how‐ever, many have a variety of side effects (Chen et al., 2014a;Chen et al., 2014b).Gualou Guizhidecoction (GLGZD),a traditional Chinese medicine, was first documented inJinkui Yaolue, an ancient Chinese medical treatise written by Zhongjing Zhang during the Han Dynasty (216 A.D.). It has been used to treat post‐stroke limb spasm with favorable therapeutic efficacy and few side effects. In clinical trials,GLGZD promotes post‐stroke recovery by ameliorating limb muscle tension and improving locomotive function,thereby enhancing the quality of life of patients with stroke(Chen et al., 2014b). Recently, a number of studies have demonstrated the neuroprotective effects of GLGZD on ce‐rebral ischemia‐induced injuryin vivoandin vitro(Zhang et al., 2014). However, the effects of GLGZD on endogenous neurogenesis and axonal remodeling following cerebral ischemia remain unknown.
In the present study, we investigate the effects and mech‐anisms of action of GLGZD on the long‐term improvement of functional outcomes in a rat model of focal cerebral isch‐emia/reperfusion (I/R) injury.
Materials and Methods
Animals
A total of 24 male Sprague Dawley rats (8—10‐week‐old),weighing 280 to 350 g, were used in this study (SCXK (Hu)2015‐0002, SLAC Laboratory Animal Co., Ltd., Shanghai,China). The rats were housed under a 12/12‐hour light/dark cycle with free access to standard diet and water. The study was conducted in compliance with the international laws on animal experimentation and approved by the Committee of Ethics of Fujian Academy of Traditional Chinese Medicine,China (approval No. FJATCM‐IAEC2016020).
Transient middle cerebral artery occlusion (MCAO)surgery and animal group assignment
Focal cerebral I/R was induced as described in our previous study (Han et al., 2015). The rats were anesthetized with chloral hydrate (10% w/v). After isolating the right common carotid artery (CCA), the external carotid artery (ECA) and the internal carotid artery (ICA), the ECA and its branch‐es were cauterized. A nylon suture with a silicone‐coated tip (0.38 mm diameter for rats of 280—350 g) (Guangzhou Jialing Biotechnology Co., Ltd., Guangzhou, China) was in‐serted into the ICA through the ECA stump, approximately 17—18 mm distal to the carotid bifurcation, to occlude the middle cerebral artery (MCA). At this point, a mild resis‐tance was felt. After 120 minutes, the suture was removed from the ICA to allow MCA reperfusion. The distal ICA was then immediately coagulated. The body temperature was maintained between 35.5 and 36.0°C using a heat pad.
To verify the success of the MCAO model, neurological behavior was assessed immediately after reperfusion using the 5‐point scoring system developed by Longa et al. (1989)as follows: 0, no de ficits; 1, difficulty in fully extending the contralateral forelimb; 2, difficulty in walking in line; 3,circling to the contralateral side; and 4, falling to the contra‐lateral side. Rats scoring 1—3 were included in the following tests. All the selected rats were randomly divided into two groups according to their scores: the vehicle group (n= 8)and the GLGZD group (GLGZD treatment,n= 8). Another group of rats subjected to all surgical procedures, but with‐out suture insertion, was used as the sham group (sham op‐eration,n= 8).
Drug administration and 5-bromo-2′-deoxyuridine(BrdU) labeling
The GLGZD formula, originally described inJinkui Yaolue,consists ofRadix Trichosanthis, Ramulus Cinnamomi, Radix Paeoniae Alba, Radix Glycyrrhizae, Rhizoma Zingiberis RecensandFructus Ziziphi Jujubae, with the respective propor‐tion of each drug as follows: 30:9:15:6:9:30. The dosage used was proven to be effective in the treatment of post‐stroke spasm in a clinical trial (Chen et al., 2014b). The herbal de‐coction ingredients were purchased from Guoyitang Drug store (Fuzhou, China), and each herbal component was identi fied by Professor Wensheng Pang (Fujian University of Traditional Chinese Medicine, Fuzhou, China) using the methods in the Chinese Pharmacopoeia, 2015 edition (Chi‐nese Pharmacopeia Commission, 2015). The effective form of GLGZD used in clinical practice is its water extract. Thus,the water extract of GLGZD was prepared by decocting the herbs twice through boiling in distilled water for 1 hour each, then combining the decocted extracts together and concentrating to obtain a final concentration of 0.78 g/mL.The dosage of intragastrically administered medicine was 7.8 g/kg, based on that used clinically for humans (Chen et al., 2014b), and was calculated using the body surface area of humans and rats.
The rats in the GLGZD group were intragastrically ad‐ministered GLGZD after the grouping of rats following the surgery, once daily for 14 consecutive days. The rats in the vehicle and sham groups were intragastrically administered vehicle (distilled water, 10 mL/kg) at the same time. BrdU(50 mg/kg; Sigma‐Aldrich, Shanghai, China) was intraperi‐toneally injected twice daily in all groups on day 7 and day 8 after cerebral I/R to label dividing cells.
Neurological behavior assessment
The rats were neurologically assessed 1, 3, 7 and 14 days after reperfusion by investigators who were blinded to the experimental groupings. The data were collected 1 hour after GLGZD administration on each assessment day. The assess‐ments included the modi fied neurological severity score, the balance beam test and the foot fault test.
Modi fied neurological severity score
The neurological severity score is used to behaviorally assess the animal’s motor, sensory, balance and reflex functions.The modified scoring system was based on that developed by Chen et al. (2001), and graded on a scale of 0 to 14 (normal score, 0; maximal de ficit score, 14) (Mora‐Lee et al., 2011).In the scoring system, the inability to perform the task or the lack of a tested re flex is awarded 1 point. Thus, a more severe injury is indicated by a higher score.
Balance beam test
To perform the balance beam test, rats were placed on a bal‐ance beam, 1.5 cm wide and 80 cm long, which was placed 15 cm above a soft platform (Shohami et al., 1995). Behav‐ioral performance was scored using a 6‐point scoring system developed by Chen et al. (2001), as follows: 0, balances with steady posture; 1, grasps side of beam; 2, hugs the beam and one limb falls down from the beam; 3, hugs the beam and two limbs fall down from the beam, or spins on beam (> 60 seconds); 4, attempts to balance on the beam but falls off(> 40 seconds); 5, attempts to balance on the beam but falls off (> 20 seconds); 6, falls off, with no attempt to balance or hang on to the beam (< 20 seconds). A higher score indi‐cates a more severe injury.
Foot fault test
The foot fault test was used to evaluate forelimb motor coor‐dination. The rats were placed on an elevated metal square grid (45 × 30 cm2, with each grid cell 2.5 × 2.5 cm2; 8 cm above the ground) (Shehadah et al., 2014). Under normal conditions, rats tend to place their paws on the wire frame when moving on the grid. The rats given MCAO surgery might place their paw inaccurately, the front limb falling onto one of the grid cells. Each time the paw falls down or slips through the wires, a foot fault is recorded. For each animal, the total number of steps on the grid (movements of each forelimb) in a 3‐minute period was recorded, as well as the total number of foot faults for the left paw. The foot fault score was calculated as the ratio (percentage) of foot faults over the total number of steps.
Immuno fluorescence staining
BrdU is a thymidine analog that is incorporated into DNA during cell division, and is therefore often used to assess cell proliferation following cerebral ischemia (Zhang et al.,2015a). Doublecortin (DCX) is a microtubule‐associated protein expressed mainly in neuronal precursors in adult rats, and is used as a biomarker of neuroblasts (Dennie et al., 2016). Glial fibrillary acidic protein (GFAP) is an inter‐mediate filament protein that is primarily expressed in as‐trocytes (Brenner, 2014), and is used as a marker of mature astrocytes. NeuN is a neuronal nuclear antigen that is com‐monly used as a biomarker of neurons (Mullen et al., 1992).Growth associated protein‐43 (GAP‐43) is an important molecule involved in neuronal pathfinding and branching during development and axonal regeneration, and is consid‐ered an indicator of axonal remodeling (Grasselli and Strata,2013).
Fourteen days after reperfusion, the rats were deeply anesthetized with chloral hydrate (10% w/v) and perfused with 500 mL 0.9% physiological saline followed by 350 mL 4% paraformaldehyde solution from the left ventricle until the limbs were stiff. The rats were decapitated, and the brains were removed and fixed in 4% paraformaldehyde, and dehy‐drated in a sucrose gradient until submerged at the bottom of the vessel. Then, 30‐μm frozen coronal sections containing the cortex, SVZ and striatum (bregma −2.0 to −1.0 mm; Pax‐inos and Watson, 2005) were cut using a freezing microtome,and then preserved in cryoprotective solution at −20°C.
Immuno fluorescence staining was performed on floating sections. For BrdU staining, the slices were incubated in 2×saline sodium citrate solution containing formamide for 2 hours at 65°C. After rinsing in 2× saline sodium citrate solution, the sections were incubated in 2 N hydrochloric acid for 30 minutes at 37°C and rinsed in 0.1 M boric acid solution (pH 8.5) twice. After washing in 0.01 M phos‐phate‐buffered saline (PBS), the sections were incubated in blocking reagent (0.01 M PBS with 5% bovine serum albumin and 0.5% Triton X‐100) (Beyotime Ins. Biotech‐nology, Haimen, China) for 1 hour at room temperature to inhibit nonspeci fic binding. Subsequently, the sections were double‐labeled with an anti‐BrdU antibody and an antibody towards a cell type‐specific marker. The following primary antibodies were used: rat monoclonal anti‐BrdU (1:200),rabbit polyclonal anti‐DCX (1:1000), rabbit polyclonal an‐ti‐GFAP (1:2000), rabbit polyclonal anti‐NeuN (1:2000) and rabbit monoclonal anti‐GAP‐43 (1:500) (all from Abcam,New Territories, Hong Kong, China). All primary antibodies were diluted in 0.01 M PBS containing the blocking reagent.The brain sections were incubated in the diluted antibod‐ies at 4°C overnight. Afterwards, the sections were washed with PBS and incubated in secondary antibodies, including the Cy3‐conjugated goat anti‐rat antibody, the Alexa Fluor 488‐conjugated goat anti‐rabbit antibody and the Cy3‐con‐jugated goat anti‐rabbit antibody (all 1:200; Beyotime Ins.Biotechnology), for 1 hour at 37°C in a humidi fied chamber.Then, sections were mounted onto slides, and images were captured on a confocal laser scanning microscope (Zeiss LSM710, Oberkochen, Germany). Two non‐adjacent sec‐tions were selected for each rat, and five non‐overlapping visual fields were selected for each section for assessment.
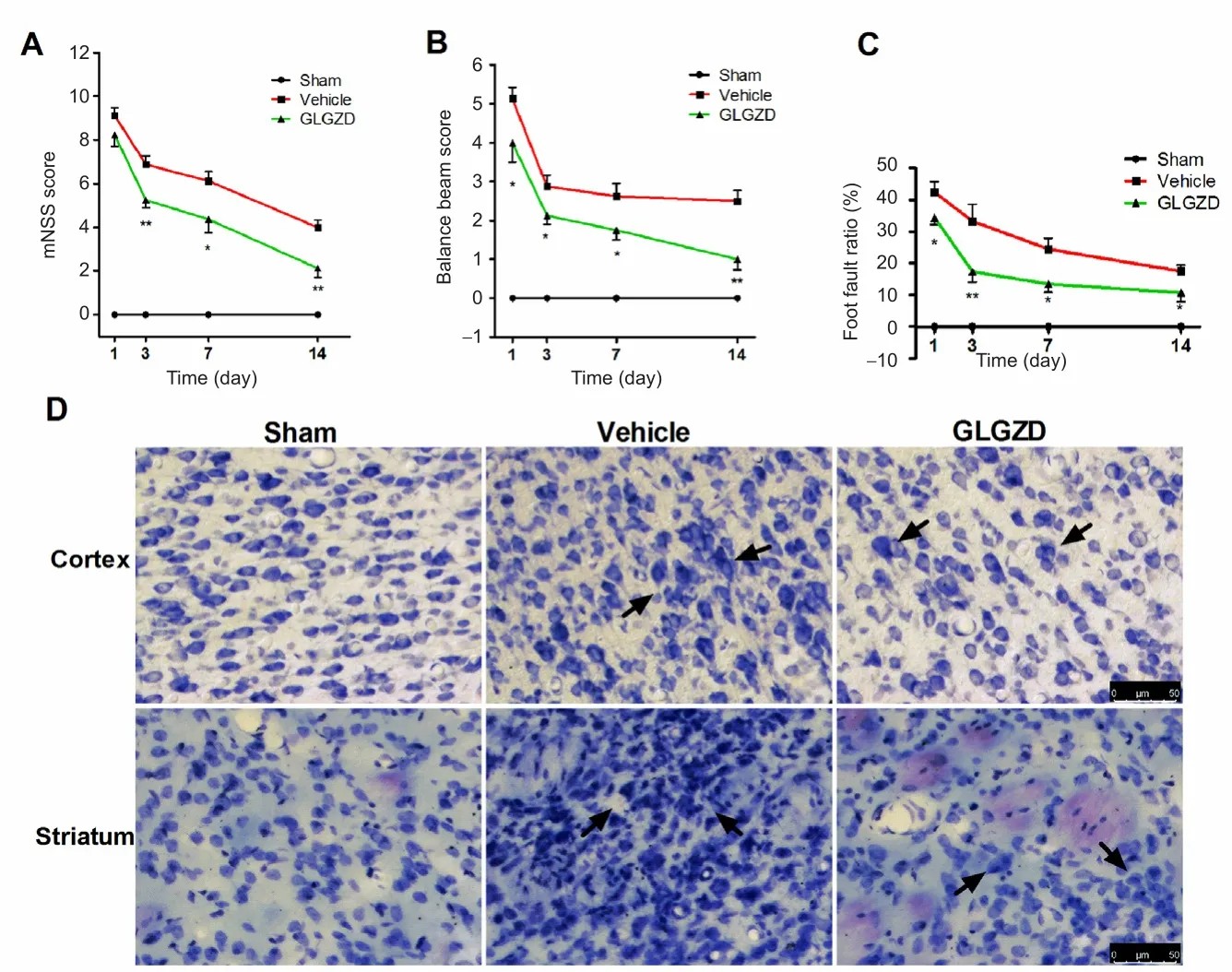
Figure 1 GLGZD attenuated histological change and improved neurological function after middle cerebral artery occlusion/reperfusion.
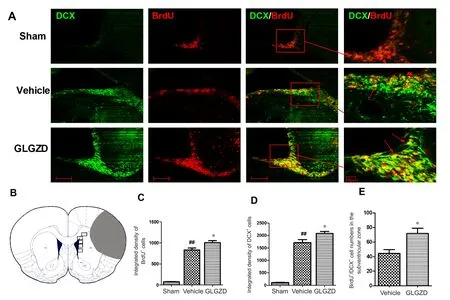
Figure 2 Immuno fluorescence staining for DCX and BrdU in the subventricular zone of rats given GLGZD treatment at 14 days after middle cerebral artery occlusion/reperfusion.
The immunofluorescence intensities of BrdU and DCX were determined using the integrated optical density of the whole photo with Image‐J 1.51K software (Wayne Rasband,National Institutes of Health, Bethesda, MD, USA). The im‐muno fluorescence intensity for each animal was calculated from the average integrated optical density of all the ac‐quired photos for that animal. BrdU+/DCX+, BrdU+/GFAP+and GAP‐43+cells were quanti fied by observers blinded to the experimental groupings. The numbers for each animal were calculated by summing the values of all the acquired photos for that animal.
Nissl staining
To evaluate histological changes, Nissl staining was carried out 14 days after MCAO on the 30‐μm frozen sections. Sec‐tions were immersed into 70% ethanol for at least 4 hours and then washed in distilled water for several minutes. Then,the sections were incubated in cresyl violet solution (Beyotime Ins. Biotechnology) for 5 minutes, and then dehydrated in 100% ethanol for 1 minute. The images were captured using a fluorescence microscope (Leica DMi8, Wetzlar, Germany).
Statistical analysis
Data are presented as the mean ± SEM, and analyses were performed with SPSS 13.0 software (SPSS Inc., Chicago, IL,USA). The measurement data were first analyzed for normal distribution. Comparisons among multiple groups were per‐formed with one‐way analysis of variance, followed by the least signi ficant difference t‐test. Comparisons between two groups were conducted using Student’st‐test.P‐values less than 0.05 were considered statistically signi ficant.
Results
GLGZD improved neurological function after I/R
The neurological assessment immediately after reperfusion showed that functional scores were not signi ficantly different between the vehicle and GLGZD groups (P> 0.05). To eval‐uate the effects of GLGZD on the long‐term improvement of functional outcomes following cerebral I/R, neurological functions were assessed 1, 3, 7 and 14 days after treatment.Rats in the sham group did not show any functional de ficits as assessed with the modi fied neurological severity score, the balance beam test and the foot fault test. I/R injury robustly increased the modified neurological severity score and the balance beam score in the vehicle group. GLGZD treatment significantly decreased the modified neurological severity score and the balance beam score after I/R (P< 0.05 andP< 0.01 at different time points; Figure 1A, B). Forelimb locomotor coordination was evaluated with the foot fault test (Shehadah et al., 2014). The rats in the GLGZD group showed a lower percentage of foot faults compared with the vehicle group at each time point after I/R (P< 0.05 andP<0.01 at the different time points; Figure 1C).
GLGZD attenuated histological changes after I/R
The effect of GLGZD on histological changes in the isch‐emic penumbra of the cortex and striatum 14 days after I/R is shown in Figure 1D. Nissl staining showed that neural cells in the sham group were regularly distributed, with a normal shape and structure. In the vehicle group, cerebral I/R caused severe histopathological changes; the normal ar‐rangement was perturbed, and there was a large number of swollen cells. Normal cell morphology was not detectable. In the GLGZD group, the morphology of the cells in the isch‐emic penumbra was better, with many normal‐shaped cells,compared with the vehicle group.
GLGZD promoted neurogenesis after I/R
The effect of GLGZD on cell proliferation 14 days after I/R was investigated using immuno fluorescence staining. BrdU+cells were mainly seen in the SVZ of the ischemic cerebral hemisphere. Some of the BrdU+cells were also observed in the ischemic striatum. There were no obvious changes in the non‐ischemic hemisphere. The intensity of BrdU immuno‐reactivity was higher in the ipsilateral ischemic hemisphere in the vehicle group compared with the sham group (P<0.01). Treatment with GLGZD further enhanced BrdU im‐munoreactivity (P< 0.05; Figure 2A, C).
To identify the proliferating cell types, we performed confocal double immunofluorescence with BrdU/DCX,BrdU/GFAP and BrdU/NeuN. In the sham group, only a few DCX+cells were found scattered in the SVZ. Cerebral I/R enhanced DCX immunoreactivity in the SVZ in the ischemic hemisphere in the vehicle group (P< 0.01). GL‐GZD treatment further augmented the intensity of DCX immunoreactivity (P < 0.05; Figure 2A, D). Notably, more BrdU+/DCX+cells were observed in the SVZ in the GLGZD group compared with the vehicle group (P< 0.05; Figure 2A, E).
GFAP is a marker of astrocytes (Brenner, 2014). Cerebral I/R robustly induced the expression of GFAP (Figure 3A).The GFAP+cells were mainly distributed in the striatum near the ischemic core (Figure 3A, B). Only a few BrdU+/GFAP+cells were observed in the ischemic striatum, with a greater number of BrdU+/GFAP+cells in GLGZD‐treated rats compared with vehicle‐treated rats (P< 0.05; Figure 3C). NeuN, a neuronal marker (Mullen et al., 1992) was examined for co‐localization with BrdU. Figure 4 shows that NeuN was not co‐localized with BrdU in the SVZ or the ischemic striatum.
GLGZD increased GAP-43 expression after I/R
GAP‐43 is a marker of axonal growth (Grasselli and Strata,2013). Immunohistochemical staining was used to examine the effect of GLGZD on GAP‐43 expression. GAP‐43 im‐munoreactivity was distributed in the cytoplasm of neurons in the cortex (Figure 5A). GAP‐43 immunoreactivity in the cortex of the ischemic hemisphere was signi ficantly greater in the vehicle‐treated rats (P< 0.01). GAP‐43+cells were increased in the GLGZD group compared with the vehicle group (P< 0.05; Figure 5C).
Discussion
In this study, we investigated the neuroprotective action of GLGZD and its effect on neurogenesis and axonal remod‐eling after stroke in the focal cerebral I/R model. GLGZD treatment improved motor functions, including limb coor‐dination and balance, following cerebral I/R. Histological observation revealed that GLGZD decreased neural damage.In addition, GLGZD increased the number of BrdU+(pro‐liferating) cells in the SVZ of the ischemic hemisphere and promoted neurogenesis (BrdU+/DCX+cells) and astrocyto‐sis (BrdU+/GFAP+cells). Furthermore, GLGZD increased GAP‐43 expression in the cortical peri‐infarct region. Some recent studies have demonstrated the neuroprotective effect of GLGZD in the focal cerebral ischemia model. GLGZD was shown to reduce infarct volume and alleviate neurologi‐cal de ficits in a rat model of cerebral I/R injury (Huang et al.,2013; Zhu et al., 2015), consistent with our current findings.During subacute and chronic stages of ischemia, it is more important to promote neural regeneration and axonal re‐modeling to improve the recovery of neurological functions(Fisher et al., 2009). Studies have shown that the adult brain has the capacity to regenerate because of the presence of neural stem/progenitor cells. These cells are mainly localized in germinal niches in the SVZ of the lateral ventricle and the subgranular zone of the hippocampus, and are able to self‐renew and differentiate into cell types that are function‐ally and tissue‐specific (Sharp et al., 2002). Under normal conditions, neuroblasts are produced by neural stem cells in the SVZ, from where they migrate through the rostral mi‐gratory stream to the olfactory bulb. There, they differentiate into mature neuronal cell types (Schmidt and Minnerup,2016). Following cerebral ischemia, neurogenesis increases in the ischemic ipsilateral SVZ, and the newly produced neuroblasts migrate from the SVZ to the ischemic penum‐bra, where they differentiate into mature neurons (Arvidsson et al., 2002). However, the neurogenesis induced by the isch‐emic injury is insufficient for repair of the damaged neural tissue.
We found that most BrdU+cells were distributed in the SVZ following cerebral ischemia, and some were also detect‐able in the ischemic striatum. The BrdU+cells in the isch‐emic striatum might be neuroblasts having migrated from the SVZ, or alternatively, they might be activated resident progenitor cells. GLGZD treatment increased BrdU immu‐noreactivity, indicating that it increases cell proliferation after cerebral ischemic injury.
Our current findings and many other studies (Wang et al.,2014, 2017) show that following cerebral ischemic injury,most BrdU+cells in the ipsilateral SVZ are positive for DCX,indicating that the proliferating cells are mainly neuroblasts.GLGZD treatment increased BrdU+/DCX+cells in the isch‐emic hemisphere, indicating that GLGZD promotes the proliferation of neuronal precursors after cerebral ischemic injury. Astrocytes play a major role in the inflammatory response in the central nervous system (Brenner, 2014).The astrocytes were activated in response to the cerebral ischemia. The astrocytes proximal to the ischemic periphery were enlarged, and branching increased. Correspondingly,GFAP was robustly expressed in the ischemic periphery.However, we did not find a major effect of GLGZD on cerebral ischemia‐induced GFAP expression in our exper‐iment. Zhang et al. (2001) reported that only 6% of BrdU+cells expressed GFAP in the cortex and subcortex, and that no BrdU+cells expressed neuronal markers after cerebral ischemia. Here, we found that a small number of BrdU+cells expressed GFAP, but no BrdU+/NeuN+cells were found in the ischemic periphery. GLGZD increased the number of BrdU+/GFAP+cells, indicating that GLGZD increases the number of neuroglia in the ischemic striatum. It has been reported that cerebral ischemia induces gliosis. Mature astrocytes and oligodendrocytes retain the ability to prolif‐erate, and are able to react to cerebral injury (Barakat and Redzic, 2016). Therefore, the increase in BrdU+/GFAP+cells might re flect the proliferation of mature glial cells or their differentiation from progenitor cells in the brain. Further research is needed to clarify the source of the proliferating astrocytes and neuroblasts and how they integrate into the existing neural networks.
GAP‐43 is a synaptic phosphoprotein that is mainly ex‐pressed in the growth cone of axons (Benowitz and Rout‐tenberg, 1997; Hou and Kang, 2016). GAP‐43 plays a key role in development and axonal remodeling in the adult brain (Denny, 2006). The expression of GAP‐43 increases during neuronal rewiring, such as following traumatic brain or spinal cord injury or stroke. The increased expression of GAP‐43 after cerebral ischemia is considered evidence of axonal regrowth and remodeling (Stokowska et al., 2017).In a recent study, GLGZD upregulated GAP‐43 expression in anin vitromodel of oxygen‐glucose deprivation‐induced injury in organotypic cortical slice culture (Nan et al., 2017).Here, we found that GLGZD increases GAP‐43 expression in our model of cerebral ischemia, suggesting that GLGZD promotes axonal regrowth and remodelingin vivo.

Figure 3 Immuno fluorescence staining for GFAP and BrdU in the striatum of rats given GLGZD treatment at 14 days after middle cerebral artery occlusion/reperfusion.

Figure 4 Immuno fluorescence staining for NeuN and BrdU in the subventricular zone of rats given GLGZD treatment at 14 days after middle cerebral artery occlusion/reperfusion.
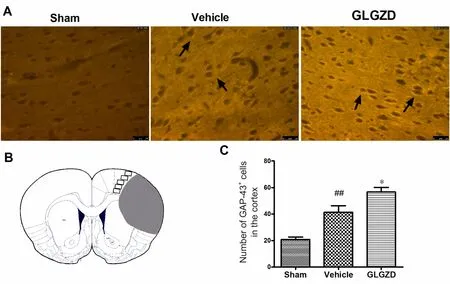
Figure 5 Immuno fluorescence staining for GAP-43 in the cortex of rats given GLGZD treatment at 14 days after middle cerebral artery occlusion/reperfusion.
GLGZD is composed ofRadix Trichosanthis, Ramulus Cinnamomi, Radix Paeoniae Alba, Radix Glycyrrhizae, Rhizoma Zingiberis RecensandFructus Ziziphi Jujubae. Most of these component herbs have antioxidant, anti‐in flammatory or anti‐apoptotic effects.Radix Trichosanthissaponins, the main component ofRadix Trichosanthis, have been reported to exhibit neuroprotective effects in a subarachnoid hemor‐rhage model because of their strong antioxidant action (Chen et al., 2015). The major component and active ingredient in Ramulus Cinnamomi is cinnamaldehyde, which inhib‐its cerebral ischemic injuryin vitroandin vivothrough its anti‐in flammatory effect (Zhao et al., 2015; Lv et al., 2017).Paeoni florin is the main active ingredient in Radix Paeoni‐ae Alba, which has neuroprotective effects against cerebral ischemic injury (Zhang et al., 2015b; Zhang et al., 2017). The main component ofRadix Glycyrrhizae, glycyrrhizic acid, is a direct HMGB1 inhibitor that attenuates cerebral ischemic injury (Gong et al., 2014; Akman et al., 2015; Xiong et al.,2016). Previous studies have demonstrated the antioxida‐tive and anti‐in flammatory effects of Zingiber and its active ingredient 6‐shogaol in animal models of cerebral ischemia(Wattanathorn et al., 2011; Na et al., 2016). The main ingre‐dients in each of the six component herbs in GLGZD are present in the decoction (Xu et al., 2015), and many of them have the ability to traverse the blood-brain barrier (Li et al.,2015). This might contribute to the neuroprotective effec‐tiveness of GLGZD against cerebral ischemic tissue damage.
In the present study, while we provide evidence that GL‐GZD promotes neuroregeneration and axonal remodeling,it remains unknown which of the many active component molecules contribute to the efficacy of the decoction. Further‐more, the molecular mechanisms by which GLGZD exerts its effects on neuroregeneration and axonal remodeling have not been investigated. Further research is needed to identify the effective ingredients and to clarify the molecular pathways and mechanisms underlying the effects of GLGZD.
In summary, our current findings demonstrate that GL‐GZD promotes recovery from cerebral ischemic injury, and suggest that the decoction enhances neuroregeneration and axonal remodeling to improve the recovery of neural func‐tions. Therefore, GLGZD might have therapeutic potential for the treatment of post‐stroke motor impairments.
Author contributions:JH designed the study and performed experiments and wrote the paper. JZZ and ZFZ helped to do the surgery of the animals, performed the immunohistochemical staining and analyzed data. ZFL helped to capture the confocal images and analyzed the data.Professor WSP instructed the identi fication of decoction pieces and the preparation of GLGZD decoction. Professor JH and Professor LDC directed the research. All authors read and approved the final paper for publication.
Conflicts of interest:No con flict of interest exits in the submission of this manuscript.
Financial support:This work was supported by a grant from the Research Project of Fujian Provincial Health and Family Planning Commission of China, No. 2014-ZQN-JC-32; a grant from the Project of Fujian Province Office of Education of China, No. JZ160442; the Natural Science Foundation of Fujian Province of China, No. 2018J01855; a grant from the Platform for Preclinical Studies of Traditional Chinese Medicine and Quality Control Engineering Technology Research Center of Fujian Province of China, No. 2009Y2003. The funding bodies played no role in the study design, collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Institutional review board statement:The procedures were performed in accordance with the international laws on animal experimentation and approved by the Ethical Committee of Fujian Academy of Traditional Chinese Medicine, China.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles aredistributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer review reports:
Reviewer 1: Lezi E, Duke University, USA.
Comments to authors:A well-written article. Although there is not much data, as it is a descriptive study, but they are of good quality and presented in a clear and effective manner. In this study, the authors evaluated the neuroprotective effects of GLGZD, a Chinese traditional prescription, in a rat model of cerebral ischemia. They reported that GLGZD can improve the neurological function, possibly through promoting neurogenesis and axon regrowth in or surrounding the lesion site, which was demonstrated by showing increased doublecortin+/BrdU+ cells in SVZ, as well as increased GAP-43 expression. Although the study does not go deep into the mechanisms, the authors present a fundamental evidence at a cellular level for GLGZD bene fiting individuals post-stroke.
Reviewer 2:Kim C-Yoon, Seoul National University, Korea.
- 中国神经再生研究(英文版)的其它文章
- In Memoriam: Ray Grill (1966–2018)
- Structural brain volume differences between cognitively intact ApoE4 carriers and non-carriers across the lifespan
- Roles of Eph/ephrin bidirectional signaling during injury and recovery of the central nervous system
- Neuroplasticity, limbic neuroblastosis and neuro-regenerative disorders
- A tissue-engineered rostral migratory stream for directed neuronal replacement
- Targeting the noradrenergic system for anti-in flammatory and neuroprotective effects:implications for Parkinson’s disease

