Current surgical treatments for Parkinson’s disease and potential therapeutic targets
Darrin J. Lee, Robert F. Dallapiazza, Philippe De Vloo, Andres M. Lozano
Division of Neurosurgery, Toronto Western Hospital, Department of Surgery, University of Toronto, Toronto, Canada
Abstract Currently, the most common surgical treatment for Parkinson’s disease is deep brain stimulation (DBS).This treatment strategy is typically reserved for bradykinesia, rigidity and tremor in patients who no longer respond to medication in a predictable manner or who suffer medication‐induced dyskinesias. In addition to DBS, ablative procedures like radiofrequency, radiosurgery and focused ultrasound are also utilized for select tremor symptoms. In this review, we discuss evolving surgical techniques, targets, and emerging technology. In addition, we evaluate potential paradigm shifts in treatment, including gene therapy, im‐munotherapy and cell transplantation. While these new techniques and treatment options are still in their infancy, advances in Parkinson’s disease treatment are rapidly expanding.
Key Words: Parkinson’s disease; deep brain stimulation; pallidotomy; thalamotomy; focused ultrasound; gene therapy; immunotherapy; cell transplantation
Introduction
Parkinson’s disease (PD) is a chronic, progressive neurodegenerative disorder that affects the motor system and has variable non‐motor components including cognitive and autonomic changes in the later stages of the disease. PD is characterized by al‐tered activity within the basal ganglia and is typi fied by profound degeneration of dopaminergic neurons within the substantia nigra pars compacta. Medi‐cations to replace or prevent degradation of dopa‐mine (i.e., levodopa, catechol‐O‐methyl transferase inhibitors, dopamine agonists, monoamine oxidase inhibitors) have had a dramatic impact on the lives of PD patients. Surgical treatments involving lesioning the basal ganglia also have a long history. Despite the initial decline in surgery following the discovery and clinical introduction of levodopa in 1961, surgery has become a standard adjunct to medical treatment.Since the late 1940’s, there have been over 12,000 ar‐ticles published on the surgical management of Par‐kinson’s disease (Lozano et al., 2018).
The first published surgical treatments for PD oc‐curred in the early 1950s, and involved lesioning regions of the basal ganglia (pallidotomy and thalam‐otomy) (Narabayashi and Okuma, 1953; Hassler and Riechert, 1954; Cooper, 1955). However, it was not un‐til 1980 that Brice and McLellan utilized chronic elec‐trical stimulation of the midbrain and basal ganglia to suppress intention tremor (Brice and McLellan, 1980).Since deep brain stimulation (DBS) is reversible, ad‐justable, and has a more favorable safety profile it has become the predominant surgical procedure for PD over pallidotomies and thalamotomies. While current treatments are effective at treating disabling motor symptoms, there currently are no treatments that alter the underlying disease pathophysiology.Here, we discuss the current management and recent surgical advances as well as potential paradigm shifts in treatment.
Current Surgical Treatments
DBS is the most common surgical procedure to ame‐liorate motor symptoms of PD. Moreover, it reduces the occurrence of “off” episodes that usually occur throughout the day in more advanced stages of med‐ically treated PD. However, DBS has variable effects on other motor symptoms, such as postural and gait disturbances or non‐motor symptoms (cognitive de‐cline, sleep, swallowing, speech or micturition distur‐bances). The two most common targets for DBS are the subthalamic nucleus (STN) and the globus palli‐dus pars interna (GPi). Large, randomized, controlled studies have demonstrated similar motor benefits between these two targets; however, after STN DBS,dopamine replacement medications can generally be reduced, while GPi DBS results in fewer cognitive and mood side effects (Follett et al., 2010). There is some evidence that targeting the pedunculopontine nucleus may improve gait instability and freezing(Stefani et al., 2007; Thevathasan et al., 2011).
Radiofrequency and radiosurgery pallidotomies were initially used to treat tremor symptoms alone.
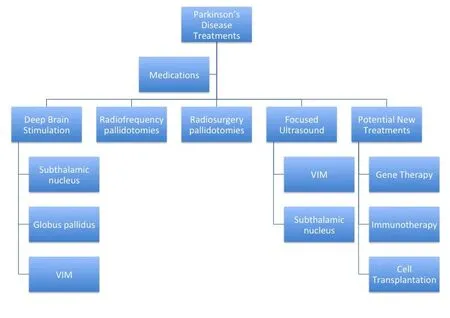
VIM: Nucleus ventero‐intermedius.
These types of lesional procedures have been used to some extent in PD patients who decline or are poor candidates for DBS. More recently, there has been increased interest in the use of focused ultrasound(FUS) thalamotomies for tremor as it does not re‐quire a craniotomy and physical brain penetration.Successful FUS thalamotomy for essential tremor has led investigators to treat highly selected tremor‐dom‐inant PD patients with FUS thalamotomy when the tremor is disabling (Bond et al., 2017) and FUS subthalamotomy when there is an asymmetric dis‐ease presentation (Martínez‐Fernández et al., 2018).While being a therapy that does not require an im‐planted device and successive operations for battery changes, lesional procedures are not reversible and thus are primarily performed unilaterally, limiting its effectiveness in a bilateral disease process.
Recent Advances
Since the first DBS surgeries, there have been many advances in technology used for surgical PD manage‐ment. The internal pulse generators (IPGs) used to charge the stimulation have become more efficient,and now have the potential to be recharged transcu‐taneously, limiting the number of surgeries the pa‐tient has to undergo for IPG changes. Newer versions of the DBS components are MRI compatible, which gives the patients and their physicians more flexi‐bility in diagnosing and treating PD as well as other conditions. The electrode leads have also become more complex, allowing for directional current com‐pared to radial current spread. Furthermore, multiple different stimulation paradigms can be programmed simultaneously, allowing for more flexibility, com‐plexity and speci ficity of stimulation. These advances in lead and IPG technology allow for more complex and patient‐speci fic programming.
DBS for PD utilizes continuous high frequency stimulation to the basal ganglia. While the mecha‐nism of DBS is not entirely understood, it is clear that DBS interferes with both pathological and physio‐logical neural circuitry. Newer IPGs can record and store local field potential data while simultaneously multiple targets, which can enable physicians and researchers to better understand the pathophysiolo‐gy of the disease and the mechanism by which DBS ameliorates symptoms (Swann et al., 2018). Current DBS utilizes an “open‐loop” (continuous stimulation)paradigm; however, its benefits can often be limit‐ed by side effects and/or partial efficacy. Moreover,patients do not suffer from the same exact motor symptoms continuously throughout the day. These potential limitations may result from the fact that DBS disrupts not only pathologic neural oscillations and connectivity, but also disrupts normal physiol‐ogy. One conceivable treatment option could be to identify aberrant local field potential biomarkers in real‐time and utilize an adaptive or “closed loop”form of stimulation to disrupt or modify that speci fic electrophysiology (Rosin et al., 2011).
Imaging the speci fic DBS targets and basal ganglia circuitry has also become more sophisticated. Mag‐netic resonance imaging continues to increase in res‐olution, which improves targeting of speci fic regions of the intended structure (STN, GPi). With advances in tractography, it is also possible to be able to tar‐get output and input circuitry as opposed to only the nodes within the basal ganglia. Studies suggest that not only is diffusion tensor imaging feasible for surgical planning of movement disorders, psychiat‐ric disorders and pain DBS, but it may also improve surgical outcome (See and King, 2017). Furthermore,advances in imaging techniques, such as functional MRI and magnetoencephalography, provide tools to better understand connectivity between various re‐gions of the brain as well as brain activity associated with electrical stimulation. This information poten‐tially could help in determining better functional and anatomic targets for DBS.
One of the principle concerns with DBS is its in‐vasive nature. A recent study by Grossman et al suggested that non‐invasive DBS might be possible by offsetting non‐physiologic high frequency stim‐ulation (i.e., 2.00 kHz and 2.01 kHz) to produce low frequency stimulation at a subcortical location(Grossman et al., 2017). In this rodent study, they were able to steer and target oscillatory activity with‐in the hippocampus, but did not affect the pathway from the source of stimulation to the target, similar to the concept of using multiple sources to lesioning a speci fic target in radiosurgery. This approach could reduce the morbidity associated with open surgery(i.e., infections, intracranial hemorrhage, lead migra‐tion/fracture,etc.), as well as decrease the need for permanent internalized batteries. Furthermore, this type of technology could theoretically enable nonin‐vasive test stimulation mapping for lesional experi‐ments and potentially be a permanent adaptable and noninvasive treatment option.
Potential Future Treatments
Current treatments and recent advancements primar‐ily treat the motor symptoms of PD; however, future disease‐modifying treatments are necessary to alter the course of the disease and treat the neurodegen‐erative and cognitive aspects of the disease. As with any disease process, it is necessary to understand the underlying pathophysiology. In that vein, there have been a number of PD gene therapy clinical trials that focus on improving motor symptomsviaaltering lo‐cal neurotransmitters or neurotrophic factors in the basal ganglia. While these trials have demonstrated that gene therapy can be safely delivered to the brain and induce speci fic neuronal protein expression, the clinical results have been less encouraging (Bartus et al., 2014).
There has also been interest in immunotherapy as a potential treatment approach. Animal studies and clinical trials have largely targeted using antibodies against misfolded α‐synuclein, the main constituent of Lewy bodies (George and Brundin, 2015). How‐ever, like the gene therapy trials, the studies merely demonstrate safety, but not necessarily efficacy.Consequently, there remain a number of hurdles to translate the promising animal studies into successful clinical trials (i.e., signi ficant placebo response, limit‐ed validated biomarkers, differences in rodent versus human biology).
Cell transplantation also offers a hypothetically intriguing treatment option. Ever since it had been shown in 1979 that fetal rat dopamine‐containing neurons could be transplanted into a PD rodent model with improved motor function, there has been significant interest in cell transplantation for PD.To date, there have been cell transplantation clinical trials using autologous and nonautologous cells, in‐cluding the use of human embryonic stem cells and induced pluripotent stem cells. The additional hurdle for cell transplant is the potential ethical consider‐ations that need to be addressed when using stem cells. Unfortunately, like the gene therapy and im‐munotherapy trials, the results have been equivocal(Yasuhara et al., 2017).
While there are currently very effective medical and surgical treatments for the motor symptoms of PD, patients continue to suffer from this progressive debilitating disease. Future research is necessary to understand the underlying disease process in order to better treat the disorder as a whole, rather than reducing very speci fic motor symptoms. While newer techniques, imaging, and medications have been able to suppress certain aspects of this motor disorder;disease‐modifying or disease‐reversing treatments still remain the ultimate goal (Figure 1).
Author contributions:DJL (guarantor), RFD, PDV and AML were involved with the development of the intellectual content and writing and editing the manuscript.
Conflicts of interest:AML is the consultant for Medtronic, St Jude,Boston Scienti fic, and Functional Neuromodulation.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Ahed Alkhatib, Jordan University of Science and Technology, Jordan.
- 中国神经再生研究(英文版)的其它文章
- In Memoriam: Ray Grill (1966–2018)
- Structural brain volume differences between cognitively intact ApoE4 carriers and non-carriers across the lifespan
- Roles of Eph/ephrin bidirectional signaling during injury and recovery of the central nervous system
- Neuroplasticity, limbic neuroblastosis and neuro-regenerative disorders
- A tissue-engineered rostral migratory stream for directed neuronal replacement
- Targeting the noradrenergic system for anti-in flammatory and neuroprotective effects:implications for Parkinson’s disease

