Roles of Eph/ephrin bidirectional signaling during injury and recovery of the central nervous system
Yue Wan , Jin-Shan Yang , Li-Cai Xu, Xiao-Jiang Huang Wei Wang Min-Jie Xie
1 Department of Neurology, The Third People’s Hospital of Hubei Province, Wuhan, Hubei Province, China
2 Department of Neurology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province,China
3 Department of Neurology, First Affiliated Hospital, Fujian Medical University, Fuzhou, Fujian Province, China
4 Department of Neurological Rehabilitation Center, The Third People’s Hospital of Hubei Province, Wuhan, Hubei Province, China
Abstract Multiple cellular components, including neuronal, glial and endothelial cells, are involved in the sophis‐ticated pathological processes following central nervous system injury. The pathological process cannot reduce damage or improve functional recovery by merely targeting the molecular mechanisms of neuronal cell death after central nerve system injuries. Eph receptors and ephrin ligands have drawn wide attention since the discovery of their extensive distribution and unique bidirectional signaling between astrocytes and neurons. The roles of Eph/ephrin bidirectional signaling in the developmental processes have been re‐ported in previous research. Recent observations suggest that Eph/ephrin bidirectional signaling continues to be expressed in most regions and cell types in the adult central nervous system, playing diverse roles.The Eph/ephrin complex mediates neurogenesis and angiogenesis, promotes glial scar formation, regulates endocrine levels, inhibits myelin formation and aggravates in flammation and nerve pain caused by injury.The interaction between Eph and ephrin is also considered to be the key to angiogenesis. This review focus‐es on the roles of Eph/ephrin bidirectional signaling in the repair of central nervous system injuries.
Key Words: nerve regeneration; Eph/ephrin; bidirectional signaling; central nervous system; injury; recovery;neurons; glial cells
Introduction
Functional recovery after injury to the central nervous sys‐tem (CNS) has been limited by failure of neurons to regen‐erate and restore. The molecular mechanisms underlying the processes are not fully understood. Over the past few decades, intensive studies have indicated that the extracellu‐lar environment of the CNS plays a pivotal role. The lack of neuronal regeneration may be explained partly by the lack of growth promoting molecules, together with inhibitors to axonal regeneration in the extracellular environment. The Eph receptors and their ligands, ephrins, are members of the receptor tyrosine kinase family. In the last decade, the ma‐jority of research has focused on their roles in development.The Eph receptors and ephrins coordinate not only devel‐opmental processes, but also are re‐expressed in a variety of CNS diseases and play a role in adult regeneration by affect‐ing the neural microenvironment (Goldshmit et al., 2006;Cramer and Miko, 2016). Imbalance of Eph/ephrin function has been implicated in a wide variety of CNS injuries and dis‐eases, summarized in Table 1. Therefore, an in‐depth study of Eph/ephrin would be of great importance for the understand‐ing of the pathophysiological mechanisms and in developing treatments of these diseases. This review discusses the effects of Eph/ephrin in the CNS, and provides an insight into devel‐oping possible routes to achieve neural repair.
Eph and Ephrins
Eph receptor family members
Since Hirai et al. (1987) first identi fied the EphA1 receptor,the Eph proteins have become the largest known recep‐tor tyrosine kinase family. Fourteen members of the Eph receptor family have been found in humans and other mammals, including the EphA subfamily (EphA1–EphA8,EphA10) and the EphB subfamily (EphB1–EphB4, EphB6).The former preferentially binds to five glycosylphosphati‐dylinositol‐anchored ephrin‐A ligands (ephrinA1–A5) and the latter possesses high affinity binding domain to three transmembrane ephrin‐B (ephrinB1–B3) ligands, with some promiscuity of the binding (Wilkinson, 2001; Goldshmit et al., 2006; Pasquale, 2008; Lisabeth et al., 2013; Cramer and Miko, 2016; Pasquale, 2016; Taylor et al., 2017). The signal‐ing pathways are directly initiated by either Eph receptor or ephrin ligand activation are termed “forward signaling ” and“reverse signaling” respectively (Aoto and Chen, 2007). The diverse roles of Eph/ephrin bidirectional signaling in various pathological and physiological processes have been eluci‐dated extensively (O’Leary and Wilkinson, 1999; Wilkinson,2001; Goldshmit et al., 2006; Chumley et al., 2007; Pasquale,2008; Lisabeth et al., 2013; Day et al., 2014; Choi et al., 2016;Cramer and Miko, 2016; Pasquale, 2016; Taylor et al., 2017;Wei et al., 2017).
Eph structure
Although there are a large number of subtypes, Eph recep‐tors share similar structures (summarized in Figure 1). The extracellular region of Eph receptors includes a ligand‐bind‐ing site and a cysteine‐rich region followed by two fibronec‐tin type III motifs (Lackmann et al., 1998; Pasquale, 2008;Lisabeth et al., 2013; Taylor et al., 2017). The intracellular part is composed of four domains: a juxtamembrane region,a kinase domain, a sterile‐alpha‐motif (SAM) domain and a PSD95/Dlg/ZO1 (PDZ)‐binding domain. The juxtamem‐brane region is critical for receptor activation (Brückner and Klein, 1998; Lisabeth et al., 2013; Taylor et al., 2017).The kinase domain is widely considered to implicate the cytoskeleton through the small GTPases (Nimnual et al.,1998). The SAM domain is involved in regulating receptor dimerization and initiating downstream signal transduction(Goldshmit et al., 2006). The fourth, PDZ, domain is critical for Eph/ephrin complexes assembly and location (Kullander and Klein, 2002; Goldshmit et al., 2006).
Ephrin structure
The ephrin ligands are also divided into two subclasses: an ephrinA subfamily and an ephrinB subfamily based on se‐quence conservation and affinities for Eph receptors. Both of them have a receptor‐binding domain (Figure 1). The eph‐rinA ligands (ephrinA1–A5) are attached to the membrane at their carboxyl terminalviaa glycosylphosphatidylinositol anchor, whereas the ephrinB ligands (ephrinB1‐B3) have a short transmembrane domain, a PDZ‐domain binding site,a short cytoplasmic tail and have five conserved tyrosine residues (Flanagan and Vanderhaeghen, 1998; Goldshmit et al., 2006; Wu et al., 2012; Lisabeth et al., 2013; Taylor et al.,2017).
Eph/ephrin complex formation and bidirectional signaling
The unique feature of Eph/ephrin signaling complexes is their ability to generate bidirectional signaling. An Eph receptor can also act as a ligand and an ephrin ligand can act as a receptor. The former signaling is termed as forward signaling, while the latter signaling is termed as reverse sig‐naling. Both forward and reverse signaling have a role in a series of physiological process such as cell migration and adhesion, neurite outgrowth, synaptic plasticity, topograph‐ic mapping, neuronal connectivity and regeneration. It also has a role in the vascular formation in the nervous system(Mellitzer et al., 1999; Huot, 2004; Klein, 2004; Eichmann et al., 2005; Pasquale, 2008; Taylor et al., 2017). The formation of highly clustered Eph/ephrin complexes is necessary for the initiation of Eph/ephrin bidirectional signaling. Previous studies demonstrated that recombinant, soluble ephrin must be pretreated to form clustered ephrin to trigger efficient activation (Pasquale, 2008; Taylor et al., 2017), since any soluble monomeric ephrins act as Eph receptor antagonists(Carter et al., 2002; Lawrenson et al., 2002; Dobrzanski et al.,2004). The same principle also applies to reverse signaling.The degree of clustering is related to signal strength and downstream pathways (Pasquale, 2005, 2008).
Eph forward signaling induced by ephrin binding initiates downstream signal transduction after autophosphoryla‐tion and phosphorylation in the tyrosine kinase domain.Signaling pathways downstream of Eph forward signaling have been extensively studied (Henkemeyer et al., 1994;Holland et al., 1997; Becker et al., 2000; Shamah et al., 2001;Kullander and Klein, 2002; Takasu et al., 2002; Tong et al.,2003; Goldshmit et al., 2004; Pasquale, 2005, 2008; Lisabeth et al., 2013; Taylor et al., 2017). Forward signaling mediated by Eph receptors has been shown to involve regenerative processes such as neurite outgrowth and cell migration by regulating the organization of dynamic cytoskeletal and rearrangement of actin (Figure 1). The Eph receptors are highly important to the Rho GTPases, including Rac, Cdc42 and Rho that are critical in regulating the actin cytoskeleton(Giniger, 2002; Pasquale, 2008; Lisabeth et al., 2013). For‐ward signaling through Eph receptors inhibits axonal regen‐eration in neuronal cells by stimulating the collapse of the growth cone, probably acting on Rac and Cdc42 (Lehmann et al., 1999; Dickson, 2001; Dergham et al., 2002; Fournier et al., 2003). On the contrary, blocking Eph receptors leads to downstream Rac and Cdc42 activation that promotes axonal outgrowth. Therefore, EphA receptors can lead to repulsive guidance for growing axons by Rho GTPases activation. Rac and Cdc42 activation promotes axonal outgrowth through blocking Eph forward signaling (Shamah et al., 2001; Sa‐hin et al., 2005; Lisabeth et al., 2013). Ephexin, which links EphA4 receptors to the Rho GTPases, is known to play piv‐otal roles in axon guidance (Shamah et al., 2001; Sahin et al.,2005). It has been demonstrated that ephrinA3 can suppress Wnt/β‐catenin signaling and inhibit the neurogenic poten‐tial of retinal stem cell through EphA4 (Fang et al., 2013).
Eph forward signaling may also be involved in the Janus kinase/signal transducer and activator of the transcription(JAK/TAT) pathway, the phosphoinositide 3‐kinase (PI3K)pathway and the mitogen‐activated protein (MAP)‐kinase pathway (Steinle et al., 2002; Lai et al., 2004; Macrae et al.,2005).
A signal through the ephrin ligand is defined as reverse signaling. Although many previous studies have shown the predominant roles played by ephrin reverse signaling,e.g.in neural progenitor proliferation, axon guidance, neuronal migration and synaptic plasticity, the intracellular signaling cascades following ephrin activation have not been investi‐gated thoroughly. With the help of the co‐receptors, ephri‐nAs ligands are able to trigger downstream activation of the PI3K and Src family kinases despite the lack of a cytoplasmic tail (Davy et al., 1999; Davy and Robbins, 2000) (Figure 1).It was recently demonstrated that associated transmembrane signaling partners,e.g., TrkB and p75 neurotrophin receptor(p75NTR), represent good candidates as co‐receptors for eph‐rinAs (Suetterlin et al., 2012).
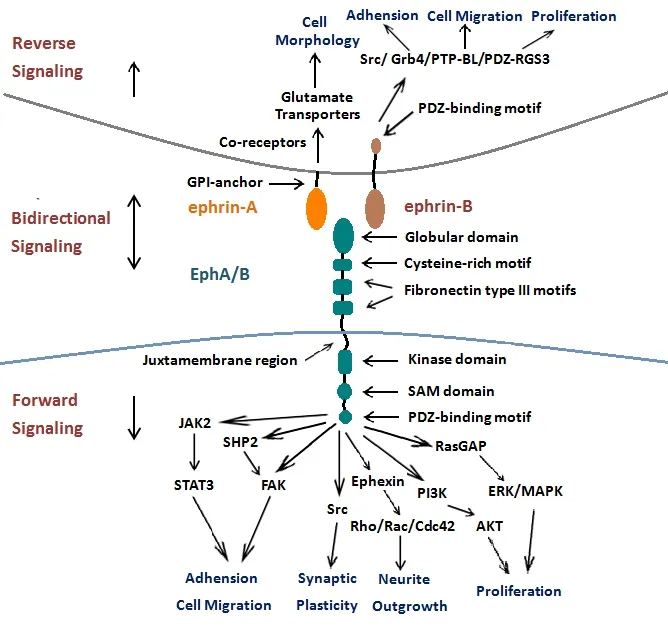
Figure 1 Bidirectional signaling of Eph/ephrin.
In contrast to ephrinA ligands, transmembrane ephrinB ligands have a single transmembrane domain and a short conserved cytoplasmic domain with a PDZ domain‐binding motif, which constitutes the structural foundation of reverse signaling. Through the activation of the Src‐family kinases,ephrinB ligands are phosphorylated. Recent advances have discovered that Src‐homology‐2‐domain‐containing adap‐tor molecules,e.g., Grb4, are recruited and then phosphor‐ylate ephrin‐B ligands, further initiating the regulation of cytoskeleton dynamics by downstream signaling (Holland et al., 1996; Bruckner et al., 1997; Cowan and Henkemeyer,2001; Kalo et al., 2001; Palmer et al., 2002). Then the protein tyrosine phosphatase is recruited through its PDZ domain to the ephrinB carboxy‐terminal tail, which dephosphorylates ephrinB and inactivates Src‐family kinases. PDZ‐binding protein, as a regulator of G‐protein signaling 3 (PDZ‐RGS3),might inhibit C‐X‐C chemokine receptor type 4 (CXCR4‐re‐ceptor)‐mediated chemo attraction by inactivating the Gαβγ‐protein complex. This regulates the endothelial migra‐tion and angiogenesis (Salcedo et al., 1999; Lu et al., 2001).
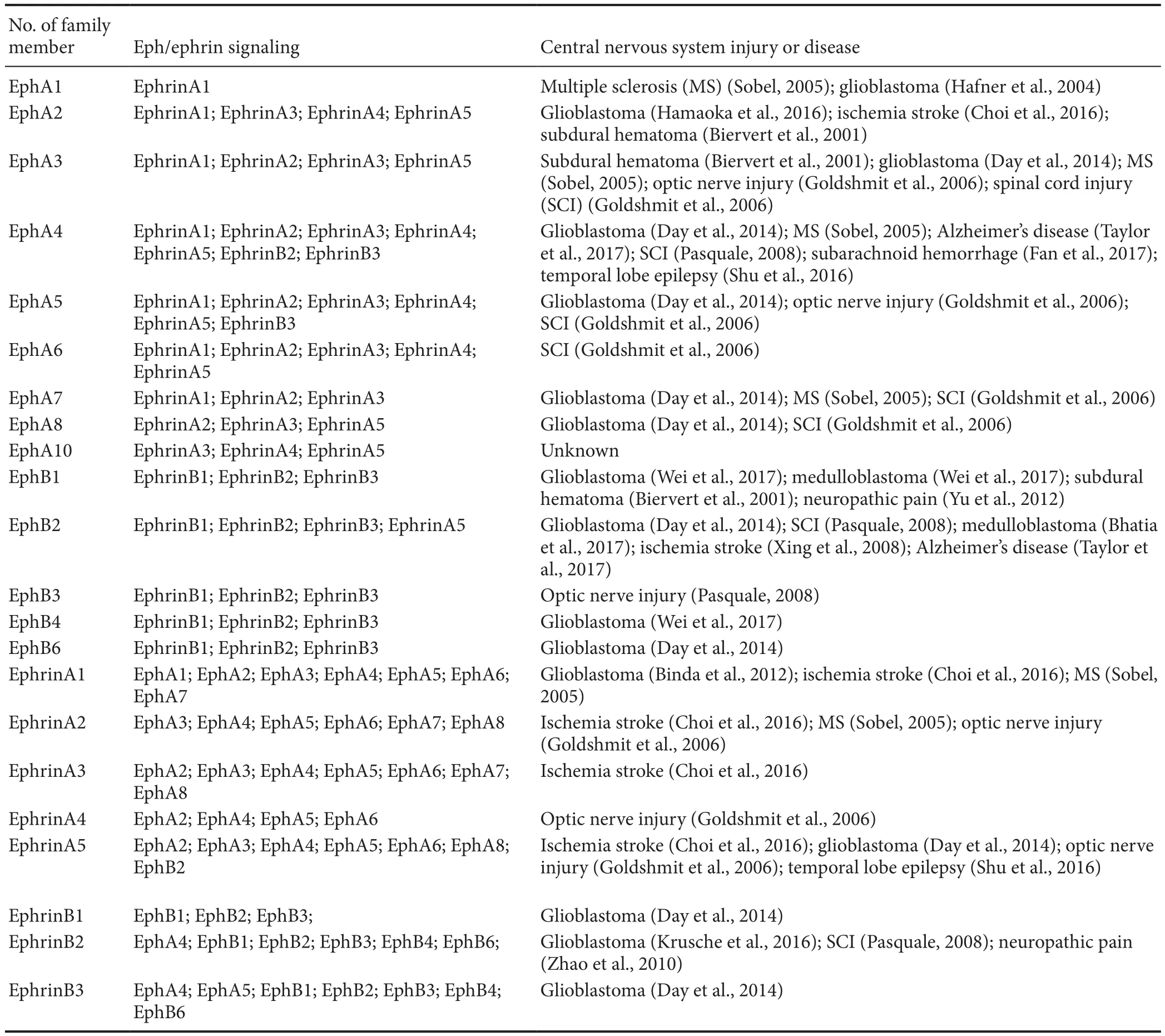
Table 1 Summary of Eph/ephrin and their associated central nervous system injuries or diseases
Eph/Ephrin Expression in Adult CNS
For many years, researchers have focused on the roles of Eph family in the developing CNS. During development, ex‐pressions of Eph receptor and ephrin ligand change signi fi‐cantly. In most regions and cell types of normal CNS, Ephs and ephrins continue to be expressed in adults, but at a low‐er level than during development (Goldshmit et al., 2006).In brain regions that show highly changeable morphological and physiological plasticity into adulthood,e.g., the amyg‐dala and hippocampus, many Eph/ephrins continue to be expressed (Liebl et al., 2003; Pasquale, 2008). Several diverse roles of Eph/ephrins in adult CNS have been elucidated(Goldshmit et al., 2006; Pasquale, 2008; Day et al., 2014; Ka‐nia and Klein, 2016; Taylor et al., 2017; Wei et al., 2017).
Eph/ephrins are widely regarded to have an important role in regulating synapse formation as well as synaptic plas‐ticity and function (Olivieri and Miescher, 1999; Murai and Pasquale, 2002; Takasu et al., 2002). This is especially so in processing spinal cord pain (Zhao et al., 2010) and main‐taining hippocampal plasticity (Taylor et al., 2017). Several studies have shown that EphA4 forward signaling activation mediates dendritic spine retraction together with a reduc‐tion in their number and size by remodeling the actin cyto‐skeleton and modifying the properties of adhesion receptors(Fu et al., 2007; Klein, 2009). EphA4 knockout mice show significantly longer and overlapping spines comparing to wild‐type mice (Murai et al., 2003). However, triple EphB(EphB1/EphB2/EphB3) knockout mice show a distinct de‐crease in spine density and formation of abnormal spines;the contrasting effects suggest that EphB forward signaling is associated with synaptic maturation and dendritic spine for‐mation (Henkemeyer et al., 2003). Extensive studies by com‐paring wild‐type mice and Eph/ephrin knockout show that Eph/ephrins affect memory and learning by controlling syn‐aptic formation (Martone et al., 1997; Murai and Pasquale,2002; Murai et al., 2003; Grunwald et al., 2004; Rodenas‐Ru‐ano et al., 2006). Eph/ephrins may recruit cell‐surface mol‐ecules such as the N‐methyl‐D‐aspartic acid (NMDA) re‐ceptor through their PDZ domain (Hsueh and Sheng, 1998;Torres et al., 1998; Buchert et al., 1999; Kalo and Pasquale,1999). EphB2/ephrinB3 bidirectional signaling can induce long‐term potentiation generation by the NMDA receptor(Grunwald et al., 2001; Henderson et al., 2001; Takasu et al.,2002; Lim et al., 2008; Taylor et al., 2017).
In the hippocampus, EphA4/ephrinA3 mediated bidirec‐tional signaling has not only been implicated in changing spine morphology and α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazole propionate receptor (Murai et al., 2003), but also in the regulation of hippocampal synaptic plasticity and the astrocytic glutamate transporter (Carmona et al., 2009).Others have shown that ephrinBs/EphB take part in process‐ing spinal cord pain through NMDA receptors, PI3K and its downstream signaling (Battaglia et al., 2003; Slack et al.,2008; Song et al., 2008; Ruan et al., 2010; Yu et al., 2012).
In the subventricular zone of the lateral ventricle and subgranular zone of the dentate gyrus, where there is con‐tinuous neurogenesis of stem cells, Eph/ephrins are present throughout adulthood (Goldshmit et al., 2006). Both the dif‐ferentiation and proliferation of neural precursor cells can be in fluenced by Eph/ephrin bidirectional signaling. EphB3/ephrinB3 regulates the differentiation and proliferation of cells in the rostral migratory stream and subventricular zone by controlling the expression level of p53 (Ricard et al.,2006; del Valle et al., 2011; Baumann et al., 2013). EphA4 knockout mice exhibit decreased cell proliferation and dif‐ferentiation disorder in the rostral migratory stream and subventricular zone, resulting in a reduced number of neu‐roblasts (Khodosevich et al., 2011). EphB1/ephrinB3 signal‐ing is found to affect the differentiation and proliferation of neural precursor cells in the subgranular zone of the dentate gyrus. This highlights a potential therapeutic target for neu‐rodegenerative diseases and brain damage (Chumley et al.,2007).
The signals transduced by Eph/ephrin also impact on angiogenesis in the CNS (Pasquale, 2008). EphA2 receptor blockage is proven to improve tight junction formation be‐tween endothelial cells and to promote angiogenesis (Zhou et al., 2011). There is evidence that intracerebroventricular injection of ephrinA1 promotes angiogenesis through EphA forward signaling (Jing et al., 2012).
Expression and Regulation of Eph/Ephrins in the CNS following Injury or Diseases
Apart from their critical roles in the developing CNS, Eph/ephrin signaling is involved in the sophisticated patholog‐ical processes following CNS injury or diseases (Table 1).Various studies have shown that Eph receptors and ephrin ligands upregulate after CNS injuries and exhibit diverse and changing patterns depending on when or where injuries happen (Miranda et al., 1999; Willson et al., 2002; Bundesen et al., 2003). After CNS damage, different cell types behave differently. Neurons attempt to regenerate the damaged connections. To maintain the homeostasis, astrocytes and microglial cells are activated and proliferate, while oligo‐dendrocytes try to initiate remyelination. The changes in expression of Ephs and ephrins under these situations may reveal how the Eph/ephrin signaling functions in response to damage. Given their effect on axon guidance in devel‐oping CNS, it is commonly believed that Ephs and ephrins may play a role in regulating axonal guidance during axonal regeneration. Eph receptors and their ephrin ligands are also expressed on mature cell types,e.g. neurons and astrocytes,to mediate astrocytic gliosis, neural regeneration, vascular remodeling and neuroin flammation.
Eph/ephrin signaling affects glial scar formation and glutamate homeostasis
Eph/ephrins also act in various ways to in fluence the struc‐tural and functional reorganization of damaged CNS. A pri‐mary response of the Ephs and ephrins is their contribution to CNS injury by promoting the formation of a glial scar,thereby inhibiting axonal regeneration. Previous studies have revealed that sophisticated processes involved in gliosis consist of glial reactivation, extracellular matrix alteration and even collagen deposition. Multi‐cellular components,including astrocytes, microglia, oligodendrocyte progenitors and fibroblasts, participate in glial scar formation (Bunge et al., 1997; Dawson et al., 2000). Ephs and ephrins are ex‐pressed on many kinds of cells related to gliosis and glial scarring and affect their cellular response to damage. Glial cells are highly sensitive to damage. During CNS injury, as‐trocytes and microglia are activated and start to proliferate,characterized by morphological and functional changes,then form the glia scar. Astrocytic activation presents as cellular hypertrophy, proliferation and gliosis (Schnell et al., 1999), which can even be observed in areas distal to the injury site (McGraw et al., 2001; Xie et al., 2011). However,the astroglial response is a double‐edged sword for neuronal cells. Of course, there are considerable benefits obtained from the glial scar formation. It prevents the injury site from spreading to the surrounding normal tissue, so holding back the damage, and filling the lesion cavity (Faulkner et al.,2004). The glial scar helps reconstruct the damaged area and reorganize blood vessels, then epithelial cells can migrate into the scar tissue. The glial scar, however, has been proven to be the main barrier to neural regeneration (Goldshmit et al., 2004; Fernández‐Klett and Priller, 2014). There is mounting evidence that Eph/ephrin signaling is involved in this process and influences glial scar formation during CNS injury or diseases. Cell‐contact‐mediated bidirection‐al signaling between EphB2 on meningeal fibroblasts and ephrinB2 on reactive astrocytes triggers cellular cascades.These result in the exclusion of meningeal fibroblasts and the development of the glial scar from the injured site and occur early in the spinal cord injury model (Bundesen et al.,2003). Other research showed that astrogliosis was reduced and regeneration of injured corticospinal axons accelerated in ephrin B2–/–mice, thereby resulting in the recovery of motor function after spinal cord injury (Ren et al., 2013). In lesioned EphA4–/–spinal cords, the astrocytic gliosis and the glial scar were greatly reduced. EphA4–/–astrocytes failed to respond to the leukemia inhibitory factor or interferon‐gam‐ma and in flammatory cytokinesin vitro. Neurons grown on EphA4–/–astrocytes extended longer neurites than on wild‐type astrocytes. The application of EphA4 inhibitors in wild‐type mice produced a moderate reduction in astrocytic glio‐sis, promoted axonal regeneration and improved functional outcome following spinal cord hemisection (Goldshmit et al., 2004; Goldshmit et al., 2011).
Glutamate is not only a pivotal excitatory neurotransmit‐ter but also a potential neurotoxin in the CNS. Excessive glutamate signaling can lead to excitotoxic cell death. Eph/ephrin signaling can help the astrocytes in the injured brain maintain the homeostasis of extracellular glutamate. Astro‐cytic glutamate transporters and astrocytic glutamate uptake capacity decreased, if the clustered EphA4 was applied (Yang et al., 2014). The EphA4‐mediated ephrinA3 reverse‐signal‐ing controls the glial glutamate transporters and prevents glutamate excitotoxicity developing under pathological con‐ditions (Yang et al., 2014).
Eph/ephrin signaling mediates neurogenesis and angiogenesis
In the mature CNS, endogenous neural precursor cells are present in the rostral subventricular zone of the lateral ven‐tricles and the subgranular zone of the dentate gyrus. Under pathophysiological conditions, proliferation of neural pre‐cursor cells in the subventricular zone and subgranular zone is triggered. These neuroblasts may migrate toward to the lesioned area, and some of them differentiate into new neu‐rons to replace damaged neurons. Eph/ephrin bidirectional signaling contributes to their response to CNS injury by in‐fluencing both the differentiation and proliferation of neural precursor cells. EphB3/ephrinB3 regulates the proliferation and differentiation of cells in the subventricular zone and in the rostral migratory stream by controlling levels of p53(Ricard et al., 2006; Theus et al., 2010; del Valle et al., 2011;Baumann et al., 2013). Blockade of EphB2 plays a role in the neurogenesis and the recovery of neurological function after cerebral cortical infarction in hypertensive rats (Xing et al., 2008). In addition to neurogenesis, neurons control their plasticity by adapting their structure and function to the microenvironmental changes. A series of experiments have shown that Eph/ephrin signaling exhibits an inhibitory effect on neurite outgrowth in damaged CNS. For example,ephrinA5 reverse signaling could induce growth cone col‐lapse and then inhibit axonal regeneration through activat‐ing RhoA (Yue et al., 2008). At the same time, ephrinA5 me‐diated EphA4 forward signaling triggers axonal growth cone collapse via the downstream RacGAP alpha2‐chimaerin‐de‐pendent signaling pathway (Wegmeyer et al., 2007). Eph/ephrin bidirectional signaling can regulate the oligoden‐drocyte precursor cells and oligodendrocytes. Eph‐ephrin interactions between axons and oligodendrocyte precursor cells control the distribution and migration of oligodendro‐cyte precursor cells in the optic axonal tracts (Prestoz et al.,2004). It is worth noting that ephrin‐B3 expressed in post‐natal myelinating oligodendrocytes acts as a myelin‐based inhibitor through the combined p75NTRneurotrophin recep‐tor (Benson et al., 2005). It is now relatively well accepted that neurogenesis and angiogenesis are coupled processes.
Eph receptors and their ephrin ligands are also involved in angiogenesis, which is critical for vascular remodeling following CNS injury (Pasquale, 2008). EphA2 can regulate post‐natal angiogenesis. EphA2‐deficient endothelial cells were unable to undergo vascular migration and assembly(Brantley‐Sieders et al., 2004). UniPR129, a competitive small molecule Eph‐ephrin antagonist, blocks angiogenesis at a low concentrationin vitro(Hassan‐Mohamed et al.,2014). The ephrinA5/EphA4 interaction is associated with the epilepsy, and can suppress microvessel remodeling and neogenesis of neurons in a mouse model of temporal lobe epilepsy (Shu et al., 2016).
Eph/ephrin signaling and neuroin flammation
Post‐injury inflammation is implicated in almost all kinds of CNS injury. Infection, neurodegeneration, trauma even though ischemia stimulate an in flammatory response, which causes the activation of microglia in the brain. In flammation is beneficial in the clearance of debris caused by necrotic cells. Nevertheless, severe inflammation causes cerebral swelling and vascular dysfunction, aggravating the neuronal damage. There is proof for the involvement of Eph/eph‐rin proteins in the inflammatory process after CNS injury(Pasquale, 2008). Inhibition of EphBs receptors by injection of EphB1‐Fc (blocker of EphB1 receptor) reduced chronic constrictive injury‐induced neuropathic pain and forma‐lin‐induced inflammation (Yu et al., 2012). Presynaptic ephrinB2 has also been found to regulate in flammatory pain by regulating the tyrosine phosphorylation of the NMDA receptor (Zhao et al., 2010).
Conclusions
Eph/ephrin bidirectional signaling plays a critical role in CNS injury. Signaling through Eph/ephrin complexes me‐diates neurogenesis and angiogenesis, promotes glial scar formation, regulates homeostasis, inhibits myelination and exaggerates inflammation linked to injury‐induced neuro‐pathic pain. However, interaction between Ephs and ephrin ligands is essential for angiogenesis. More specific roles of Eph/ephrin in the injured nervous system are only begin‐ning to be appreciated, such as the paradoxes of EphB1 re‐ceptor in the malignant brain (Wei et al., 2017) and the role of EphA10 in the CNS injury or related diseases. Therefore,further studies will generate more comprehensive data. We will continue to explore more roles of Eph/ephrin in the re‐pair of the injured CNS.
Author contributions:YW and JSY wrote most of the chapters and drew the figure and the table. LCX, XJH, WW and MJX supervised and proofread the entire review. All authors approved the final version of the paper.
Conflicts of interest:The authors declare no competing financial interests.
Financial support:The investigation was supported by the National Natural Science Foundation of China, No. 81371312, 81030021, and the National Basic Research Development Program (973 Program) of China, No. 2011CB504403. The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer review report:
Reviewer:Sanusi Mohammad Bello, King Faisal University, Saudi Arabia.
Comments to authors:The article brought forward the emerging ever expanding roles of Eph/Ephrin bidirectional signaling abilities in the injury and repair of the central nervous system. This area of Ephrin signaling has the potential for exploration for more speci fic roles in the repair of injured nervous system with huge potential for clinical application in the near future. I therefore encourage the author to continue to explore other useful potential applications of the Ephrin signaling. It is a good move to explore the wonders of this important member in the repair of nervous system.
- 中国神经再生研究(英文版)的其它文章
- In Memoriam: Ray Grill (1966–2018)
- Structural brain volume differences between cognitively intact ApoE4 carriers and non-carriers across the lifespan
- Neuroplasticity, limbic neuroblastosis and neuro-regenerative disorders
- A tissue-engineered rostral migratory stream for directed neuronal replacement
- Targeting the noradrenergic system for anti-in flammatory and neuroprotective effects:implications for Parkinson’s disease
- Targeting the mitochondrial permeability transition pore in traumatic central nervous system injury

