Drug repurposing for neuroregeneration in multiple sclerosis
Multiple sclerosis (MS) is a chronic, in flammatory and neu‐rodegenerative disease of the central nervous system (CNS)affecting at least 2.5 million people worldwide. While the relapsing subtypes of MS are well treatable, the disease per se remains incurable and results in progressive disability. Its etiology is complex and far from being understood. How‐ever, it is well‐established that its central histopathological hallmark is demyelination ‐ the autoimmune destruction of myelin sheaths. These elaborate structures wrap around ax‐ons electrically isolating them and provide accelerated elec‐trical transmission as well as physical and trophic support in the brain and spinal cord (Lassmann, 2018). Demyelination impairs axonal integrity which leads to permanent disability(Lassmann, 2018). Whereas relapsing MS (RMS) which is most common at disease onset is characterized by episodes of autoimmune attacks (relapse) followed by spontaneous partial functional recovery (remission), most patients even‐tually develop a progressive disease course. Progressive MS stages, however, are mainly characterized by reduced or absent immune cell in filtration but ongoing neurodegenera‐tion. Neuroregeneration in MS, on the other hand, basically refers to myelin repair ‐ a process that can repair some of the existing lesionsviarecruitment of resident oligodendroglial precursor cells (OPCs) and neural stem cells (NSCs) which can differentiate and produce new axonal myelin sheaths restoring axonal integrity (Franklin and Ffrench‐Constant,2017). However, the repair capacity of precursor‐ and stem cells declines with age and disease progression. Moreover,differences in the extent of myelin regeneration can be ob‐served between lesions and patients, potentially indicating heterogeneous underlying mechanisms which interfere with myelin restoration (Franklin and Ffrench‐Constant, 2017).In this regard, several oligodendroglial differentiation in‐hibitors have been identi fied which are supposed to prevent successful cell maturation in an in flammatory environment(Kremer et al., 2011).
Of note, whereas a number of RMS treatments exist that successfully reduce relapse rate, none of the currently avail‐able disease‐modifying therapies (DMT) has been shown to effectively enhance lesion repair. Hence, there is an unmet clinical need to develop new disability‐reversing therapies aiming at the preservation of both axons and oligodendrog‐lial cells. Different strategies for enhancing remyelination are conceivable including cell‐based therapies relying on exogenous cell supply (Scolding et al., 2017), the neutral‐ization of differentiation inhibitors (Kremer et al., 2011) or direct stimulatory approaches for improved adult oligoden‐drogenesis (Kremer et al., 2016). As cell‐based therapies are restricted in their practical feasibility, stimulation and pro‐motion of endogenous cell‐based repair represents a more promising avenue. To this end, the development of new drugs, acting on inhibitory or stimulatory glial pathways,and the repurposing of existing drugs represent possible approaches. The strategy of drug repurposing offers the ad‐vantage of identifying new targets for known drugs already clinically approved for other indications minimizing risks and costs. Hence, high‐throughput drug‐screenings (Mei et al., 2014) as well as computational drug‐repurposing ap‐proaches (Azim et al., 2017) are being used. In this context,recent screenings identi fied the ability of the histamine H1 receptor blocker clemastine, the muscarinic receptor antag‐onist benztropine and the atypical neuroleptic quetiapine, to promote myelin repair (Mei et al., 2014). On the other hand,ocrelizumab, a monoclonal antibody directed against CD20 on B cells and initially designed for treatment of rheumatoid arthritis (RA), demonstrated efficacy on disability progres‐sion in primary progressive MS patients (Montalban et al.,2017; Kremer et al., 2018). Moreover, the phosphodiester‐ase inhibitor ibudilast, an approved asthma treatment, was found to reduce brain atrophy in progressive MS patients(Kremer et al., 2018). Investigating its effects in secondary progressive MS, a clinical study found that siponimod, a novel sphingosine‐1‐phosphate (S1P)‐receptor modulator,reduced con firmed disability progression by 21% over three months of treatment (Kappos et al., 2018). Finally, in our recent own contribution to the field, we closer investigated the effect of teriflunomide, currently used as an immuno‐modulatory DMT for RMS. In our preclinical study we fo‐cused on oligodendroglial cells, their differentiation capacity and their ability to differentiate and wrap around central nervous system (CNS) axons. We could demonstrate that teriflunomide ‐ beyond its role as an immune modulator actingviainhibition of pyrimidine biosynthesis in activated lymphocytes ‐ can also significantly promote OPC differ‐entiation and internode formation (Figure 1), particularly when applied early and in pulses (Göttle et al., 2018). Futurein vivostudies will reveal to what degree teri flunomide also holds promise for remyelination in hostilein vivosettings and whether such repair processes could explain the re‐duced disability progression as observed in the terifluno‐mide multiple sclerosis trials (TEMSO, ClinicalTrials.gov number NCT00134563; TOWER, ClinicalTrials.gov number NCT00751881) (Freedman et al., 2018). At the current time‐point it can therefore be concluded that, given the hetero‐geneity of clinical MS subtypes and the dynamics of lesion development in time and space, it will be challenging to find the appropriate window of opportunity for therapy — no matter if using newly designed drugs or repurposed ones.
Cited research work in the laboratory of the authors was supported by the Jürgen Manchot Foundation Düsseldorf, by the research commission of the medical faculty of Heinrich-Heine-University of Düsseldorf and by a grant of Sano fiGenzyme. The MS Center at the Department of Neurology is supported in part by the Walter and Ilse Rose Foundation and the James and Elisabeth Cloppenburg, Peek&Cloppenburg Düsseldorf Stiftung.
Patrick Küry, David Kremer, Peter Göttle*Department of Neurology, Medical Faculty, Heinrich‐Heine‐University, Düsseldorf, Germany
*Correspondence to:Peter Göttle, Ph.D.,Peter.Goettle@uni-duesseldorf.de.
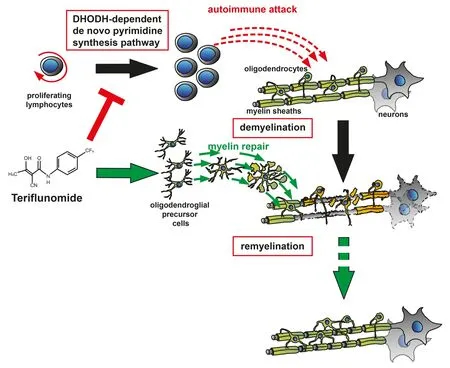
Figure 1 Teri flunomide’s mode of action.
orcid:0000-0001-6830-0956 (Peter Göttle)0000-0002-2654-1126 (Patrick Küry)
Accepted:2018-06-02
doi:10.4103/1673-5374.235242
Copyright transfer agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Tetsuya Akaishi, Tohoku University, Japan; Anna Maria Colangelo, University of Milano-Bicocca, Italy.
Additional file:Open peer review reports 1, 2.
- 中国神经再生研究(英文版)的其它文章
- In Memoriam: Ray Grill (1966–2018)
- Structural brain volume differences between cognitively intact ApoE4 carriers and non-carriers across the lifespan
- Roles of Eph/ephrin bidirectional signaling during injury and recovery of the central nervous system
- Neuroplasticity, limbic neuroblastosis and neuro-regenerative disorders
- A tissue-engineered rostral migratory stream for directed neuronal replacement
- Targeting the noradrenergic system for anti-in flammatory and neuroprotective effects:implications for Parkinson’s disease

