Targeting the mitochondrial permeability transition pore in traumatic central nervous system injury
Joe E. Springer, Pareshkumar Prajapati, Patrick G. Sullivan
Spinal Cord and Brain Injury Research Center, Department of Neuroscience, University of Kentucky, Lexington, KY, USA
AbstractThe mitochondrion serves many functions in the central nervous system (CNS) and other organs beyond the well‐recognized role of adenosine triphosphate (ATP) production. This includes calcium‐dependent cell signaling, regulation of gene expression, synthesis and release of cytotoxic reactive oxygen species, and the release of cytochrome c and other apoptotic cell death factors. Traumatic injury to the CNS results in a rapid and, in some cases, sustained loss of mitochondrial function. One consequence of compromised mitochondrial function is induction of the mitochondrial permeability transition (mPT) state due to formation of the cyclosporine A sensitive permeability transition pore (mPTP). In this mini‐review, we summarize evidence supporting the involvement of the mPTP as a mediator of mitochondrial and cellular demise following CNS traumatic injury and discuss the bene ficial effects and limitations of the current ex‐perimental strategies targeting the mPTP.
Key Words: mitochondrial permeability transition; cyclophilin-D; cyclosporine A; NIM811; spinal cord injury;traumatic brain injury; secondary injury; functional recovery
Introduction
The ability of cells in the central nervous system (CNS) to survive and maintain a functional level of homeostasis fol‐lowing a traumatic injury depends on a number of critical factors. At a relatively gross level, the type of insult (vascular,structural,etc.) as well as the severity and proximity of cells to the injury site are obvious factors. However, many pathophys‐iological events occur at the subcellular and molecular levels that ultimately determine cellular demise or recovery. These events have been well studied and include, but are not limited to, glutamate excitotoxicity, Ca2+overload, in flammation, free radical mediated oxidative damage, and a loss of mitochon‐drial bioenergetics. Some of these secondary injury events are intimately interconnected, which is one rationale for develop‐ing neuroprotective therapeutic strategies targeting cell death signaling pathways at the molecular and cellular level.
The Mitochondrial Permeability Transition
Our research has focused on characterizing changes in mi‐tochondrial function after spinal cord (SCI) and traumatic brain (TBI) injuries (Sullivan et al., 2005; McEwen et al.,2011). This is based on well‐documented observations that mitochondria play a critical role in determining cellular fate, and compromised mitochondrial function is a prom‐inent feature in both SCI and TBI. Under physiological conditions, mitochondria exhibit a high transmembrane potential generated by the proton pumping components of the respiratory electron transport system. This transmem‐brane potential is a driving force in the phosphorylation of adenosine diphosphate (ADP) and sequestering Ca2+from the cytosol. However, following SCI or TBI, mitochondria rapidly become dysfunctional resulting in a loss of cytosolic Ca2+buffering capacity due to in flux of massive pathophysi‐ological levels of Ca2+through glutamate receptor subtypes,free radical mediated oxidative damage to mitochondrial complex proteins, and a subsequent compromise in bioen‐ergetic capacity. This process becomes insidious as the loss of energy production further reduces the ability of adenosine triphosphate (ATP)‐dependent Ca2+channels to regulate Ca2+cytosolic levels. Prolonged Ca2+overload can push mito‐chondria to the next pathophysiological stage‐ induction of the mitochondrial permeability transition (mPT) state due to mPT pore (mPTP) formation (Halestrap and Brenner, 2003).The mPT state uncouples respiration from ATP production and by this point recovery of mitochondrial function is limit‐ed at best. Cell survival is then compromised when sufficient numbers of mitochondria undergo mPT. Given the impor‐tance of mitochondria in cellular function, inhibiting forma‐tion of the mPTP is a strategy for limiting cell death in both SCI and TBI. In this perspective article, we summarize what is known about the mPTP and discuss the possible pros and cons of targeting the mPTP as a therapeutic strategy in CNS injury.
The mPTP is a multiprotein mega‐channel complex span‐ning both the inner and outer mitochondrial membranes,essentially allowing for communication of small molecules between the matrix and cytosol. The structural components of the pore are not well understood and the subject of con‐tinuing debate. Initial studies suggested that the pore was made up of the voltage‐dependent anion channel (VDAC)located on the outer mitochondrial membrane, the adenine nucleotide translocator (ANT) on the inner mitochondrial membrane, and cyclophilin‐D (Cyp‐D) in the matrix (Hal‐estrap and Brenner, 2003). However, follow‐up genetic dele‐tion studies revealed that ANT and VDAC are not required for mPTP formation, which may be explained, in part, by the presence of different isoforms of these putative pore components (Bernardi et al., 2015). Despite these observa‐tions, there is a general consensus that Cyp‐D functions as a major contributor to mPTP formation.
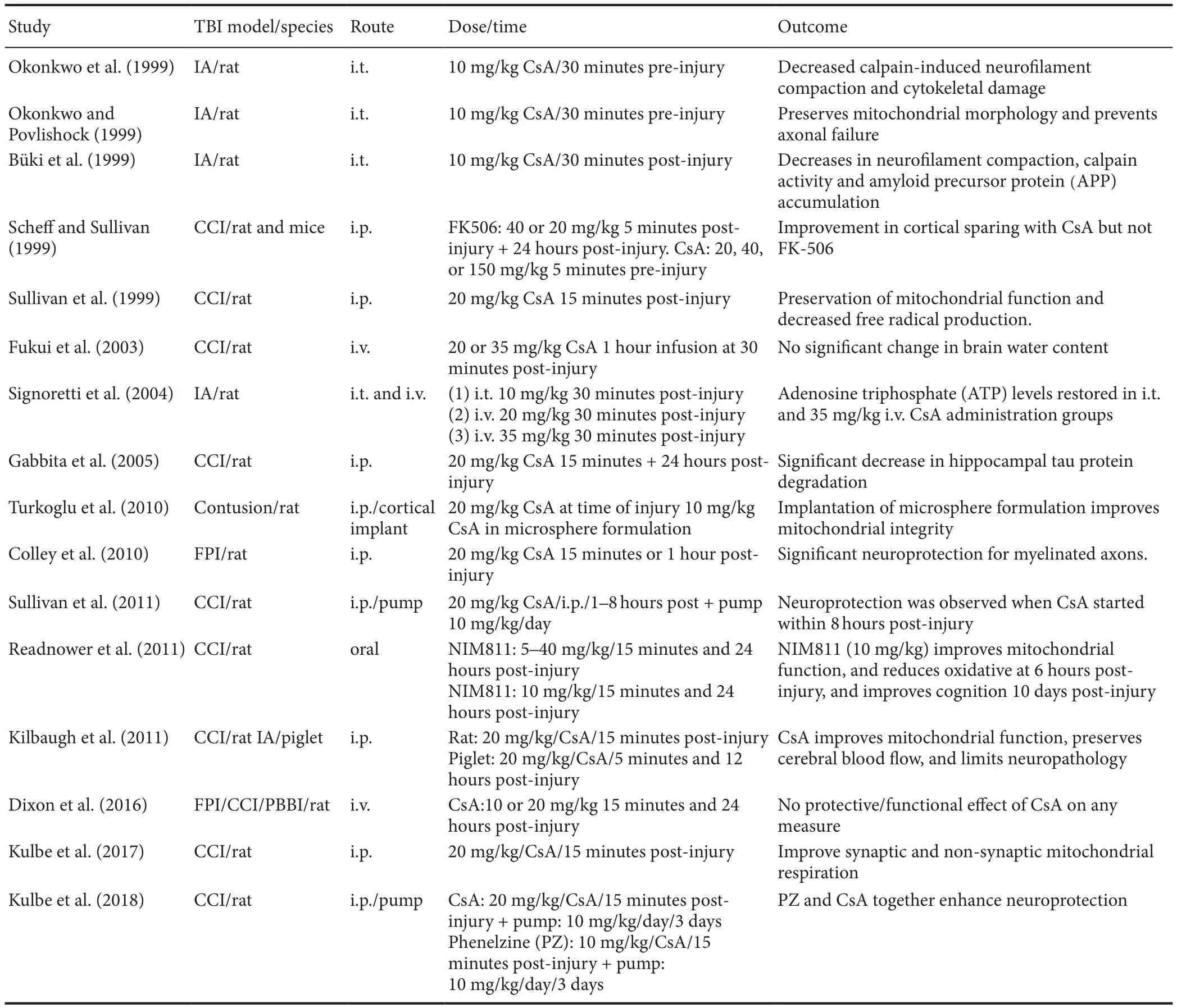
Table 1 Experimental studies testing the efficacy of CsA or NIM811 in the treatment of TBI
Inhibiting mPTP Formation in TBI and SCI
Cyclosporine A (CsA) is an immunosuppressant that inhib‐its mPT by binding to Cyp‐D. It has been shown repeatedly to be neuroprotective in TBI, although similar effects in SCI remain controversial based on con flicting reports. This differential efficacy of CsA in TBI versus SCI may be relat‐ed to properties inherent to mitochondria in the respective CNS regions. Moreover, it is difficult to examine a wide dose range of CsA due to its potent side effects and poten‐tial toxicity. In fact, doses of CsA above 20 mg/kg were,for the most part, ineffective in providing neuroprotection following TBI. Given these caveats, our group started inves‐tigating the use of NIM811, a Cyp‐D binding CsA derivate lacking any immunosuppressive properties and having rel‐atively minimal toxicity (Waldmeier et al., 2002). Our early studies provided evidence that NIM811 reduces oxidative damage while improving mitochondrial function and tissue sparing following TBI or SCI (McEwen et al., 2007; Mbye et al., 2008). The outcome of these studies provided strong evidence that NIM811 and CsA exhibited neuroprotective effects by inhibiting mPTP formation. A recent report by our group revealed a dose dependent effect of post‐injury NIM811 treatment in experimental SCI (Springer et al.,2018). Interestingly, we observed that a low dose of NIM811 significantly improved locomotor recovery, while higher doses significantly increased tissue sparing and reflexive bladder control but not recovery of locomotor function. The reasons for these dose‐related differences are not clear at this time, although it can be suggested that conducting a fol‐low‐up study examining additional doses in the range used in this study might prove revealing.
As stated above, there is strong evidence that targeting the mPTP has therapeutic potential in the treatment of TBI andSCI and numerous studies have examined this hypothesis with mixed results. We conducted a PubMed search to iden‐tify published studies investigating CsA or NIM811 in either TBI or SCI and the results of this search are summarized in Tables 1 and 2, respectively. What is clear from this analysis is that the many labs found CsA or NIM811 to be effective in the treatment of various TBI‐related outcomes across a range of doses, routes of administration and injury models.NIM811 was reported to be effective in one TBI study while all but two studies (see Fukui et al. (2003) and Dixon et al.(2016) in Table 1) reported structural, physiological, or functional efficacy with CsA treatment. It is interesting to point out that these two negative outcome studies used an intravenous route of delivery, while the positive outcome studies employed intrathecal, subcutaneous or intraperito‐neal routes of administration. Given the pharmacokinetics,clearance rates,etc., associated with different routes of ad‐ministration, it is possible to speculate that an intravenous route may not be optimal. One of these studies (Fukui et al.,2003) recommended that another route of administration be considered as there was no effect of intravenous CsA administration at least in terms of reducing brain edema.In addition, we have avoided an intravenous route of ad‐ministration in our TBI and SCI studies due to the relative toxicity of the vehicle. However, the results of a prospective randomized clinical trial indicate a good safety pro file when using intravenous CsA administration in severe TBI patients(Mazzeo et al., 2009).

Table 2 Experimental studies testing the efficacy of CsA or NIM811 in the treatment of traumatic SCI
CsA treatment in compression, contusion, or transec‐tion models of SCI has been reported to be either effective or ineffective in promoting functional recovery and tissue sparing (see Table 2). For example, work from Ibarra and colleagues report that CsA reduces lipid peroxidation, in‐creases survival of rubrospinal tract axons, and improves functional recovery, while Rabchevsky et al. (2001) report no effect of CsA treatment on any outcome measure in‐cluding functional recovery, tissue sparing. The reason for the discrepancies is not entirely clear, but may be related to differences in the injury models, injury severities, dosing,and routes of administration. We also demonstrated that the Cyp‐D and oxidative stress levels are higher in uninjured spinal cord relative to cortex, and that it takes a higher dose of CsA to inhibit mPTP in mitochondria isolated from spi‐nal cord compared to cortex (Sullivan et al., 2004). This is an important observation as our recent and previous studies have focused exclusively on NIM811, which can be used at higher doses than CsA. These studies demonstrate that NIM811 treatment increases mitochondrial function, spinal cord tissue sparing, and functional recovery in a contusion injury model (Table 2).
Future Considerations
One caveat of comparing CsA efficacy is the fact that many negative reports go unpublished making it difficult to as‐certain the true impact of CsA treatment in TBI and SCI.Regardless, given the detrimental side effects of CsA and its vehicle, as well as its immunosuppressive properties, we support the idea of targeting mPTP in both TBI and SCI using reformulated CsA and non‐immunosuppressive CsA derivatives such as NIM811. However, some effort should go into understanding the impact of targeting mPTP forma‐tion in cells that are signi ficantly compromised and may not survive without intervention. There is evidence that mito‐chondrial dysfunction persists well beyond the acute stages following TBI and SCI, and it is not clear whether rescuing compromised mitochondria in minimally functioning cells is bene ficial. For example, the bene fit of CsA administration seems to follow an inverted U‐shape highlighting the need for robust therapeutic window data in preclinical studies. It also is not clear what cellular energy demands are placed on surrounding cells in order to maintain minimally functioning cells that might die off in the absence of mPTP targeted drugs.Finally, the current mPTP drugs do not exhibit any cell spec‐i ficity, which might prove detrimental if treatment promotes survival and life span of pro‐inflammatory and destructive microglia, neutrophils, macrophages, and astrocytes.
In conclusion, there is compelling evidence that targeting the mPTP with CsA or NIM811 limits secondary injury and promotes functional recovery after TBI and SCI. Future studies examining the therapeutic potential of this strategy will rely, in part, on 1) identi fication of other CsA analogues or other compounds that selectively target the mPTP, 2)replication of existing studies using identical methodologies,and 3) examination of mPTP targeted drugs in other species and other injury models in which mPTP is known to occur.
Author contributions:JES, PP, and PGS contributed to the review of the literature, generation of the tables, and writing of the manuscript.
Conflicts of interest:None declared.
Financial support:This work was supported by a grant from the Kentucky Spinal Cord and Head Injury Research Trust.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Petra Henrich-Noack, Otto von Guericke Universitat Magdeburg, Germany.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- In Memoriam: Ray Grill (1966–2018)
- Structural brain volume differences between cognitively intact ApoE4 carriers and non-carriers across the lifespan
- Roles of Eph/ephrin bidirectional signaling during injury and recovery of the central nervous system
- Neuroplasticity, limbic neuroblastosis and neuro-regenerative disorders
- A tissue-engineered rostral migratory stream for directed neuronal replacement
- Targeting the noradrenergic system for anti-in flammatory and neuroprotective effects:implications for Parkinson’s disease

