Scutellarin protects oxygen/glucose-deprived astrocytes and reduces focal cerebral ischemic injury
Jing-Bo Sun , Yan Li , Ye-Feng Cai Yan Huang Shu Liu, Patrick KK Yeung, Min-Zhen Deng Guang-Shun Sun,Prince LM Zilundu, Qian-Sheng Hu, Rui-Xin An, Li-Hua Zhou, Li-Xin Wang Xiao Cheng
1 Department of Neurology, Guangdong Provincial Hospital of Traditional Chinese Medicine, Guangzhou, Guangdong Province, China
2 Department of Second Institute of Clinical Medicine, Guangzhou University of Traditional Chinese Medicine, Guangzhou, Guangdong Province, China
3 Guangdong Provincial Academy of Chinese Medical Sciences, Guangzhou, Guangdong Province, China
4 Guangdong Provincial Chinese Emergency Key Laboratory, Guangzhou, Guangdong Province, China
5 Department of Anatomy, An Hui Medical University, Hefei, Anhui Province, China
6 Department of Biomedical Sciences, The University of Hong Kong, Hong Kong Special Administrative Region, China
7 Department of Preventive Medicine, School of Public Health, Zhong Shan School of Medicine, Sun Yat‐Sen University, Guangzhou, Guangdong Province, China
8 Guangzhou Department of Anatomy, Zhong Shan School of Medicine, Sun Yat‐Sen University, Guangzhou, Guangdong Province, China
Abstract Scutellarin, a bioactive flavone isolated from Scutellaria baicalensis, has anti‐in flammatory, anti‐neurotoxic, anti‐apoptotic and anti‐oxida‐tive effects and has been used to treat cardiovascular and cerebrovascular diseases in China. However, the mechanisms by which scutellarin mediates neuroprotection in cerebral ischemia remain unclear. The interaction between scutellarin and nicotinamide adenine dinucleotide phosphate oxidase 2 (NOX2) was assessed by molecular docking study, which showed that scutellarin selectively binds to NOX2 with high affinity. Cultures of primary astrocytes isolated from the cerebral cortex of neonatal Sprague‐Dawley rats were pretreated with 2, 10 or 50 μM scutellarin for 30 minutes. The astrocytes were then subjected to oxygen/glucose deprivation by incubation for 2 hours in glucose‐free Dulbecco’s modi fied Eagle’s medium in a 95% N2/5% CO2 incubator, followed by simulated reperfusion for 22 hours. Cell viability was assessed by cell counting kit‐8 assay. Expression levels of NOX2, connexin 43 and caspase‐3 were assessed by western blot assay. Reactive oxygen species were measured spectrophotometrically. Pretreatment with 10 or 50 μM scutellarin substantially increased viability, reduced the expression of NOX2 and caspase‐3, increased the expression of connexin 43, and diminished the levels of reactive oxygen species in astrocytes subjected to ischemia‐reperfusion. We also assessed the effects of scutellarin in vivo in the rat transient middle cerebral artery occlusion model of cerebral ischemia‐reperfusion injury. Rats were given intraperitoneal injection of 100 mg/kg scutellarin 2 hours before surgery. The Bederson scale was used to assess neurological de ficit, and 2,3,5‐triphenyltetrazolium chloride staining was used to measure infarct size. Western blot assay was used to assess expression of NOX2 and connexin 43 in brain tissue. Enzyme‐linked immunosorbent assay was used to detect 8‐hydroxydeoxyguanosine (8‐OHdG), 4‐hydroxy‐2‐nonenal (4‐HNE) and 3‐nitrotyrosin (3‐NT) in brain tissue.Immuno fluorescence double staining was used to determine the co‐expression of caspase‐3 and NeuN. Pretreatment with scutellarin im‐proved the neurological function of rats with focal cerebral ischemia, reduced infarct size, diminished the expression of NOX2, reduced levels of 8‐OHdG, 4‐HNE and 3‐NT, and reduced the number of cells co‐expressing caspase‐3 and NeuN in the injured brain tissue. Fur‐thermore, we examined the effect of the NOX2 inhibitor apocynin. Apocynin substantially increased connexin 43 expression in vivo and in vitro. Collectively, our findings suggest that scutellarin protects against ischemic injury in vitro and in vivo by downregulating NOX2,upregulating connexin 43, decreasing oxidative damage, and reducing apoptotic cell death.
Key Words: nerve regeneration; scutellarin; cerebral ischemic injury; oxygen glucose deprivation/reoxygenation; nicotinamide adenine dinucleotide phosphate oxidase 2; reactive oxygen species; connexin 43; neural regeneration
Introduction
The latest data on cardiovascular disease in China in 2017 show that an estimated 290 million people suffer from cardio‐vascular disease, including 13 million stroke patients. Stroke is the most common cardiovascular disease and is character‐ized by high incidence, high recurrence, high disability, high mortality and high economic burden (Donnan et al., 2008).The treatment of stroke is still a global challenge (Goldstein et al., 2006). Among stroke cases, 85% are ischemic, while the remaining 15% are hemorrhagic (Roger et al., 2011). For ischemic stroke, thrombolysis is the only approved effective therapy, but it is limited by risk of hemorrhage and a short therapeutic time window (Wardlaw et al., 1997). Thus, re‐searchers have sought to develop drugs to treat cerebral isch‐emia through neuroprotection (Schmidt et al., 2013).
Increasing evidence shows that oxidative stress is a major cause of cerebral ischemic injury (Buch et al., 2012; Ma et al., 2013) resulting from increased production of reactive oxygen species (ROS) (Kahles and Brandes, 2013; Bren‐nan‐Minnella et al., 2015). Nicotinamide adenine dinucleo‐tide phosphate (NADPH) oxidase 2 (NOX2) is the most im‐portant NOX subtype involved in cerebral ischemic injury(Kahles and Brandes, 2013; Brennan‐Minnella et al., 2015).Activation of myosin light chain kinase has been found to increase NOX2‐mediated oxidation in cerebral ischemia/reperfusion injury (Zhang et al., 2015). Shichinohe et al.(2015) showed that cilostazol ameliorates ischemic tissue damage by inhibiting NOX2‐mediated oxidative stress.
Numerous studies have focused on the gene regulatory ef‐fects of ROS. However, the effects of ROS on gap junctional intercellular communication are relatively unknown. Gap junctions, the channels that connect the cytoplasm of neigh‐boring cells, such as endothelial cells that form the bloodbrain barrier and junctions between astrocytes and neurons,play an important role in central nervous system injury (Li et al., 2015). Connexin 43 (Cx43) is one of the most abun‐dant gap junction proteins in astrocytes (Nagy and Rash,2000; Contreras et al., 2004). However, the role of Cx43 in astrocytes under ischemic conditions is unclear (Farahani et al., 2005). Many studies have shown that Cx43 protects neu‐rons from cerebral ischemic injury (Schulz et al., 2015; Zhou et al., 2015). For example, selectively knocking out Cx43 in astrocytes results in large infarct volumes in rats subjected to transient middle cerebral artery occlusion (tMCAO) (Na‐kase et al., 2003). In contrast, other studies suggest that Cx43 worsens neuronal injury caused by cerebral ischemia (Chen et al., 2014a; Gao et al., 2015). In addition, there is a link be‐tween Cx43 and NOX in the pathology of some diseases, such as heart failure and diabetic hypogonadism (Xu et al., 2016).
Scutellarin (Figure 1) is a bioactive flavone isolated fromScutellaria baicalensisand has been used clinically to treat cardiocerebrovascular diseases in Asia for many years (Cuz‐zocrea et al., 2001). Several studies have reported that scutel‐larin has anti‐in flammatory, anti‐neurotoxic, anti‐apoptotic and anti‐oxidative actions (Hong and Liu, 2004; Long et al.,2015; Yuan et al., 2015). However, the mechanisms by which scutellarin mediates neuroprotection against cerebral isch‐emia remain unknown. In the present study, we investigate the effects of scutellarin on NOX2 and Cx43 expression inin vitroandin vivomodels of cerebral ischemic injury.
Materials and Methods
Molecular docking study
Molecular docking is a frequently used method to investigate the interaction between drugs and proteins (Garcia et al.,2014). There are many available docking approaches from which to choose. In this study, automated docking calcula‐tions were assessed with AutoDock 4.0 (Molecular Graphics Laboratory, La Jolla, CA, USA), and the three‐dimension crystal structure of NOX2 was obtained from the Protein DataBank (PDB ID: 3A1F). The protein crystal structure had water molecules and all other heteroatoms removed.
The docking site chose the active site with low average B‐values. Three‐dimensional affinity grids were calculated (60× 50 × 66), centered on the active site with 0.375 Å spacing for each of the atom types [C, A (aromatic C), N, O, S and H(electrostatic)] using AutoGrid 4.0. In this study, the absolute stereochemistry of scutellarin was reported in the literature,and geometry optimizations were performed using the B3LYP density functional with 6‐31G (d) basis set in a vacuum using the NWChem Quantum chemistry package (Pacific North‐west National Laboratory, Richland, WA, USA). Lamarckian genetic algorithm was selected for ligand conformational search using the AutoDock program. A 1.5 Å root‐mean‐square deviation was chosen for final docked conformations.
Culture of primary astrocytes
Primary cultures of astrocytes were prepared as described previously (Boitier et al., 1999). Astrocytes were isolated from the cerebral cortices of 40 specific‐pathogen‐free 0—2‐day Sprague‐Dawley rats (Sun Yat‐sen University, Guangzhou,China; license No. SCXK2016‐0029). The study was approved by the Animal Care and Use Committee of Sun Yat‐sen Uni‐versity (approval No. 2016006). Brie fly, cortices were harvest‐ed, while the meninges and blood vessels were removed. The cerebral cortex was cut into small pieces (1 mm3) and then dissociated with 0.125% trypsin at 37°C for 5 minutes. The reaction was terminated by addition of Dulbecco’s modi fied Eagle’s medium F12 (DMEM‐F12; Gibco, Grand Island, NY,USA) containing 10% fetal bovine serum (Gibco). After filter‐ing through a 100 μm strainer mesh, the cells were centrifuged at 300 ×gfor 5 minutes. The supernatants were removed, and the cell pellets were resuspended in complete culture medium.The cells were then seeded into a 100 μg/μL poly‐lysine‐coated flask at a density of 1 × 105/cm2, and incubated at 37°C in a 5%CO2incubator. Culture media was replaced after 24 hours and changed every 3 days until the cells reached con fluence.A variety of morphologically distinct astrocytes with many long processes and small cell bodies were obtained, and an astrocytic network was established through cellular process connections.
Purity assessment of cultured primary astrocytes
Immuno fluorescence labeling for glial fibrillary acidic pro‐tein (GFAP) was performed to assess the purity of primary astrocytes, as previously described (Wang et al., 2011; Cheng et al., 2012, 2013). Astrocytes on coated coverslips in 24‐well plates were gently washed for 15 minutes with phosphate‐buff‐ered saline (PBS; 0.01 M, pH 7.4), three times for 5 minutes each, and fixed with 4% paraformaldehyde for 15 minutes.After rinsing with PBS, astrocytes were permeabilized with 0.3% Triton X‐100, treated with 0.1% bovine serum albumin,and then incubated for 30 minutes at room temperature.The cells were thereafter incubated with anti‐GFAP antibody(rabbit; 1:400; Cell Signaling Technology, Beverly, MA, USA)at 4°C overnight, and washed with PBS. The astrocytes were then incubated with secondary antibody (TRITC‐conjugated anti‐rabbit IgG; 1:800; Cell Signaling Technology) at room temperature in the dark for 2 hours and then washed. Hoechst 33258 was used to stain the nuclei. After the final wash, the cells were imaged under a fluorescence microscope (Leica Microsystems GmbH, Wetzlar, Germany). Negative controls lacking primary or secondary antibody were used.
Oxygen/glucose deprivation/reperfusion (OGD/R) and treatment of astrocytes
OGD/R was performed with primary astrocytesin vitroto mimic cerebral ischemic injury (Zhang et al., 2018). In brief,cultured astrocytes were seeded in 6‐well or 96‐well plates at a density of 2.5 × 104/mL. After 24 hours, the medium was replaced with fresh culture medium without fetal bovine se‐rum for an additional 24 hours. The cells were then pretreated with vehicle (control) or scutellarin (2, 10 or 50 μM; purity 99%; Shanghai Winherb Medical Technology, Shanghai, Chi‐na) for 30 minutes or the NADPH oxidase inhibitor apocynin(0.05, 0.25 or 0.5 mM; purity 99%; Sigma‐Aldrich, St. Louis,MO, USA) for 2 hours (Song et al., 2013a; Lu et al., 2014;Wang et al., 2016b). All drugs were dissolved in saline and were diluted to appropriate concentrations with culture me‐dium before administration. Afterwards, the astrocytes were subjected to OGD/R. Cells were incubated in deoxygenated glucose‐free DMEM (Gibco) in a hypoxic incubator contain‐ing 95% N2and 5% CO2for 2 hours followed by simulated reperfusion (normal culture conditions) for 22 hours. Control cells were incubated in a 5% CO2incubator for 24 hours.
Evaluation of cell viability by cell counting kit-8 assay
Cell viability was evaluated after 2 and 22 hours of reperfu‐sion using a cell counting kit‐8 assay (Dojindo Laboratories,Kumamoto, Japan). All the procedures followed the man‐ufacturer’s instructions. After 4 hours of incubation, the viability of astrocytes was assessed at 450 nm optical density(OD) with a SpectraMax M5 plate reader (Molecular De‐vices, Sunnyvale, CA, USA) using a 96‐well plate, and five replicate wells were assessed in each experiment.
Measurement of ROS
Intracellular levels of ROS were detected with 2′,7′‐dichlo‐rodihydrofluorescein diacetate (DCFH‐DA; Beyotime, Ji‐angsu, China) according to the manufacturer’s instructions.At the end of OGD/R, the medium was replaced with fresh serum‐free medium containing 10 μg/mL DCFH‐DA. The DCF fluorescence was measured in a microplate reader(PerkinElmer, Waltham, MA, USA) with excitation at 488 nm and emission at 525 nm.
Animal group assignment and scutellarin treatment
A total of 69 adult male specific‐pathogen‐free Sprague‐Dawley rats weighing 250—270 g and 6—8 weeks old were purchased from the Experimental Animal Center of Sun Yat‐sen University of China. The rats were bred in a room at 21—25°C and a humidity of 55—65% under a 12‐hour dark/light cycle. The rats were allowed free access to food and water.
To investigate the protective effect of scutellarin on cere‐bral ischemic injury, rats were randomly divided into four groups: sham (sham operation + saline), MCAO (MCAO +saline), low‐dose scutellarin (MCAO + 50 mg/kg scutellarin)and high‐dose scutellarin (MCAO + 100 mg/kg scutellarin)(n= 15). The rats in each group were given an intraperito‐neal injection of scutellarin dissolved in saline at 2 hours before and at 12, 24, 36, 48 and 60 hours after tMCAO.
To assess whether NOX2 regulates Cx43 in the animal model, nine rats were randomly divided into three groups:sham (sham operation + saline), MCAO (MCAO + saline)and MCAO + apocynin (50 mg/kg). Apocynin (50 mg/kg)was injected intraperitoneally 30 minutes before tMCAO(Jing et al., 2015; Chen et al., 2016).
Rats were killed 3 days after MCAO for western blot assay,enzyme‐linked immunosorbent assay (ELISA) and immu‐nohistochemical staining. Sham rats received saline intra‐peritoneally in the same volume and same time points as the MCAO groups, but the MCA was not occluded.
Cerebral ischemic injury model
The tMCAO model was produced as previously described(Guo et al., 2012). Brie fly, the rats were anesthetized with gas anesthesia (2% halothane in 70% N2O/30% O2for induction and 1% halothane in 70% N2O/30% O2for maintenance).A nylon mono filament (Johnson & Johnson, Brussels, Bel‐gium) coated with impression material (3 M Dental Prod‐ucts, St Paul, MN, USA) was inserted into the right internal carotid artery to block blood flow at the origin of the middle cerebral artery. Regional cerebral blood flow was monitored during surgery. After 2 hours of occlusion, the filament was pulled out to allow reperfusion, and the rats were kept in an intensive care system (ThermoCare Inc., Incline Village,NV, USA) for 4 hours.
Neurologic de ficit score
Three days after tMCAO, the neurologic deficit score was assessed by an examiner who was unaware of the treatment protocols. Neurological deficit was evaluated according to a five‐point scale established by Bederson et al. (1986), as follows: 0 = no neurological symptoms; 1 = unable to fully extend the front paw on the contralateral side; 2 = circling to the contralateral side; 3 = falling to the contralateral side; 4= unable to walk spontaneously.
Measurement of infarct size
Cerebral infarct size was measured by 2,3,5‐triphenyltetra‐zolium chloride (TTC) staining 3 days after tMCAO (Pei et al., 2012). Brie fly, the rats were decapitated after neurological evaluation, and the brains were removed quickly and then sliced into six uniform coronal sections of 2 mm thickness each. The sections were stained in 2% TTC (Sigma‐Aldrich)at room temperature for 15 minutes and then fixed in 10%formaldehyde solution. Normal brain tissue was stained red,while the infarcted areas were pale. The posterior surface of each slice was photographed and analyzed with Sigma Scan Pro5.0 software. Infarct area was calculated as a percentage of the area of the contralateral hemisphere.
Western blot assay for brain tissues and astrocytes
Western blot assay was performed as previously described(Wang et al., 2011). Protein was obtained from tissues from the ischemic hemisphere (ipsilateral side of the brain) and the non‐ischemic hemisphere (contralateral side of the brain), from the corresponding areas of sham‐operated rats, as well as from astrocytes in each group using a total protein extraction kit (Jiancheng Biological Institute, Nan‐jing, China). Protein concentrations were determined with a bicinchoninic acid protein assay kit (Jiancheng Biological Institute). Equal amounts of protein were separated by sodi‐um dodecyl sulfate polyacrylamide gel electrophoresis, and transferred onto polyvinylidene fluoride membranes (Pall,Port Washington, NY, USA). All membranes were blocked with 5% non‐fat dry milk diluted in Tris‐buffered saline con‐taining 0.1% Tween‐20 at room temperature for 2 hours and incubated overnight at 4°C with rabbit anti‐NOX2 polyclonal antibody (1:1000; Abcam, Cambridge, UK), rabbit anti‐Cx43 polyclonal antibody (1:1000; Abcam), rabbit anti‐caspase‐3 polyclonal antibody (1:1000; Abcam) or mouse anti‐rat glyc‐eraldehyde‐3‐phosphate dehydrogenase (GADPH) mono‐clonal antibody (1:1000; Boster, Pleasanton, CA, USA). The membranes were then incubated with a horseradish perox‐idase‐conjugated polyclonal goat anti‐rabbit or monoclonal goat anti‐mouse secondary antibody (1:3000; Cell Signaling Technology) for 2 hours at 37°C. After washing three times in 0.1% Tween‐20 in Tris‐buffered saline, immunoreactive bands were detected by a chemiluminescence system (Bio‐Rad, Hercules, CA, USA) and analyzed with ImageJ software(National Institutes of Health, Bethesda, MD, USA). Protein expression was normalized against GAPDH. All experiments were performed in triplicate.
ELISA detection of oxidative stress-related protein expression
The ipsilateral brain samples (n= 6 for each group) were weighed and homogenized with PBS. All the procedures followed the manufacturer’s instructions. Briefly, the su‐pernatants of the cerebral cortex samples were collected to determine the total protein concentration using the bicin‐choninic acid kit (Jiancheng Biological Institute). The levels of 8‐hydroxydeoxyguanosine (8‐OHdG), 4‐hydroxy‐2‐non‐enal (4‐HNE) and 3‐nitrotyrosin (3‐NT) were determined using ELISA assay kits (Cusabio Biotech, Wuhan, China)and were quanti fied with a microplate reader at 450 nm. The levels of 8‐OHdG, 4‐HNE and 3‐NT were expressed as a percentage of the control group.
Double immuno fluorescence labeling of caspase-3 and NeuN
Brains were harvested and paraffin sections were prepared.Following dewaxing, rehydration and incubation with anti‐gen retrieval buffer (pH 8.0), sections were treated with 0.3%Triton X‐100 and 2% bovine serum albumin in PBS at room temperature for 30 minutes. For double‐labeling, sections were incubated with mouse anti‐NeuN antibody (1:500; Cell Signaling Technology) plus rabbit anti‐caspase‐3 antibody(1:1000; Abcam) for 24 hours at 4°C. Following three rinses with PBS, the sections were incubated with TRITC‐conju‐gated anti‐rabbit IgG (1:500; Sigma‐Aldrich) and FITC‐con‐jugated anti‐mouse IgG (1:200; Sigma‐Aldrich) at room temperature for 1 hour in the dark. Finally, the slides were coverslipped in antifade mounting media (Wuhan Goodbio Technology Co., Ltd., China) and examined by fluorescence microcopy (Zeiss, Oberkochen, Germany) by an investiga‐tor blinded to the experimental design. Control experiments included absence of the primary or secondary antibody. The sham group served as a negative control.
Statistical analysis
Statistical analyses were performed using SPSS 16.0 software(SPSS, Chicago, IL, USA). All variables are expressed as the mean ± SD. One‐way analysis of variance followed by a Tukey‐Kramer multiple comparison test was used to analyze the differences among groups. A value ofP< 0.05 was con‐sidered statistically signi ficant.
Results
Three-dimensional docking model of scutellarin binding with NOX2
The interaction between scutellarin and NOX2 was exam‐ined using automated docking calculations, and the com‐plete view of the three‐dimensional docking model of scutel‐larin with NOX2 is shown in Figure 1. The chemical structure of scutellarin, a well‐known flavonoid, is shown in Figure 1A.The three‐dimensional structures of NOX2 and scutellarin fit well together (Figure 1B). Scutellarin, as a flavonoid com‐pound, has antioxidant potential. The docking model also in‐dicated that scutellarin selectively binds to NOX2 with a high affinity. Enzymatic assay showed that scutellarin is a NOX2 inhibitor, with a half maximal inhibitory concentration value of 151.6 ± 11.2 mM. These results indicate that NOX2 is a speci fic target of scutellarin (Figure 1).
Scutellarin protected astrocytes against OGD/R
Purity of cultured astrocytes
A variety of morphologically distinct astrocytes with many long processes and small cell bodies were obtained, and an astrocytic network was established through cellular process connections (Figure 2). Immuno fluorescence for GFAP was used to observe the morphology of astrocytes and to assess their purity. All cell nuclei were labeled with 4′,6‐diamidi‐no‐2‐phenylindole (DAPI). The percentage of GFAP‐posi‐tive cells was greater than 98%.
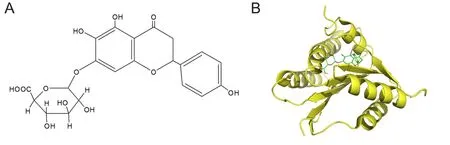
Figure 1 Scutellarin selectivity binds to NOX2 with high affinity.

Figure 2 Expression of GFAP in astrocytes detected by immuno fluorescence staining.
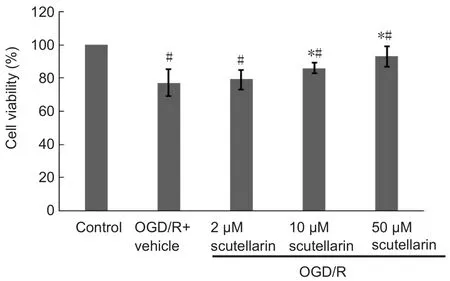
Figure 3 Scutellarin protects astrocytes against oxygen/glucose deprivation/reoxygenation (OGD/R)-induced cytotoxicity (cell counting kit-8 assay).
Scutellarin protected astrocytes against OGD/R-induced cytotoxicity
To evaluate the effect of scutellarin pretreatment on OGD/R injury in astrocytes, astrocyte cultures were incubated with culture medium in the presence or absence of scutellarin(2, 10 or 50 μM) for 30 minutes, followed by OGD/R expo‐sure (2‐hour period of OGD followed by a 22‐hour period of reperfusion). The protective effects of scutellarin against OGD/R‐induced astrocyte injury were determined using the cell counting kit‐8 assay. As shown in Figure 3, pretreat‐ment with 10 or 50 μM scutellarin significantly improved the viability of astrocytes subjected to OGD/R, compared with the vehicle group (P< 0.05).
The potential cytotoxicity of scutellarin on normal cul‐tured astrocytes was evaluated using the cell counting kit‐8 assay. Normal astrocytes were incubated with scutellarin at concentrations of 2, 10 or 50 μM, and viability was com‐pared with that of untreated cells. There was no signi ficant difference in viability among the three concentration groups(data not shown), demonstrating that scutellarin is not cyto‐toxic to cultured astrocytes. Taken together, these findings show that scutellarin protects against OGD/R‐induced cyto‐toxicity in a dose‐dependent manner.
Scutellarin inhibited NOX2 expression in astrocytes subjected to OGD/R
We investigated whether scutellarin treatment affects the ex‐pression of NOX2 in OGD/R‐exposed astrocytes. The protein levels of NOX2 in astrocytes in the presence or absence of scutellarin (2, 10 or 50 μM) were evaluated by western blot assay. NOX2 expression was significantly greater in OGD/R‐exposed astrocytes than in control astrocytes (P< 0.05). Fur‐thermore, the expression of NOX2 in cells treated with 10 or 50 μM scutellarin was significantly decreased compared with the vehicle group. There was no signi ficant difference in NOX2 expression between the 2 μM scutellarin group and the vehicle group (Figure 4A). These results indicate that scutellarin inhib‐its NOX2 expression in astrocytes subjected to OGD/R injury.
Scutellarin enhanced Cx43 expression in astrocytes subjected to OGD/R
The changes in Cx43 protein expression induced by OGD/R(2 hours of OGD followed by 22 hours of reperfusion) and the effect of scutellarin (2, 10 or 50 μM) on these changes were in‐vestigated by western blot assay (Figure 4B). There was an up‐regulation of Cx43 expression in astrocytes subjected to OGD/R compared with the control group (P< 0.05). Furthermore,Cx43 expression in cells treated with 10 or 50 μM scutellarin was signi ficantly increased compared with the vehicle group.There was no significant difference in Cx43 expression be‐tween the 2 μM scutellarin group and the vehicle group. These results demonstrate that scutellarin enhances Cx43 expression in astrocytes subjected to OGD/R‐induced injury.

Figure 4 Scutellarin inhibits NOX2 (A) but enhances Cx43 (B) expression in astrocytes subjected to OGD/R (western blot assay).

Figure 5 Scutellarin inhibits the OGD-induced intracellular accumulation of ROS (A) and caspase-3 expression (B) in astrocytes subjected to OGD/R detected using spectrophotometric assay and western blot assay, respectively.
Scutellarin inhibited intracellular accumulation of ROS in astrocytes exposed to OGD/R
Intracellular ROS accumulation caused by OGD exposure was markedly reduced by scutellarin pretreatment. As shown in Figure 5A, compared with normoxic astrocytes,astrocytes subjected to OGD/R (2 hours of OGD followed by 22 hours of reperfusion) showed increased ROS produc‐tion. Scutellarin (10, 50 μM) markedly reduced this increase in ROS generation, while 2 μM scutellarin had no effect.Scutellarin at 50 μM reduced ROS levels more than at 10 μM. These data indicate that scutellarin plays a role in ROS elimination after OGD/R.
Scutellarin downregulated caspase-3 in astrocytes subjected to OGD/R
Caspase‐3 expression in OGD/R‐exposed astrocytes and the effect of scutellarin were investigated by western blot assay(Figure 5B). Caspase‐3 expression was upregulated in astro‐cytes subjected to OGD/R compared with the control group(P< 0.001). Furthermore, the expression levels of caspase‐3 in cells treated with 10 or 50 μM scutellarin were signi ficantly decreased compared with the vehicle group (P< 0.05). There was no signi ficant difference in expression levels of caspase‐3 between the 2 μM scutellarin group and the vehicle group (P> 0.05). These results indicate that scutellarin downregulates caspase‐3 in astrocytes subjected to OGD/R, suggesting that scutellarin has an anti‐apoptotic effect in these cells.
Protective effect of scutellarin against cerebral ischemic injury in rats
Effects of scutellarin on neurological function and infarct area in rats with cerebral ischemic injury
As shown in Table 1, the sham group did not have any neu‐rological de ficits. In comparison, severe neurological de ficits were observed in the MCAO group at 1 day. Neurological de ficits were signi ficantly improved by scutellarin treatment,whether low‐ or high‐dose, 1 day after reperfusion (P< 0.01,vs.MCAO group). In addition, there was no recovery or change in neurological de ficit score in any group 3 days after reperfusion(data not shown). As shown in Figure 6A, B, there was no de‐tectable infarction in the sham group, but a large infarct area was observed in the MCAO group at 3 days. Compared with the MCAO group, infarct area was significantly reduced by scutellarin treatment, whether low‐ or high‐dose, 3 days after reperfusion (P< 0.01,vs. MCAO group).
Scutellarin inhibited NOX2 expression in the brain of rats with cerebral ischemic injury
As shown in Figure 7A, the effects of scutellarin (50 or 100 mg/kg) on NOX2 expression in the brain 3 days after reper‐fusion were evaluated by western blot assay. NOX2 expres‐sion was similar on the contralateral side of the brain among the various groups, suggesting no effect of drug treatment on expression in this hemisphere. On the ipsilateral side of the brain, the expression levels of NOX2 were greater in the MCAO group than in the sham group (P< 0.05). NOX2 expression in the ipsilateral hemisphere was lower in rats treated with scutellarin (50 and 100 mg/kg) compared with the MCAO group (P< 0.05).
Scutellarin enhanced Cx43 expression in the brain of rats with cerebral ischemia/reperfusion injury
Western blot assay was used to examine the effect of scutel‐larin (50 or 100 mg/kg) on Cx43 protein levels in the ipsilat‐eral side of the brain. Cerebral ischemia/reperfusion induced a robust increase in Cx43 expression at 3 days (P< 0.05).Cx43 expression was markedly greater in the scutellarin 50 and 100 mg/kg groups than in the MCAO group at 3 days (P< 0.05; Figure 7B).
Scutellarin reduces 8-OHdG, 4-HNE and 3-NT levels after cerebral ischemic injury
Oxidative damage to DNA was assessed by measuring 8‐OHdG levels. Lipid peroxidation was assessed by measur‐ing 4‐HNE levels. Protein oxidative damage was evaluated by quantifying 3‐NT levels. The levels of 8‐OHdG, 4‐HNE and 3‐NT were higher in the MCAO group than in the sham group 3 days after reperfusion. Scutellarin markedly sup‐pressed this ischemic injury‐induced increase in the levels of these three indicators (Figure 8).
Scutellarin downregulated caspase-3 in neurons in the ischemic penumbra
Immunofluorescence double labeling for caspase‐3 and NeuN was carried out to detect apoptosis in neurons in the penumbra after tMCAO injury and to evaluate the ef‐fect of scutellarin. Immunostaining showed that capsase‐3 was localized in the nuclei of apoptotic cells, while NeuN had a cytoplasmic and perinuclear localization in mature neurons (Figure 9). Cells double‐labeled for caspase‐3 and NeuN in the penumbra were more numerous in the MCAO group compared with the sham group (P< 0.05). Caspase‐3 expression was significantly decreased in the scutellarin(50 and 100 mg/kg) treatment groups compared with the MCAO group (P< 0.05). There was no signi ficant difference in caspase‐3/NeuN‐double labeling between the scutellarin group and the sham group (P> 0.05). These findings indi‐cate that scutellarin protects against neuronal apoptosis in the penumbra region after tMCAO injury (Figure 9).
Treatment with apocynin increased Cx43 protein expression in OGD/R-exposed astrocytes and cerebral ischemic rats
Cx43 protein expression in the presence or absence of apocynin (0.05, 0.25, 0.5 mM) in OGD‐exposed astrocytes 22 hours after reperfusion was evaluated by western blot as‐say. Cx43 expression in OGD/R‐exposed astrocytes was sig‐ni ficantly greater than that in the control group (P< 0.001).Furthermore, the expression levels of Cx43 in cells treated with 0.05, 0.25 or 0.5 mM apocynin were significantly in‐creased compared with the vehicle group (Figure 10A).
The effect of apocynin (50 mg/kg) treatment on Cx43expression 3 days after reperfusionin vivowas evaluated by western blot assay. NOX2 expression in the ipsilateral hemisphere was greater in the MCAO group than in the sham group (P< 0.001). Cx43 expression was greater in rats treated with apocynin than in the MCAO group (P< 0.001;Figure 10B). These results suggest that Cx43 expression is regulated by NOX2 in astrocytes and in the brain of rats ex‐posed to ischemic injury.
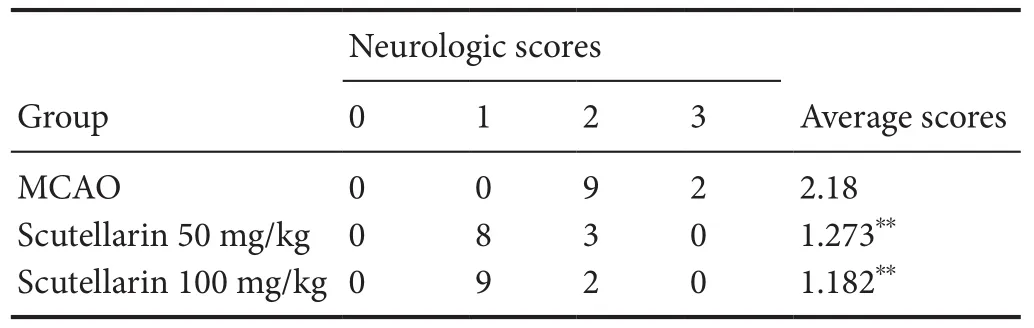
Table 1 Effects of scutellarin on neurological de ficit score in rats with cerebral ischemic injury 3 days post-injury
Discussion
In the present study, molecular docking study showed that scutellarin and NOX2 are effectively docked with a good fit at the active site. Scutellarin decreased the neurological de fi‐cit score, infarct area, apoptosis and ROS levels following ischemic injury in a dose‐dependent manner. These effects of scutellarin were associated with the downregulation of NOX2 and the upregulation of Cx43 in the brainin vivoand in astrocytesin vitro. Treatment with apocynin markedly in‐creased Cx43 expression both thein vivoandin vitromod‐els, suggesting that scutellarin has a potent NOX2 inhibiting activity. Given this unique neuroprotective action of scutel‐larin, it may have therapeutic effectiveness, in combination with thrombolysis, for the treatment of ischemic stroke.
Our results are consistent with previous studies showing that scutellarin is protective in cerebral ischemic injury modelsin vivoandin vitro(Guo et al., 2011, 2012; Wang et al., 2011,2016a; Pei et al., 2012; Chai et al., 2013; Fang et al., 2015).In ourin vitroexperiment, pretreatment with 10 and 50 μM scutellarin signi ficantly improved the viability of OGD/R‐ex‐posed astrocytes (85.81 ± 3.20% and 92.75 ± 6.05%, respec‐tively).in vivo, high‐dose scutellarin (100 mg/kg) was more effective than low‐dose scutellarin (50 mg/kg) at reducing the neurologic de ficit and infarct area. Therefore, high‐dose scute‐llarin effectively protects against tMCAO injury. Our finding that the neuroprotective effect of scutellarin is concentra‐tion‐dependent is in accordance with previously studies (Jiang et al., 2011a, 2011b).
Oxidative stress is one of the main pathogenetic events in cerebral ischemic injury (Manzanero et al., 2013; Feng et al.,2016). Oxidative stress occurs when ROS formation exceeds endogenous antioxidant capacity (Tian et al., 2016). ROS signaling has been shown to be bene ficial, and therefore, the timing of anti‐oxidant therapy is critical and is likely a key reason for the failure of clinical trials. Moreover, intracellu‐lar ROS cause the oxidation of a large number of molecules,such as DNA, lipids and proteins. 8‐OHdG, 4‐HNE and 3‐NT are oxidative stress makers that indicate the degree of oxi‐dative or peroxidative damage to DNA, lipids and proteins,respectively (Chen et al., 2014b; Fan et al., 2016). Scutellarin protects against neuronal injury in rat cerebral ischemia models partly because of its antioxidant property (Tang et al.,2014), which contributes to reducing oxidative stress (Wang et al., 2016c). Here, we found that scutellarin suppresses the increase in oxidative stress makers induced by cerebral ischemia/reperfusion in rats. Our results demonstrate that the neuroprotective effect of scutellarin is through oxidative stress suppression, consistent with a previous study (Guo et al., 2013). At excessive levels, intracellular ROS alter cellular metabolism and the internal environment, ultimately leading to apoptosis (Olmez and Ozyurt, 2012). Among the caspase family members, caspase‐3 is a critical mediator of apoptosis(Elmore, 2007). This suggests that the neuroprotective effect of scutellarin involves an anti‐apoptotic action.
NOX is a key enzyme producing ROS (such asand H2O2) in cerebral ischemic injury (Abramov et al., 2007), and NOX activity is among the three distinct processes that gen‐erate ROS (Kahles et al., 2007). Several studies strongly indi‐cate an important role of NOX2 in stroke‐related oxidative stress injury (Chen et al., 2009). NOX is known to be involved in the pathogenesis of stroke (Tang et al., 2012). In partic‐ular, NOX2 plays a critical role in cerebral ischemic injury(Carbone et al., 2015). Several studies show that inhibition of NOX2 expression protects against cerebral ischemic damage(Song et al., 2013b; Shichinohe et al., 2015). Here, we found that cerebral ischemic injury upregulates NOX2, and that scutellarin exerts neuroprotection likely by downregulating the protein. Our results suggest that scutellarin can be used as an inhibitor of NOX2 to treat cerebral ischemic injury.
Gap junctions, an important component in direct cell‐to‐cell communication, play a central role in the pathogenesis of ischemic brain injury (Deng et al., 2014). Connexin proteins constitute the main components of gap junctions, and Cx43 is one of the most abundant gap junction proteins found in the brain (Contreras et al., 2004; Giaume and Theis, 2010). Al‐though Cx43 is involved in cerebral ischemic injury, the role of the protein in ischemia remains controversial. Nakase et al.(2003) showed that Cx43 protects neurons from cerebral isch‐emic injury. In contrast, another study showed that inhibition of Cx43 upregulation is neuroprotective in cerebral ischemic injury (Deng et al., 2014). Our previous study showed that upregulation of Cx43 by Tongxinluo administration was neu‐roprotective against cerebral ischemic injury (Cheng et al.,2017). In our current study, scutellarin upregulated Cx43 ex‐pression in rat primary astrocytes and the tMCAO rat model.This suggests that Cx43 has a neuroprotective role in cerebral ischemic injury.
While oxidative stress is associated with stroke, it is unclear how it impacts cell signaling. Oxidative stress affects not only intracellular signaling pathways but also intercellular signaling mechanisms, such as gap junctions. Oxidative stress‐depen‐dent interactions between signal transduction pathways and intercellular gap junctional communication has been shown to regulate gene expression (Upham and Trosko, 2009). There is crosstalk between Cx43 and NOX in the pathology of some diseases, such as heart failure and diabetic hypogonadism (Xu et al., 2016). Our present findings show that scutellarin treat‐ment downregulates NOX2 but upregulates Cx43. Further‐more, NOX2 inhibition increased Cx43 expression in both thein vivoandin vitroischemia models. This novel finding shows that NOX2 regulates Cx43 expression in ischemic injury.
The mechanism by which NOX2 interacts with Cx43 in the cerebral ischemia models is unclear. Cerebral ischemic injury activates NOX enzymes, which not only generate radicals,such asbut also induce the production of otherROS. These ROS play important roles in the regulation of cell function, and several of these induce Cx43 expression. The mechanism may involve ROS‐regulated activation of several kinases and transcription factors, such as c‐Jun N‐terminal kinase, mitogen‐activated protein kinase and P38. These mol‐ecules regulate Cx43 expression (Berthoud and Beyer, 2009).NOX2 is widely distributed in brain tissue. However, cerebral ischemic injury induces not only NOX2 expression but also other NOX proteins such as NOX4. Participation of these molecules in the induction of Cx43 expression is also likely.The roles of these individual proteins need to be investigated by selective gene knockdown.
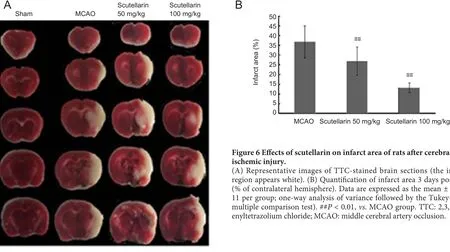
Figure 6 Effects of scutellarin on infarct area of rats after cerebral ischemic injury.

Figure 7 Scutellarin inhibited NOX2 but enhanced Cx43 expression in the ipsilateral hemisphere of rats with cerebral ischemic injury 3 days after reperfusion (western blot assay).
There are some limitations to this study. For example, the role of Cx43 in central nervous system injury is unclear. A large number of studies demonstrate that the role of Cx43 in cerebral ischemic injury is complex, involving phosphorylat‐ed, cytoplasmic and plasma membrane forms of the protein as well as translocation from the cell membrane to the cyto‐plasm, which could not be examined in this study. Second,we did not investigate whether Cx43 knockdown abolishes the neuroprotective effect of scutellarin in astrocytes. Fur‐ther studies are needed to tackle these unresolved issues.
Author contributions:XC and YFC designed this study. XC, YL and JBS drafted the manuscript. PKKY and MZD helped to edit and modify the language. GSS and QSH performed the vitro part study. SL performed molecular docking study. YL, XC, LHZ, YH and LXW performedthe vitro part study and reviewed this paper. XC completed this paper revision. All authors approved the final version of this paper.

Figure 8 Scutellarin reduces the increase in the levels of 8-OHdG (A), 4-HNE (B) and 3-NT (C) induced by cerebral ischemic injury(enzyme-linked immunosorbent assay).
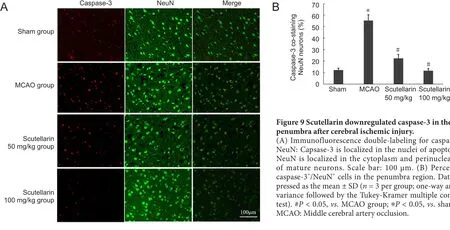
Figure 9 Scutellarin downregulated caspase-3 in the penumbra after cerebral ischemic injury.
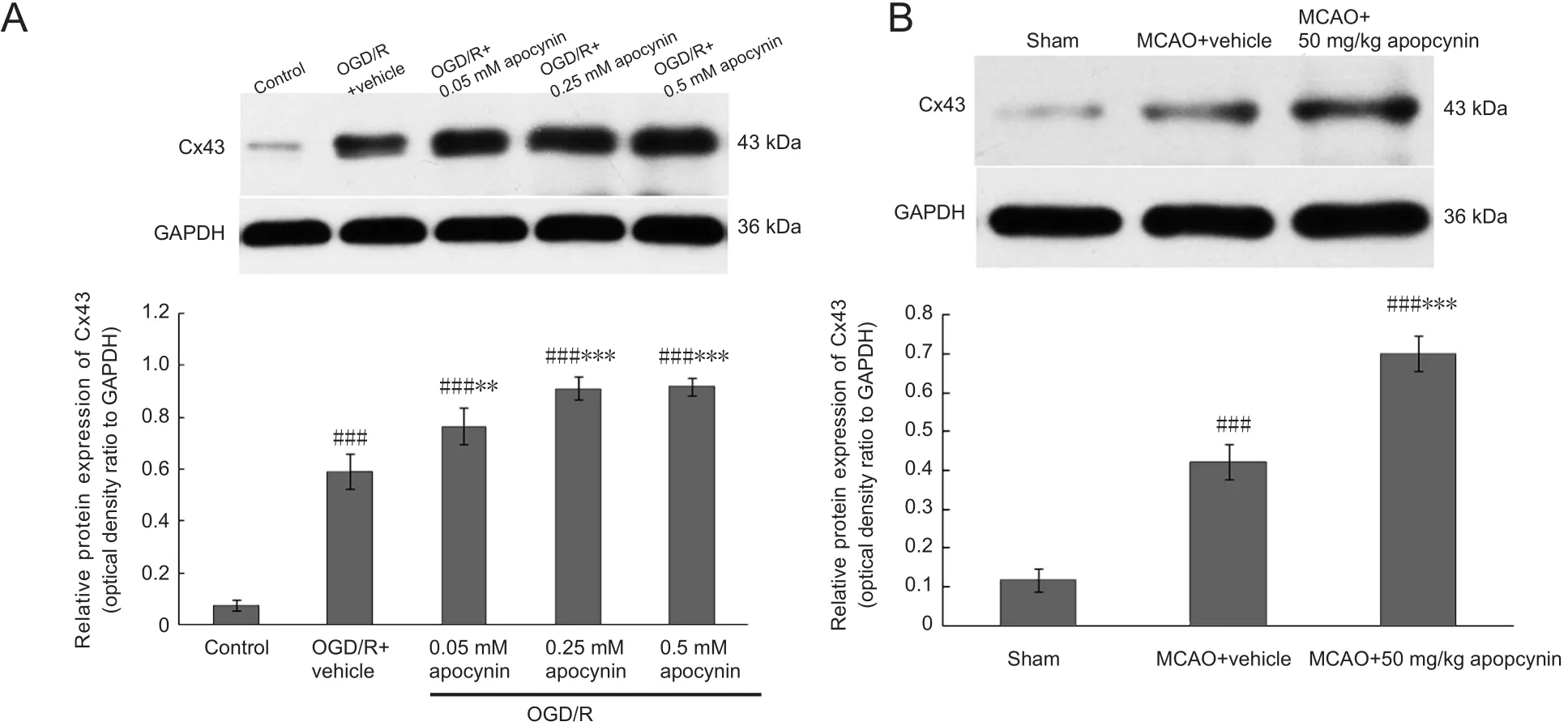
Figure 10 Apocynin markedly increased Cx43 protein expression in OGD-exposed astrocytes and in rats with cerebral ischemia/reperfusion injury (western blot assay).
Conflicts of interest:None declared.
Financial support:This study was financially supported by the National Natural Science Foundation of China, No. 81303115, 81774042,81771353; the Natural Science Foundation of Guangdong Province of China, No. S2013040016915; the Science and Technology Program of Guangzhou City of China, No. 201508020050, 201604020003; the Pearl River S&T Nova Program of Guangzhou, the Postdoctoral Foundation of China, No. BBK42913K09, 201003345, BBH429151701; a grant from the Hong Kong Scholar Program, Guangzhou University of TCM 2017 High Level University Construction Program, No. A1-AFD018171Z11096;a grant from the Specialty Program of Guangdong Province Hospital of Traditional Chinese Medicine of China, No. YN2016MJ07, YN-2015QN16, YN2015B2025. The funders had no roles in the study design,conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement:The study was approved by the Animal Care and Use Committee at Sun Yat-sen University of China(approval No. 2016006).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- In Memoriam: Ray Grill (1966–2018)
- Structural brain volume differences between cognitively intact ApoE4 carriers and non-carriers across the lifespan
- Roles of Eph/ephrin bidirectional signaling during injury and recovery of the central nervous system
- Neuroplasticity, limbic neuroblastosis and neuro-regenerative disorders
- A tissue-engineered rostral migratory stream for directed neuronal replacement
- Targeting the noradrenergic system for anti-in flammatory and neuroprotective effects:implications for Parkinson’s disease

