Effect of body material and temperature variation on the performance of the time delay pyrotechnic compositions
Azizullah Khan,Abdul Qadeer Malik,Zul fiqar Hameed Lodhi,Zain Ul Abdin
School of Chemical and Materials Engineering(SCME),National University of Sciences and Technology(NUST),H-12,Islamabad,Pakistan
1.Introduction
End result of a pyrotechnic delay device demands that it must function reliably and produce consistent burning time[1-4].Silicon and Boron fuels based pyrotechnic compositions attracted interest for delay devices and detonators due to their excellent end results[5-9].The material of the delay body affects the burning rate of pyrotechnic delay composition.The rigid delay body acts as a heat sink during burning of the delay composition.Metals are generally better conductors of heat than the delay compositions[10].The accuracy of pyrotechnic delay composition can be improved at given temperature by improving the design and controlling the dimensions of the delay column especially the length and diameter.Material in respect of the body of the delay material is also very important while designing a pyrotechnic delay device.Better accuracy is achieved by using brass delay body compared to stainless steel,aluminum and carbon steel.Main concern in pyrotechnic delay compositions is the accuracy that ranges between±10%to±20%of the mean value over the normally military operating temperature ranges of-40°C to+70°C[11].In some pyrotechnic compositions,the time delay increased up to 25%from mean at low temperature of-54°C[12-15].The development of pyrotechnic delay composition consists of mixing an oxidizer,fuel,binder and solvent to form slurry.It is then dried to remove the liquid to obtain solid product and then into desired grains sizes.Now a trend has been developed of replacing lead as a body material with delay body made of rigid metals such as aluminum,zinc,steel or brass.Delay body made of rigid metal can facilitate ease of loading the delay composition.Rigid delay body into which a pyrotechnic composition is loaded has higher thermal conductivity and act as a heat sink because of better conductors of heat than pyrotechnic delay compositions.There is a risk of failure of combustion propagation especially when the delay device is operating in low temperature environment.The pyrotechnic composition should produce more heat than the heat loss to the surrounding due to thermal conductivity of the delay body,to sustain combustion propagation[16,17].Modern pyrotechnic delay devices require reliable initiation and to produce consistence burning time[18].Pyrotechnic delay compositions due to controlled chemical reactions are also strongly affected by the ambient temperature.Change in the ambient temperature varies the burning time of a pyrotechnic composition.High ambient temperature normally produces smaller change in burning time than when a temperature change occurs at lower ambient temperature[19].To the best of our knowledge not much data is available on the effect of ranges of ambient temperatures and body material on burning performance of these types of pyrotechnic delay compositions.The main objective of this research work was to study:
(a)The effect of body material on the burning time and burning rate of Si/PbO/Pb3O4/FG and B/BaCrO4/FG pyrotechnic delay compositions.
(b)The effect of temperature variation on the burning time and burning rate of Si/PbO/Pb3O4/FG pyrotechnic delay compositions.
2.Experimental part
2.1.Materials used
2.1.1.Si/PbO/Pb3O4/FG delay mixture
High purity analytical grade silicon powder as fuel,lead(II)oxide(PbO)and lead oxide Pb3O4as oxidizers and commercial grade fish glue as a binder were used during this research work.All these chemicals were procured from Sigma Aldrich Company.Purity of these ingredients was≥99%.Particle sizes of silicon(Si),lead(II)oxide(PbO)and lead oxide Pb3O4were ≤44μm,1-2μm and<10μm respectively.
2.1.2.B/BaCrO4/FG delay mixture
Boron was used as a fuel and barium chromate was used as oxidizer.The purity of the boron and barium chromate is between 95 and 98%.The fuel and oxidizer used were fine powder,whereas commercial grade fish glue as a binder was used during this study.
2.2.Formulation of pyrotechnic delay composition
2.2.1.Mixing of pyrotechnic delay compositions°
Fuels and oxidizers were dried in heating oven at 80C for 2 h to remove the moisture content.The ingredients of the mixture were weighted according to the required percentages,and mixed the ingredients in the three dimensional(3-D)automatic Tumbler Mixing Machine.Small batches of 5-10 g each were further processed by mixing the chemicals in Mortar and Pestle in a mechanized mixing machine for 30 min to further homogenize the compositions.These operations were performed in a specially designed fuming hood.Binder solutions of 0.30wt%and 1.0 wt%Fish Glue were prepared in distilled water for Si/PbO/Pb3O4/FG and B/BaCrO4/FG delay composition respectively.Binder solutions were then mixed in delay compositions.A homogenous paste was prepared by using the spatula in agate container.The composition was semi dried in the Drying oven at 80°C.To avoid the formation of lumps,the semi dried composition was broken carefully by spatula in an agate container.The composition was then sieved gently through 212 mesh to get grains sizes of≤65μm.Haver test shaker EML 200-89 digital was used for preparation of grains of required particle sizes[20,21].Grains were dried for 8 hat 80°C to remove the moisture content.The finished compositions were stored in special containers and placed in desiccators for 24h to stabilize the compositions.
Binder was added to collect the particles to bind together in the form of free flowing grains.The binder protects the fuel and oxidizer from environment effect such as humidity.Additionally,binder also increase cohesion between particles of fuels and oxidizers to protect them from being segregated due to their density difference especially during storage.The grains also provide ease of loading of the composition in the cartridge body.Free flowing grains of the mixture have the ability to flow freely when poured from one container to another.
2.2.2.Safety precautions during mixing of compositions
These compositions are sensitive to friction,especially the processes of dry mixing and grains formation.The following safety precautions were observed during handling of these compositions.
(a)The exposure of a minimum number of operators was ensured during processing steps.
(b)The quantity of pyrotechnic composition for each batch was kept to 5-10g.
(c)A mechanized mixing machine installed with Mortar and Pestle was used.
(d)A Blast proof screen was used while mixing the compositions.
(e)Fire proof goggles and gloves were used while handling and preparing these compositions.
2.2.3.Pressing of delay composition in delay tube
Finalized compositions were pressed into stainless steel and brass delay tubes of internal bore diameter of 4.0 mm.The bulk density of the Si/PbO/Pb3O4/FG delay composition is more than that of B/BaCrO4/FG delay mixture;therefore,the Si/PbO/Pb3O4/FG and B/BaCrO4/FG delay mixtures were loaded in six and four equal increments respectively.The weight of each increment was kept 100 mg.Each increment was weighted separately and pressed at 30000 psi in the delay tubes one by one.A hydraulic press machine installed with a calibrated pressure gauge was used to press the delay compositions in the delay tubes.The surface of the delay column was kept flushed with the end of the delay tube.Extra delay composition was removed by sliding the end of the delay tube.Delay device filled with delay composition is shown in Fig.1.
2.2.4.Functional testing and data recording
Delay device used in this study consisted of rigid delay body,percussion primer,pyrotechnic delay composition and flame composition.These delay compositions were tested in mechanically initiated delay devices.Percussion cap of this mechanically initiated delay device was hit with a striking pin.The firing system consisted of electromechanical switch.Burning time started when the pin hit the percussion primer,and stopped when the Light Dependent Resistor(Sensor)detected the flame of the delay composition.To protect the senor from the slag,a Perspex disc was assembled in front of it.The burning time was measured with digital Oscilloscope.Measured results were in milliseconds,therefor the sensitivity of the instruments used were in micro second in order to avoid motion blur.A customize chronometer was also used for the measurement of the burning time simultaneously.Schematic diagram of the burning time measurement system is shown in Fig.2.Five measurements for each composition were carried out and the results were averaged.
3.Results and discussion

Fig.1.Pyrotechnic delay device.
In this study two pyrotechnic delay compositions Si/PbO/Pb3O4/FG and B/BaCrO4/FG were experimentally investigated.Stainless steel and Brass were used as body materials to determine the effect of the body material on combustion propagation of these compositions.Effect of temperature variation on burning time of Si/PbO/Pb3O4/FG composition was also studied.The recipes of both these compositions are shown in Table 1.
Lead oxide reacts with Silicon to give solid products SiO2and Pb[21].The chemical reaction is given below


Fig.2.Firing and delay time measurement system of pyrotechnic delay device.
Boron-Barium chromate(B-BaCrO4)is also a gasless delay composition.Burning propagation of pressed delay column of this gasless delay composition is a combustion reaction.Boron(B)and Barium Chromate(BaCrO4)react to give solid products[22].The chemical reaction between B and BaCrO4is given below:

3.1.Study of effect of body material on burning time and burning rate of Si/PbO/Pb3O4/FG delay mixture
Pyrotechnic delay mixture Si/PbO/Pb3O4/FG was first studied in Stainless steel tube.The internal diameter of the delay tube was 4 mm while the column length was 11 mm.The mean charge weight of the delay composition was 544 mg.Each increment was accurately weighted with a calibrated weighing balance.The maximum variation in the weight of the delay composition was 3.86%.The applied loading pressure to press the delay composition in the column was 30000 psi.Measured results of burning time and burning rate of this delay mixture are shown in Table 2.Result illustrates that the average burning time and burning rate of this mixture was 0.377s and 29.21mm/s respectively.Maximum variation in burning time and burning rate was 7.43%and 7.57%respectively.Standard deviation in the charge weight,burning time and burning rate was 0.008,0.010 and 0.81 respectively.Results show the burning time and burning rate is inversely proportional to each other.No igniter composition was used as first fire between percussion and delay composition for initiation of Si/PbO/Pb3O4/FG pyrotechnic delay composition.This delay mixture is sensitive to percussion primer and was easily initiated by output energy of the standard percussion primer.
Pyrotechnic delay composition Si/PbO/Pb3O4/FG=18/21/60.7/0.3 was then loaded in the brass tube at same loading pressure of 30000 psi and subjected to functional tests for the measurement of burning time and burning rate.Results of these parameters are shown in Table 2.Variation in the internal bore of the delay body significantly changes the burning time,therefore variation in the internal diameter of the tube was reduced from 7.7%to 2.6%during manufacturing process.Results as shown in Table 2 reveal that,the mean burning time and burning rate of the Si/PbO/Pb3O4/FG delay mixture recorded was 0.384 s and 28.64mm/s respectively.The percent variation in the burning time and burning rate was 4.17%and 4.12%respectively.By comparing,burning time and burning rate of Si/PbO/Pb3O4/FG delay mixture in both body materials,results show that using brass as body material,variation in the burning time decreased from 7.43%to 4.17%.The variation in burning rate reduced from 7.57%to 4.12%.An overall reduction of 43.88%in burning time,and 45.57%in burning rate was recorded.The standard deviation in charge weight,burning time and burning rate measured was 0.013,0.006 and 0.456 respectively.
Results also show that when the composition was tested in the brass delay body,the average burning time increased from 0.377s to 0.384 s and average burning rate decreased from 29.21 mm/s to 28.64mm/s.This slight increase in burning time and decrease in burning rate in brass delay tube could be due to the more heat lost from the delay composition in brass tube than in the stainless steel tube.The thermal conductivity of the brass(109 W/mk)is much higher than Stainless steel(16W/mk).Higher thermal conductivity of the brass material caused more heat loss from the delay composition to the surrounding and thus slightly decreased the burning rate and increased the burning time.
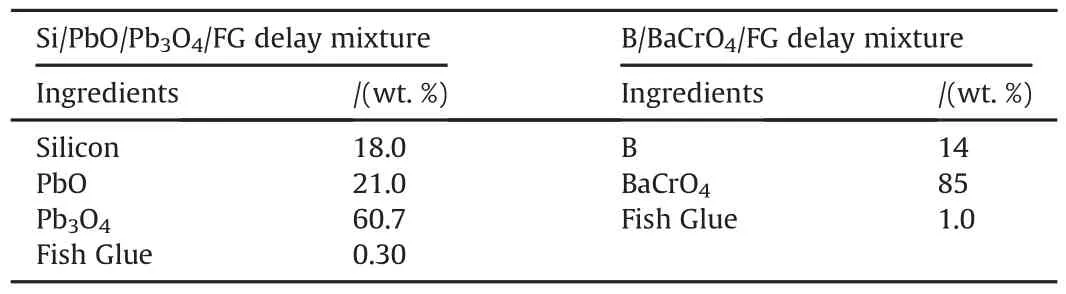
Table 1Ingredients and their percentages for delay mixtures.
3.2.Study of effect of body material on burning time and burning rate of B/BaCrO4/FG delay mixture
Delay composition B/BaCrO4/FG=14/85/1.0 was also studied in the stainless steel and brass delay bodies to determine the effect of body material on the burning performance of this delay mixture.Table 3 represents the burning time and burning rate of this delay in the Stainless steel tube.Mean charge weight of the delay composition was 283mg.The average burning time and burning rate of B/BaCrO4/FG delay composition were 0.208 s and 52.95 mm/s respectively.Maximum variation in burning time and burning rate was 16.83%and 17.0%respectively,whereas the standard deviation in the burning time and burning rate was 0.013 and 3.38 respectively.Results in Tables 2 and 3 also show that B/BaCrO4/FG=14/85/1.0 is a fast pyrotechnic delay composition and its average burning rate is 81.3%faster than Si/PbO/Pb3O4/FG=18/21/60.7/0.3 delay mixture in stainless steel delay body.The average burning rate of B/BaCrO4/FG=14/85/1.0 is 80.2%faster than Si/PbO/Pb3O4/FG=18/21/60.7/0.3 delay mixture in Brass delay body.
Similarly pyrotechnic delay mixture B/BaCrO4/FG was also filled and tested in brass delay body.Results are shown in Table 3.Mean charge weight of the delay composition was 280 mg.Average burning time and burning rate of this delay mixture was 0.213s and 51.61 mm/s respectively.Maximum variation in burning time and burning rate was 9.39%and 9.69%respectively.Standard deviation in the burning time and burning rate was 0.0085 and 2.124 respectively.Variation in burning time and burning rate reduced from 16.83%to 9.69%.Results also show that the burning rate decreased from 52.95 mm/s to 51.61 mm/s when tested in brass delay body,which shows 2.53%decrease in burning rate.This slight reduction in burning rate may be due to the more heat loss through brass delay body than through the stainless steel delay body.The more is the heat lost from a delay composition,the slower the burning rate and vice versa.
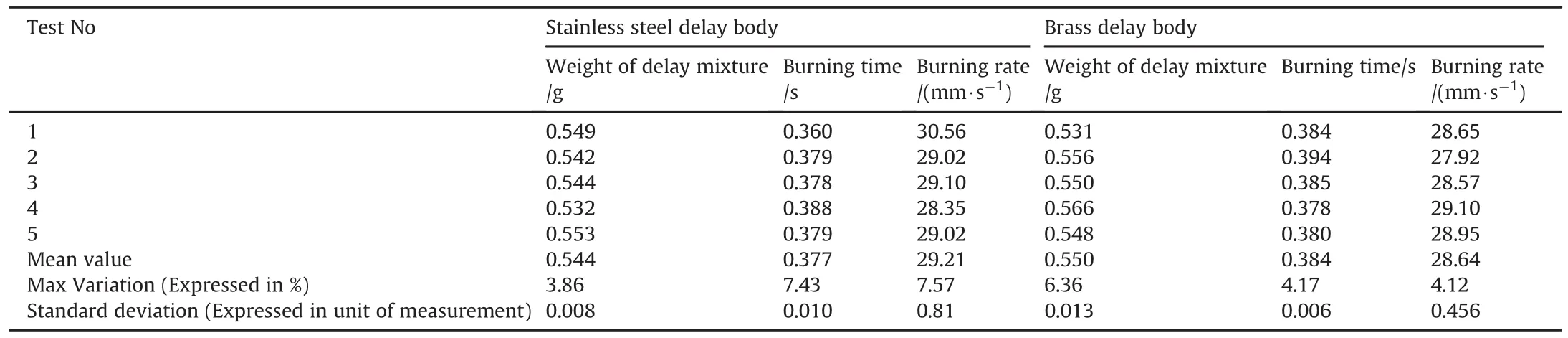
Table 2Test results of Si/PbO/Pb3O4/FG delay mixture in Stainless Steel and Brass delay body.
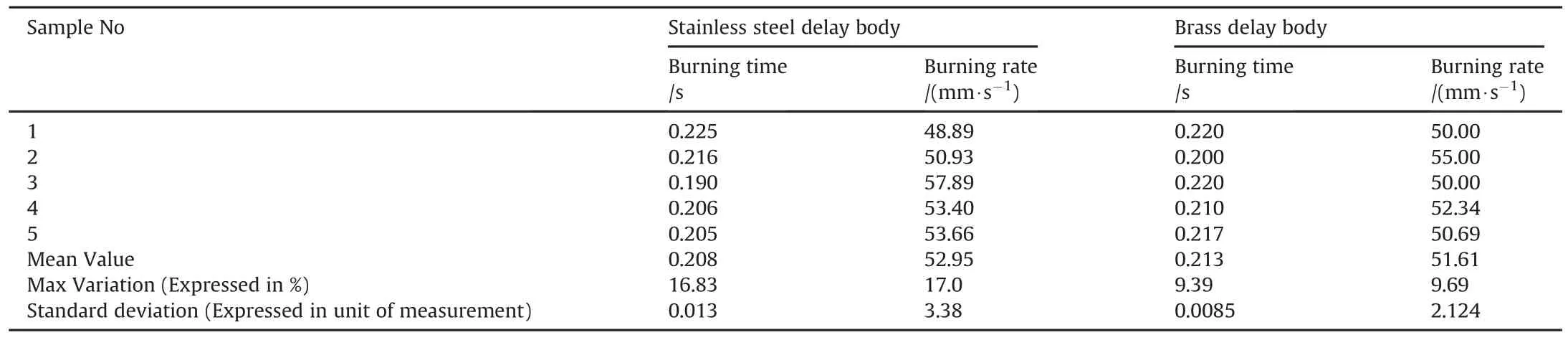
Table 3Test results of delay B/BaCrO4/FG in Stainless Steel and Brass delay body.
3.3.Effect of temperature variation on the burning rate of Si/PbO/Pb3O4/FG delay mixture
Si/PbO/Pb3O4/FG pyrotechnic delay mixture was loaded in a brass tube and conditioned in calibrated environmental chamber at-54°C,-40°C,-20°C,25°C,70°C and 100°C for 4h each.After conditioning at these temperatures,the samples were shifted and tested for burning time measurement within three minutes after removal from the chamber to meet requirement of applicable Military Standard[23].The average measured burning time and burning rate are shown in Table 4.Each data point listed in Table 4 represents an average of five measurements.The mean charge weight of delay composition ranged from 535mg to 548 mg.Results in Fig.3 and Fig.4 reveal that the burning time decreased and the burning rate increased with increase in the ambient temperature.The minimum and maximum burning rates were recorded at-54°C and 100°C respectively.The temperature dependence of the burning rate of Si/PbO/Pb3O4/FG delay mixture is nearly linear.
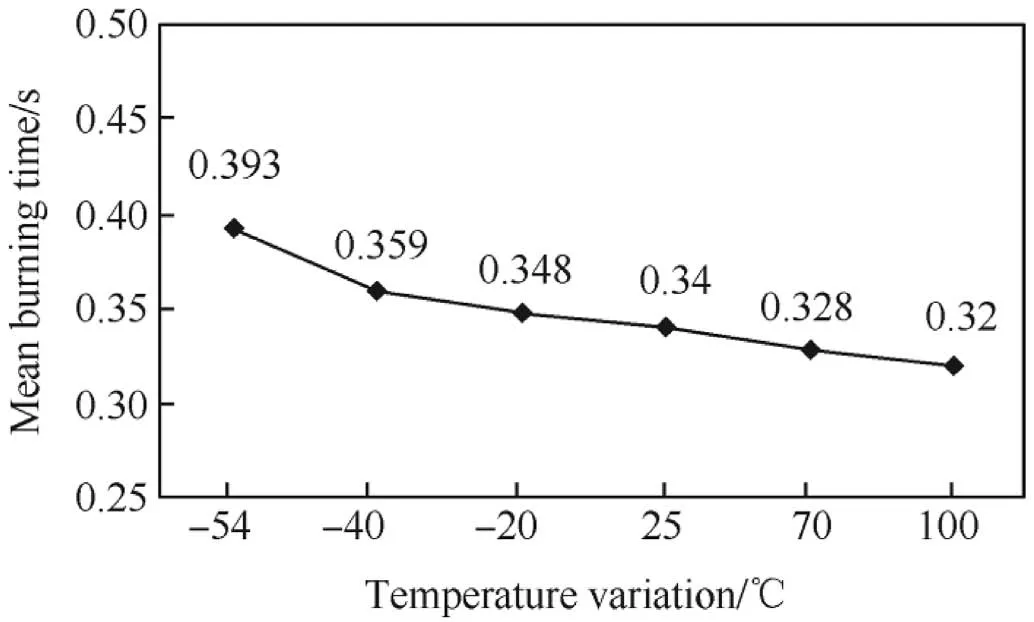
Fig.3.Plot of burning time vs temperature for Si/PbO/Pb3O4/FG mixture.

Table 4Temperature dependency of delay Si/PbO/Pb3O4/FG in Brass delay body.
Temperature of the surrounding and also the temperature of the unreacted delay composition affected burning rate.As ambient temperature is raised,activation energy is lowered because less energy is required to raise a composition to its ignition temperature.Thus the burning rate increased and less time was required to reach the ignition temperature.Burning rate increased from 28.01mm/s at-54°C to 34.38 mm/s at 100°C which shows an overall increase of 23.0%in the burning rate.The burning time decreased from 0.393 s to 0.320 s when the temperature was increased from-54°C to 100°C,which show an overall decrease of 18.58%.
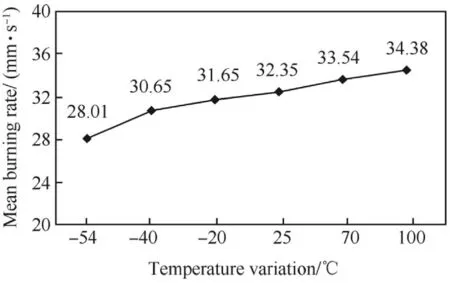
Fig.4.Plot of burning rate vs temperature for Si/PbO/Pb3O4/FG mixture.
4.Conclusions
From the analysis of both,Si/PbO/Pb3O4/FG=18/21/60.7/0.3 and B/BaCrO4/FG=14/85/1.0 delay compositions in the stainless steel and brass delay body,it is concluded that using brass delay body the variation in both burning time and the burning rate reduced.The burning time slightly increased in brass delay body due to the high thermal conductivity.Temperature variation also affected the burning time and the burning rate of Si/PbO/Pb3O4/FG delay mixture.Burning time increased and the burning rates decreased as the conditioning temperature was decreased and vice versa.
[1]Jakubko J.Pressure and temperature effects on burning rate of the silicon red lead system.J Energetic Mater 1997;15(2-3):151-61.
[2]Ricco IMM,Focke WW,Conradie C.Alternative oxidants for silicon fuel in time-delay compositions.Combust Sci Technol 2004;176(9):1565-75.
[3]Da-wei X,Da-hai W,Zhi-gang C.Characteristic and application of boron type delay charge.Explos Mater 2005;3:11-4.
[4]Wu Y,Song J.Technology of delay compositions.Explos Mater 2000;29(2):23-7.
[5]AL-Kazraji SS,Rees GJ.Differential Thermal Analysis Studies of the reactions of silicon and lead oxides.J Therm Anal 1979;16:35-9.
[6]Jakubko J.Combustion of the silicon-red lead system,temperature of burning,kinetic analysis and mathematical model.Combust Sci Technol 1999;146:37-55.
[7]Jakubko J,Cernoskova E.Differential thermal analysis of the mixtures of silicon and red lead.J Therm Anal 1997;50:511-5.
[8]Howlett SL,May FGJ.Ignition and reaction of boron fueled pyrotechnic delay compositions.Thamochimica Acta 1974;9:213-6.
[9]Charsley EL,Chen CH.Differential thermal analysis and temperature pro file analysis of pyrotechnic delay systems:ternary mixtures of silicon,boron and potassium dichromate.Themochimica Acta 1980;35:141-52.
[10]Pamplet AMC.US Army material command engineering design hand book.In:Military pyrotechnic series Part-A,theory and application;1967.p.39.Washington D.C.[Chapter 5].
[11]Danali SM,Palaiah Raha KC.Developments in pyrotechnics.Defence Sci J 2010;60(2):152-8.
[12]Military Specification.Manganese delay composition.MIL-M-21383A.1976.
[13]Military Specification.Delay composition T-10.MIL-D-85306A(AS).1991.
[14]Military Specification.Delay composition,tungsten- fluorocarbon copolymer.MIL-D-82710(OS).1984.
[15]Comyn Raymond H.Pyrotechnic research at DOFL Part-II.Pyrotechnic delays.Washington D.C:Diamond Ordinance Fuze Laboratories;1962.
[16]Aube R,Lachute CA.US Patent,0223242A1.Delay compositions and detonation delay device utilizing same;2008.
[17]Kalombo L.Evaluation of Bi2O3and Sb6O13oxidants for silicon fuel in time delay detonators.2005.
[18]Li Y,Ceng Y,Hui Y,Yan S.The effect of ambient temperature and boron content on the burning rate of the B/Pb3O4 delay compositions.J Energetic Mater 2010;28:77-84.
[19]Eller W,Frank,Valenta F.US Patent,3851586.Temperature Compensated Pyrotechnic delays 1974.
[20]Operating instructions.Haver test shaker EML 200-89 digital.1993.
[21]AI-Kazraji SS,Rees GJ.The fast pyrotechnic reaction of silicon and red lead:heats of reaction and rates of burning.Fuel 1979;58(2):139-43.
[22]John AC.Chemistry of pyrotechnics basic principles and theory.Maryland,USA:Department of Chemistry,Washington College Chestertown;1985.p.125-40[Chapter 6].
[23]Military Specification.Design and evaluation of cartridge for stores suspension equipment.MIL-81303(AS).1966.
- Defence Technology的其它文章
- Effects of ply orientation and material on the ballistic impact behavior of multilayer plain-weave aramid fabric targets
- Effect of magnesium on FOX-7 and its tautomers-A DFT treatment
- Influence of welding consumables on tensile and impact properties of multi-pass SMAW Armox 500T steel joints vis-a-vis base metal
- Effect of functional composite coating developed via sulphate and chloride process parameter on the UNS G10150 steel for structural and wear mitigation in defence application
- Optimizing submerged arc welding using response surface methodology,regression analysis,and genetic algorithm
- Virtual ballistic impact testing of Kevlar soft armor:Predictive and validated finite element modeling of the V0-V100probabilistic penetration response

