Pitting and stress corrosion cracking studies on AISI type 316N stainless steel weldments
Anit Toppo,M.G.Pujr,*,N.Sreevidy,John Philip
aCorrosion Science and Technology Division,Indira Gandhi Centre for Atomic Research,Kalpakkam,603 102,Tamil Nadu,India
bMaterials Development and Technology Division,Indira Gandhi Centre for Atomic Research,Kalpakkam,603 102,Tamil Nadu,India
1.Introduction
Austenitic stainless steels(SSs)are the one of the most commonly used structural materials,apart from carbon steel,in many industries because of their excellent resistance to general corrosion,adequate high temperature mechanical properties,good fabricability and weldability.Austenitic SSs are generally regarded as readily weldable materials with considerable tolerance for variations in welding conditions.Austenitic SS of type 316LN is the structural material for Prototype Fast Breeder Reactor(PFBR),Kalpakkam,India,which is in commissioning stage.Welding was extensively employed in the fabrication of PFBR components.The components of PFBR are fabricated using 316LN SS using 316N SS welding consumables so as to make[1]the weldments free from sensitization along with improved creep strength.Solution annealing(SA)treatment was carried out for complete restoration of mechanical properties after working on the components.In some of the PFBR components,nickel base,cobalt free hardfacing is used toavoid sliding movement and concurrent self-welding due to high temperature and high velocity sodium.Hardfacing builds-up high stresses at the substrate-coating interface and hence,stress relieving is carried out on the weldments at 750°C/1h.550°C/4 h is the dimensional stabilization(DS)treatment for PFBR components to relieve peak residual stresses to prevent distortion during final machining and assembly.The temperature selected is based on the highest temperature the component is likely to experience during service.The heat treatments,such as SA,DS(550°C/4h)and stress relief(750°C/1 h),cause delta ferrite in the as-welded weld metal(WM)to undergo transformation to secondary phases like M23C6carbide,chi(χ)and sigma(σ).Earlier it was assumed that WM,which solidified either in the primary ferritic solidification mode or in the primary austenitic solidification mode with a duplex structure,showed stress corrosion cracking(SCC)resistance which was comparable to the base metal[2].However,now it is established that welding process introduces features,such as slag and other inclusions,dendritic microstructure,residual stresses,secondary phases,defects,phase transformations etc.,which can degrade the corrosion properties of the WM as compared to the wrought base metal.Generally,a weld is required to perform either equal to or better than the base metal it joins.The most important property of the weld joint is corrosion resistance.There are many factors that influence corrosion resistance of the weld metal namely microsegregation of alloying elements[3,4],heat input[5,6]and the welding technique.Weld metal with duplex austenoferrititic microstructure does not get sensitized due to chromium carbide precipitation and if at all it gets sensitized,homogenization of sensitized microstructure would take place due torapid diffusion of chromium from the chromium rich delta-ferrite phase.However,welding followed by heat treatments can result in sensitization and subsequent intergranular corrosion(IGC)or intergranular stress corrosion cracking(IGSCC)of austenitic SS weldments.The SCC resistance of WM depends on the content and distribution ofδferrite and the solidification mode of the WM[.Investigations by Flowers[7]indicated that globular ferrite present in the cast duplex SS provided a “keying”action making the steel more resistant to SCC than single-phase austenitic SS.Sherman et al.[8]and Gooch[9]reported improved SCC resistance of 304L SS due to presence of δ/γ interface which acted as an anode and austenite phase acts as cathode.Stalder and Duquette[10]and Ray et al.[11]reported comparable SCC resistance for Type 304 SS and its weld metal in boiling magnesium chloride(MgCl2)solution(427K).In tropical and humid coastal atmospheres,welded components of austenitic SSs have reportedly failed by SCC during storage,transportation,erection and pre-commissioning.Based on their extensive work on 316N SS weld metal with similar chemical composition as well as heat treatments,Parvathavarthini et al.[12]observed that the susceptible microstructure to IGC resulted only when critical cooling rate(CCR)was below 200°C/h.
The structural materials selected for fast reactors are nitrogen containing 316LN SS owing to the fact that nitrogen addition delays sensitization as well as intergranular stress corrosion cracking(IGSCC).The major components of such reactors are grid plate,main vessel,inner vessel,safety vessel and intermediate heat exchanger(IHX).During fabrication of these vessels,one of the major fabrication procedure employed is welding.After fabrication of these components theyare stored in large service assembly areas for an indefinite period.Kalpakkambeing a coastalplace,it has high airborne chloride content.Thus,during the storage,erection and commissioning stage,due to the high humidity(RH as high as 90-95%)coupled with high airborne chloride the components can undergo pitting and IGSCC/TGSCC.The present work was carried out to understand the uniform,pitting and SCC behavior of 316 N SS weld metals in AW and thermally aged conditions in two aqueous environmental conditions.The critical cooling rate was maintained above 200°C/h during heat treatments to avoid sensitization of the weld metals.
2.Experimental details
Weld pads were prepared by gas tungsten arc welding(GTAW)process initially by using ER16-8-2 filler wires and subsequent passes with E316-16(modified)electrodes[1].The welding parameters are presented in Table 1.
The weld joint was prepared by multipass welding using a double-V-groove joint configuration.The deposition was carried out on both sides of the V-groove,wherein each side was completed using multipass deposition.Thus,the complete weld pad was prepared by double side deposition.Liquid penetrant and radiographic examinations were carried out to ensure that the fabricated weld pad was free from porosity and defects.Aqua regia was used to reveal the macrostrcuture of the weld joint.Fig.1(a)shows the macrostructure of the 316N SS weldment in AW condition,where no porosity and blow holes are seen.The WM was extracted from the all-weld region to determine its chemical composition as well as delta-ferrite content.Fig.1(b)shows the schematic of the weld pad and the locations of the weld metal portions used for extracting SCC and pitting corrosion test specimens.The chemical compositions of the base as well as 316N SS WM were analyzed using optical emission spectroscopy(OES)and are shown in Table 2.Tensile specimens were fabricated from weld pad such that the weldment portion was at the centre of the gage length.Fig.2 show the dimension of the round tensile specimen(RTS).Just as RTS,small coupons of dimension 10×10×5mm were also machined from 316N SS all-weld metals.Some of the coupons and RTSs were solution annealed at 1065°C/1 h,whereas the remaining were thermally aged at 550°C/4 h and 750°C/1 h in a vacuum furnace,by maintaining the critical cooling rate(CCR)to be above 200°C/h while cooling.Microstructural observations were carried out using an optical microscope after chemically etching the weldments in modified Murakami reagent[30g K3Fe[CN]6+30 g KOH+150 ml water,immersion in the boiling solution for 15-45 s]to study the microstructural changes due to heat treatment.Delta-ferrite measurements were carried out on polished specimens(1μm finish)of weld metals in AW,SA as well as thermally aged(550°C/4 h and 750°C/1 h)conditions using a Ferritoscope.Total of 30 readings were taken and an average of these data was reported in ferrite number(FN).Tensile tests were carried out for 316N SS weldments in AW and thermally aged conditions in air at 1.0×10-4sec-1strain rate using a Universal Testing Machine.Yield strength(YS),ultimate tensile strength(UTS)and ductility(percentage total elongation)(%TE)were calculated using stress-strain plots.
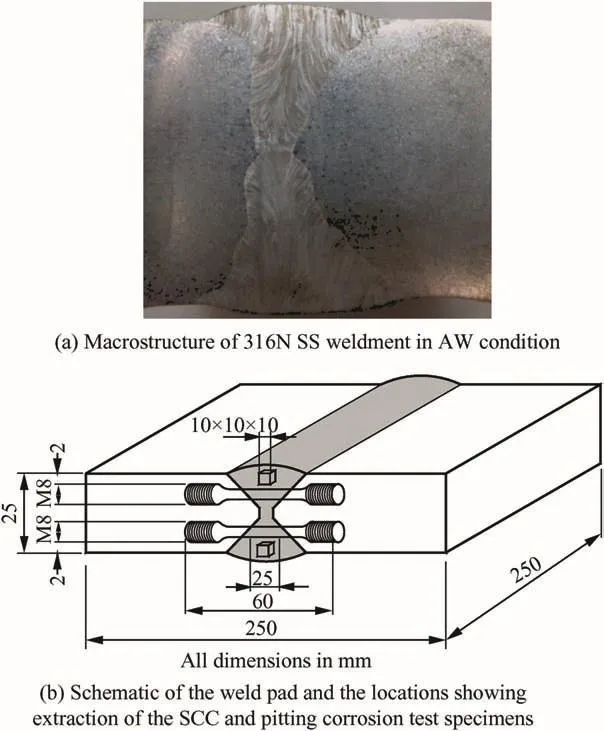
Fig.1.a:Macrostructure of 316N SS weldment in AW condition.b:Schematic of the weld pad and the locations showing extraction of the SCC and pitting corrosion test specimens.

Table 1Welding parameter for welding 316LN SS.
Localized corrosion behavior such as pitting and SCC strongly depends on the passive film formed on the surface on exposure to a corrosive medium.Initiation and propagation of localized corrosion attack is subject to the stability of the passive film formed on the surface in the environment under consideration.The stability of the passive film is routinely studied by,a potential drop technique,wherein the specimen passivated at a preset passive potential was allowed to react with the environment chemically by breaking the circuit and allowing the potential to decay,till specimen reached its corrosion potential,Ecorr.The passive film stability was evaluated in a deaerated solution of 0.5M H2SO4+100 mg NH4SCN,where the passive film could breakdown and dissolve relativelyeasily thereby the potential decay experiment could be completed in a reasonable time frame.All the potentials were measured against a standard saturated calomel(SCE)electrode.The 316N SS weld metals in SA,AW and thermally aged conditions were potentiodynamically anodically polarized to know their stable passive regions.Thereafter,these specimens were prepolarized up to the desired passive potential(+500 mV(SCE))and maintained passive by impressing the+500 mV(SCE)potentiostatically for 30 min.After 30 min,the circuit was broken and the potential decay recorded till the specimen reachedEcorr.
Potentiodynamic anodic polarization experiments were carried out for 316N SS weld metals in AW and thermally aged conditions at ambient temperature in deaerated 0.5 MH2SO4(acidic)and 0.5M H2SO4+0.5 M NaCl(acidic chloride)solutions to study the uniform and pitting corrosion behavior,respectively.Deaerationwas carried out bypurging nitrogen gas through the solution for 1 h before start of the polarization test.After cathodic cleaning at a current density of-1 mA/cm2,the open circuit potential(OCP)of the specimens was allowed to stabilize.Thereafter,potential was scanned at a rate of 0.1 mV/s,from a potential of-600mV(SCE)to a more positive potentials beyond pitting potential(Epit)till a maximum current reached up to 0.1 mA.The double loop electrochemical potentio kinetic reactivation(DLEPR)technique was used to measure the extent of chromium depletion resulting from the precipitation of Cr23C6in ferrite[13]due to thermal ageing treatments.The experimental set-up for DLEPR tests was the same as that described above for potentiodynamic anodic polarization studies.DLEPR tests were carried out in a deaerated solution of 0.5M H2SO4+0.01 M NH4SCN at room temperature.However,the potential scan rate was 6V/h in both the anodic and cathodic directions.After the wellpolished(1μm finish)specimen was immersed in the above solution,a cathodic potential of-0.5 V(SCE)was applied for 2 min potentiostatically in order to clean the surface of any residual oxide film.Thereafter the anodic scan was carried out from-0.5 V(SCE)till+0.3 V(SCE).This was immediately followed by the cathodic scan from+0.3 V(SCE)till-0.5 V(SCE).The peak current values during the anodic or activation(IA)and cathodic or reactivation(IR)scans were noted in order to quantify the degree of Cr-depletion(DOC).

Table 2Chemical composition of base and WM,wt.%.
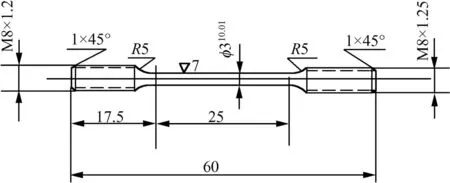
Fig.2.Schematic of the round tensile specimen(RTS).
SCC studies were carried out for 316N SS weld metals in AW and thermally aged conditions in boiling 5 M NaCl+0.15 M Na2SO4solution acidified with 2ml/l of concentrated HCl.Constant load testing technique was used to carry out SCC studies and time to failure(tf)was used as the assessment criterion.During SCC,linear variable differential transformer(LVDT)was fixed to the wave guide welded to the specimen undergoing SCC,which logged the elongation with respect to time data.SCC studies were carried out at 80%of the YS of material.Immediately after SCC failure one of the fractured surfaces was used for laser Raman spectroscopic(LRS)studies.LR spectra of the corrosion products present on failed 316N SS weld metal SCC specimens were recorded with an HR 800(Jobin Yvon)Raman spectrometer equipped with 1800 grooves/mm holographic grating,He-Ne laser of 633nm was used as an excitation source.The laser spot size focused on the surface was approximately 10μm and laser power at the specimen was 8.6mW.Raman spectra were recorded using super cooled(<-110°C)1024×256 pixels CCD detector.The system consisted of an Olympus optical microscope mounted at the entrance of the Raman spectrograph.Both Raman excitation and emission were performed using a 10x long distance objective(180°back scattering).The holographic grating in the spectrograph covers the spectral regions from 150 to 4000 wave numbers having 4cm-1spectral resolution,with each exposure of the CCD detector.Data acquisition was controlled by a software package(Kaiser-Optical System).The fractured surfaces were also examined using a scanning electron microscope(SEM)to study the SCC failure mode.
3.Result and discussion
In order to understand the mode of weld solidification Suuatala[14],DeLong[15]and WRC-92[16]formulae can be used.Solidi fication is represented in a simplified way through the plots of chromium equivalent(Creq)versus nickel equivalent(Nieq).Suuatala[14],DeLong[15]and WRC-92[16]used Creq/Nieqvalues to obtain the solidification mode.These formulae are given below:1.25<(Creq/Nieq)<1.48(AF mode),both welds solidified in the austenitic-ferritic mode with a residual ferrite content depending on the heat-input.
The average delta-ferrite content for AW 316N SS WM was 4.69±0.39 FN which reduced to 4.24±0.41 FN,0.92±0.07 FN and 0.47±0.06 FN after thermal ageing for 550°C/4h,750°C/1h and SA treatments,respectively.The optical photomicrograph in Fig.3(a)of 316N SS AW weld metal shows the austeno-ferritic microstructure with vermicular δ-ferrite.The δ-ferrite network was found to be continuous in AW specimen.In AW condition,the M23C6carbides were observed because during multipass welding the temperature in the lower passes reaches the range of 450-750°C due to reheating resulting in carbide precipitation.On thermal aging at 550°C/4 h,the connectivity ofδ-ferrite network


Non equilibrium conditions prevailing during weld metal solidification and the presence of alloying elements other than Cr and Ni limit the use of available phase diagrams(such as the ternary Fe-Cr-Ni diagram).The various mechanisms proposed were related to chemical composition,as shown below.
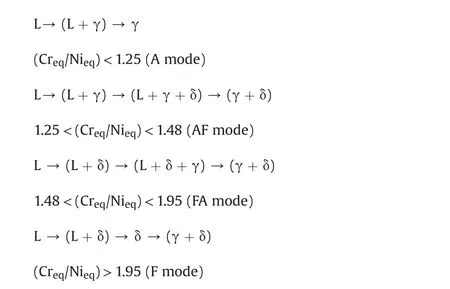
where A and F are austenitic and ferritic modes of solidification respectively.It was observed(Table 3)that the Creq/Nieqratios obtained using DeLong's and WRC-1992 formulae for 316N SS weld were comparable as against the value obtained by Suuatala's formula.Hence,these values were considered for determining the mode of solidification.Thus,it could be observed that,since decreased and fine precipitates of M23C6,χ,σ were observed as shown in Fig.3(c).The bluish green coloration[17-19]inδ-ferrite indicates presence ofχphase.Some amount ofσwas also present as the reddish brown colored precipitate along with M23C6precipitates in the Fig.3(c){inset}.Escriba et al.[20]observed that ferrite initially transformed toχphase when duplex stainless steels(DSS)were thermally aged at temperatures of 700-750°C and on prolonged ageingχ-phase was consumed byσ-phase precipitation.χ-phase was observed in the ternary(Fe-Cr-Mo)or quaternary(Fe-Cr-Ni-Mo)and(Fe-Cr-Ni-Ti)systems as reported by other workers[21-23].Lee et al.[24]reported that the precipitation ofχ-phase occurred before theσ-phase precipitation during irradiation of 316+Ti alloys.The heat-treatments given subsequently to the specimens served to increase the transformation of ferrite to various extents depending upon the temperature and time of exposure.The heat treatment at 750°C/1 h transformed the δferrite further intoσalong with some secondary austenite.Precipitation ofσincreased with increasing heat-treatment temperature as shown in Fig.3(d).On solution annealing at 1065°C/1 h,δferrite transformed completely to austenite leaving behind slag particles as shown in Fig.3(b).
The plot of mechanical properties of 316N SS weldments in AW,1065°C/1 h,550°C/4h and 750°C/1 h conditions is shown in Fig.4.AW 316N SS weldment showed comparable mechanical properties with that of the weld metal thermally aged at 550°C/4 h.Since δferrite present in the weldment contributes towards strength[25]and both AWand 550°C/4h weldments showed almost comparable ferrite contents,thus,their mechanical properties were comparable.However,ductility was relatively higher for AW weldment.On comparing the YS of 316N SS weldments in different thermallyaged conditions,it was noted that SA weldment exhibited lowest YS value,due to the absence of secondary phases/precipitates.Among thermally aged weldments,the one aged at 550°C/4h showed higher YS value than that aged at 750°C/1 h.However,UTS values were comparable for the thermally aged 316N SS weldments but SA weldments showed relatively lower UTS value.This is in agreement with the results obtained by other investigators[26,27].Vitek et al.[27]found no significant change in tensile properties after aging 308 weldment at 475°C for up to 20,000h.Increase in the strength concurrently decreases ductility which was clearly noted in 316N SS weldments.Ductility decreased for thermally aged weldments(up to 38%)whereas,AW and SA weldments showed higher ductility(>50%).It was observed from microstructural studies,thatδ-ferrite transformed completely to austenite after solution annealing,thereby resulting in the reduction in the YS with concurrent increase in ductility as austenite is a softer phase.Work carried out by Shaikh et al.[28]suggested that the amount of ferrite,degree of interconnectivity,distribution,and shape ofδferrite per se did not influence the tensile properties of the weldments,however,extensive microscopic defects and cold work influenced the YS and ductility significantly.The work carried out by Ward[25]showed that the presence of ferrite resulted in increased YS in type 316 SS,which was attributed tothe presence of second phase which acted as a barrier to deformation.Thus,it can be surmised that,presences ofδ-ferrite caused increase in the YS.Although,thermal ageing resulted in partial transformation of delta-ferrite in weldments aged at 550°C/4 h and 750°C/1h weldments,due to sluggish kinetics of transformation at these temperatures untransformed ferrite could be observed,which contributed towards the overall increase in the strength of the weldment.The specimen aged at 550°C/4 h showed marginal increase in YS due to presence of higher amount of ferrite.Similarly,UTS values for thermally aged 316N SS weldments were higher as compared to SA 316N SS weldment.Similar observation was made by Dutta et al.[29]in their work on long term aging of 316N SS weldment with comparable chemical composition.

Table 3Creq/Nieqratios obtained for 316N SS WM using different formulae.

Fig.3.Optical photomicrographs of 316 N SS weld metals in(a)AW(b)SA(c)550 °C/4h and(d)750 °C/1 h conditions after chemically etching in Murakami's reagent.

Fig.4.Mechanical properties of 316 N SS WM in SA and heat treated condition.
3.1.Electrochemical studies
The 316N SS weldment was prepared using a multipass welding technique,thus,even the AW weldment would have some carbides andσin the lower passes due to the thermal cycles while laying the higher passes.The precipitation of carbides andσphases would leave some amount of Cr-depletion in their vicinity,which can be detected using DLEPR test.From the DLEPR test results,the DOC%was calculated usingIR/IAx 100,whereIRandIAare the reactivation and activation peak current density values,respectively.The DOC%values for 316N SS weld metals with different heat treatments are shown in Table 4.The DOC%was obtained from the ratio of reactivation peak current to activation peak current[30].AsIR/IAapproaches 1,the material is considered more susceptible to grainboundary attack.However,in the present study DOC values were found to be very low,which suggested that 316N SS weldments in AW,SA and thermally aged conditions had very insignificant Cr depletion,in spite of the precipitation of carbides and σ(Fig.3(a-d)).
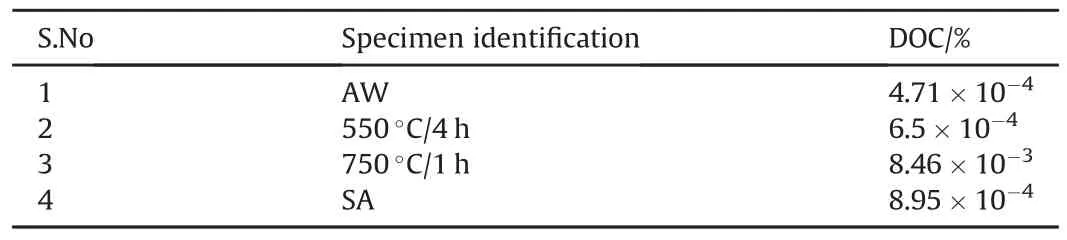
Table 4The DOC%values for 316N SS WM with different heat-treatments.
It is well known a medium like sulphuric acid promotes establishment of active-passive states of SSs,which are active-passive alloys.The potentiodynamic anodic polarization studies in sulphuric acid medium could throw light on the important parameters like range of passivity,passive current density,corrosion potential(Ecorr),critical current density(Icrit)etc.The potentiodynamic anodic polarization curves for 316N SS weld metals in AW and thermally aged conditions are shown in Fig.5.TheEcorrvalues were comparable for all the weld metals.Transpassive potential was almost same for 316N SS weld metals in different conditions.The passive current density values(Ipass)were obtained as an average of current density values in a potential range of 0.0-0.4 V(SCE).It was observed that theIpassvalues decreased in the order of weld metals as AW,750°C/1h,550°C/4h and SA(248,150,80 and 59 μA/cm2,respectively).The passive current density values showed significant rise,due to increased precipitation ofσwith concurrent alloying element depleted regions with increase in thermal ageing temperature.Although significant DOC%values could not obtained during DLEPR tests,precipitation of carbides andσhad taken place resulting in the alloying element depletion in the vicinity of these precipitates that caused nonuniform distribution of alloying elements,which reflected in the variation in the passive current density values.The fluctuations observed in the passive current density values could be attributed to the same cause.Potentiodynamic anodic polarization studies were carried out in acidic chloride medium(Fig.6)in order to evaluate the pitting corrosion resistance of the weld metals in AW and thermally aged conditions.The average of three pitting potential(Epit)values and the standard deviation values were plotted as error bars as shown in Fig.7.It was noted that SA weld metal showed highest pitting corrosion resistance.A marginal difference was observed between the pitting corrosion resistance of AW weld metal and 550°C/4h heat treated weld metal.Weld metal thermally aged at 750°C/1h showed lowest pitting corrosion resistance.Similar observation was made by Pujar et al.[31]on their work on influence of solution annealing and stress relieving on the pitting corrosion resistance of modified 316N SS weld metals which was studied using electrochemical noise(EN)technique.SA weld metal is fully austenitic without any microstructural heterogeneities which,makes the passive film on SA quite stable resulting in higher pitting corrosion resistance.It is well-known that delta-ferrite in duplex SS weld metal transforms to secondary phases,such as M23C6,σ,χ,etc.,upon thermal aging[32].Thus,thermally aged specimens have microchemical inhomogeneities due toδ-ferrite along with carbide precipitates.The secondary precipitates coupled with the alloy depleted zones around them,make the passive film extremely unstable resulting in increased pitting corrosion attack.Thus,with increasing thermal ageing temperature,Epitvalue was found to become more active.Passive film studies were carried out for 316N SS weld metals in AW,SA and thermally aged conditions to study the passive film behavior(Fig.8).The stability of film towards chemical dissolution was higher for solution annealed.SA 316N SS weld metal being microstructurally and microchemically homogeneous its passive film was found to be very stable,on the other hand,750°C/1h shows lowest passive film stability due to precipitation of carbides,other than high concentration of microscopic and macroscopic defects which destabilizes the passive film.Both AW and 550°C/4h weld metals showed an intermediate arrest potential of about 0.2 V and 0.05V(SCE),respectively.AW and 550°C/4h weld metals the passive film remained stable for 4000 and 6000 s respectively and then slowly dissolution and reached the OCP.These observations support the previously described data on pitting corrosion resistance.
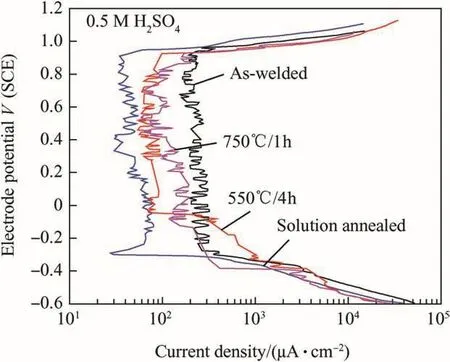
Fig.5.Uniform corrosion behavior of 316 N SS WM in AW and thermally aged condition in 0.5 M H2SO4solution.

Fig.6.Pitting corrosion behavior of 316 N SS WM in AW and thermally aged condition in 0.5 M H2SO4+0.5 M NaCl solution.
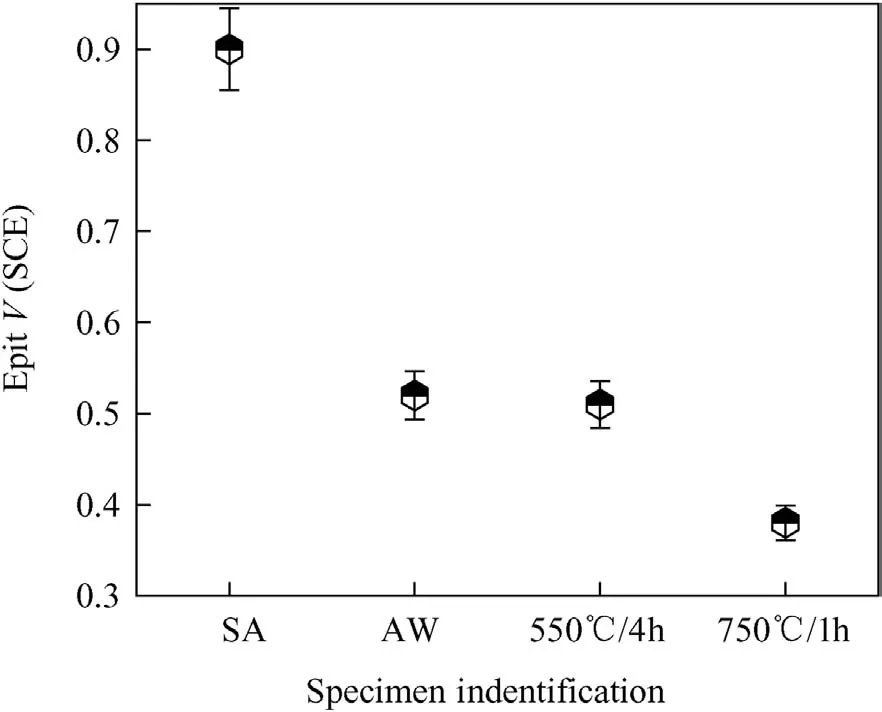
Fig.7.Epitvalues for 316 N SS WM in AW and thermally aged condition obtained in 0.5 MH2SO4+0.5M NaCl solution.

Fig.8.Passive film study on 316 N SS WM in AW and thermally aged conditions in 0.5 MH2SO4+100 mg NH4SCN solution.

Fig.9.A schematic corrosion elongation curve showing different regions.
3.2.SCC studies

Fig.10.Corrosion elongation curve for 316N SS WM in SA and thermally aged conditions.
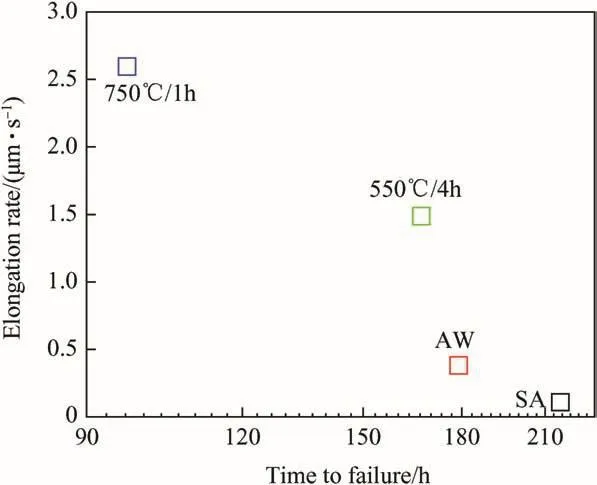
Fig.11.Elongation rate vs.SCC time to failure.

Fig.12.SCC resistance of 316N WM in AW and thermally aged condition.

Fig.13.SEM photomicrographs along with EDS analysis of(a)750 °C/1h(b)550 °C/4h(c)AW 316N SS WM.
During SCC studies using constant load tests,elongation of the specimen is measured using LVDT.A reference plot of elongation vs.time to failure(tf)is shown in Fig.9.This plot is known as corrosion creep curve which can be divided into three regions as shown in Fig.9[33].From the corrosion creep curve steady state elongation rate(iss)can be calculated from the secondary region of the curve.The corrosion creep curves for the weldments were plotted(Fig.10),issvalues were obtained from these curves and plotted in Fig.11.However,in present study SCC experiments were carried out only at 80%of YS for all the weld metals,therefore life estimation could not be done.However,using creep elongation curves,the relation betweenissand SCC as well astfcan be elicited(Fig.11).The SCC resistance of 316N SS weld metals in AW and thermally aged conditions in terms oftfis shown in Fig.12.The SA specimen showed the highest SCC resistance followed by the specimens in AW,550°C/4 h conditions,whereas weldment in 750°C/1h condition showed the least resistance.SCC behavior of a weldment is influenced by a variety of factors,which include amount,size,morphology and continuity of theδ-ferrite network,residual stresses and density of metallurgical defects present in the weld metal,alloy element partitioning and segregation at the austenite/δ-ferrite interfaces.Kwok et al.[34]in their work on SCC behavior of laser welded 304 and 316 stainless steel showed that presence of δ-ferrite adversely influenced its resistance.Manning et al.[35]suggested that pitting corrosion resistance in 304L weld metal was influenced by the presence ofδ-ferrite adversely.Their study showed that higher theδ-ferrite content,lower was the pitting resistance,however,absence ofδ-ferrite had no effect onEpitvalues.Since,a pit is a precursor to SCC,this study could be correlated with the observations in the present work too which showed highest SCC resistance for SA weld metal.In SA weld metal absence of ferrite as well as homogenization of the microstructure(elimination of microsegregation as well as precipitates)significantly influenced its SCC resistance.Also,the additional boundaries present due to γ/δinterface dissolved completely eliminating the complexities,which made the passive film much more stable thereby increasing its SCC resistance.Presence ofδ-ferrite in 550°C/4 h aged weldments,acts as an additional interface where the cracks initiate thereby retarding the SCC resistance.Similar observation was made by Cai et al.[36]on their work on 308L and 309L Mo fusion zone of weld metal.Also,δ/γ interface with several imperfections due to partial or complete transformation of deltaferrite to χ/σ with resultant alloying element depleted zones,in heat treated weldments would create unstable passive film.It is well known that interface boundaries are generally preferred sites for precipitation of new phases,adsorption of impurities and chemical attack,which destabilize the passive film.These factors lead to imperfections along the grain boundaries and weaken the passive film.The imperfections probably affect the structure and properties of the passive film,resulting in a moreimperfect or more susceptible film which undergoes localized corrosion attack.It has been suggested that sulfur and phosphorus segregation at the δ/γ interface weakens the passive film and enhances its breakdown and subsequent metal dissolution resulting in environment assisted crack initiation.At the δ/γ interface,segregation of the impurity elements also increases the partitioning of alloying elements resulting in heterogeneities which cause instability of the passive film,thereby deteriorating the SCC resistance of other WM specimens as compared to SA 316N SS weld metal.
Analysis of failed Specimens using SEM-EDS:Heat treatment at 750°C/1h transformed δ-ferrite to other intermetallic phases such asσand secondary austenite as observed in Fig.13(a).Pujar et al.[37]reported the transformation kinetics of delta ferrite in 316L weldments and its influence on pitting and intergranular corrosion resistance.Studies showed that as-deposited weld metal withδ-ferrite had superior pitting corrosion resistance.However,on heat treatment it transformed to carbides andσthereby resulting in chemical inhomegenity and inferior pitting corrosion resistance.On comparing the SCC resistance of thermally aged weldments at 550°C/4h and 750°C/1h,the former showed better SCC resistance because it had higher amount of ferrite which has higher diffusivity for minor alloying elements like sulphur and phosphorous,thereby reducing their microsegregation at the δ/γ interface boundaries[38].On the other hand,thermally aging at 750°C/1h,results in decomposition of delta-ferrite along with γ/δinterface causing alloying element partitioning and segregation at the interface leading to further detoriation in its SCC resistance.AW 316N SS weldment showed presence of carbides as shown in Fig.13(c).This is because during multipass welding,the weld will experience very high temperatures between 450 and 850°C resulting in nucleation of carbides.
3.3.Laser Raman spectroscopic analysis

Fig.14.LRS analysis of SCC failed 316N SS WM specimens in(a)SA,(b)AW,(c)550 °C/4h,(d)750 °C/1h conditions.
Laser Raman spectroscopic analysis was carried out on the fractured surface after SCC failure to analyze the corrosionproducts responsible for SCC failure.LRS plots are shown in Fig.14(a-d).Analysis of various compounds and their peak positions for solution annealed,as deposited,550 ºC/4 h,750 ºC/1 h 316N SS WM is shown in Table 5.Oxides,hydroxides and oxyhydroxides of Fe,Cr were detected from LRS analysis of the weld metals.The contributions of other elements apart from Fe,Cr,Mo and Ni were not expected to be detected in surface layer,since their contents in the alloy were low.Peak positions for oxides and hydroxides of iron were assigned based on available literature[39-44].SA 316N SS weld metal showed minimum amount of corrosion products indicating the formation of an adherent and stable passive film,which resisted pitting corrosion,since there were no inhomogeneities on the SA weld surface,as solution annealing treatment had homogenized the segregation thereby delaying the initiation of cracks.In case of AW 316N SS weld metal,the presence ofγ-Fe2O3(maghemite)was observed at around 267 and 350 cm-1.Cr2O3and MoO3peaks were identified based on the literature reports[45,46].During localized corrosion attack,β-FeOOH(akaganeite)is expected to form.Some authors[47]reported presence ofβ-FeOOH in solutions containing high chloride concentrations just as in the present case.Corrosion products formed on heat treated weld metals showed oxides of Cr,Mo and Fe.However,the intensity of these oxides was higher in 750°C/1 h suggesting increased dissolution of the passive film,which is possibly due to number of heterogeneities present.Two different crack morphologies were observed in the stress corrosion samples,as shown in Fig.15(a and b):1.Transgranular stress corrosion cracking of austenite where some cracks propagated intergranularly as shown by an arrow.2.Interdendritic cracking or dissolution of the interface betweenδferrite and austenite was observed,wherein the crack path followed the dendritic pattern of ferrite distribution as shown in Fig.15(b).Two competing factors influence the mode by which SCC takes place i.e.slip step formation and dissolution of slip steps[48].The transition to IGSCC occurs when the slip step formation rate is greater than the corrosion rate of the slip step.This leads to dislocation pileups at the grain boundaries,causing stress concentration resulting in intergranular dissolution.Such interface cracking has been reported by Sherman et al.[8],Stalder and Duquette[10]and Shaikh et al.[49]Sherman et al.[8]reported that cracking proceeded along the interfaces that were oriented near normal to the tensile stress.They observed similar,but less severe,interfacial attack in unstressed specimens.Thus,theyattributed the SCC growth to the stress-assisted dissolution of the δ/γ interfaces.Stalderand Duquette[10]attributed the preferential attack of theδ/γinterface to the nonequilibrium segregation of solute elements in the weld metal.It is suggested that when a transgranularly initiated crack in austenite phase of the weld metal encounters an interface of a δ/γ,it is arrested since δ-ferrite is resistant to SCC in chloride containing environment.This causes increasing dislocation pileups at the crack tip,leading to a stress concentration at the interface and causing its dissolution.Interfacial characteristics,such as high dislocation density,vacancies and impurity atom concentration would assist the interfacial dissolution.
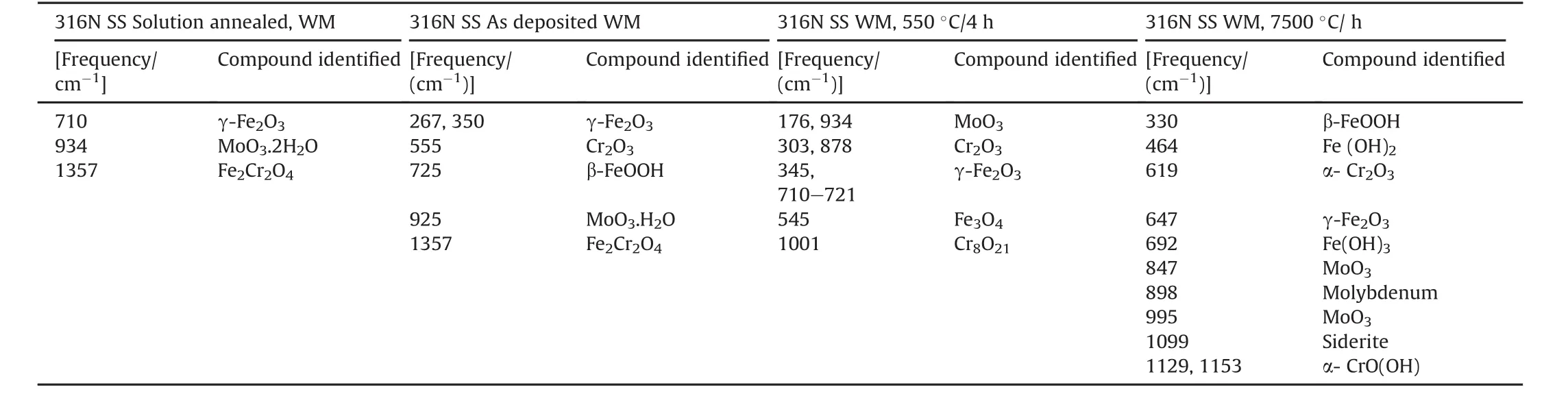
Table 5The LRS peak analysis for 316N SS WM after SCC tests.

Fig.15.SEM fractrographs which showed transgranular cracking of(a)austenite and(b)stress assisted dissolution ofδ-ferrite.
Thus,it could be realized that high-temperature heat-treatment could be detrimental to the welded components,whereas solutionannealing treatment would be helpful in restoring the mechanical as well as corrosion properties of the weldments.
4.Conclusions
1.Fraction ofδ-ferrite in as-welded 316N SS weld metal transformed to M23C6,χandσphases on thermal aging which influenced the mechanical properties of 316N SS weld metals.
2.DLEPR studies showed that welding did not result in Crdepletion in the 316N SS welds metal and thermal ageing treatments also did not result in sensitization.
3.Corrosion creep curves indicated that as theissincreased,time to failure(tf)increased.There is one to one correlation between the two i.eissαtf.
4.SCC resistance was highest for SA weld metal followed by AW weld metal.Heat treatment deteriorated the SCC resistance due to M23C6andσprecipitation.
5.Fractographic studies showed transgranular failure for austenite and stress assisted dissolution ofδ/γ interfaces.
6.LRS studies showed oxides,hydroxides and oxyhydroxides formed on the surface of SCC failed specimen in proportion to their SCC resistance.
The study concludes that irrespective of maintaining appropriate cooling rate during heat-treatment,a small amount of transformation ofδ-ferrite can deteriorate the localized corrosion resistance(pitting and SCC)of 316N SS weld metals.
[1]Specification For Modified E316-15 Austenitic Stainless Steel Welding Electrodes,PFBR/30000/SP/1032/R-2.
[2]Baeslack III WA,Savage WF,Duquette DJ.Effect of nitrogen on the microstructure and stress corrosion cracking of stainless steel weld metals.Weld J 1979;58:83s-90s.
[3]Farrar RA.Influence of microsegregation on phase transformations and properties of type 316 weld metals at elevated temperatures,stainless steels’84.London:The Institute of Metals;1986.p.336-42.
[4]Pujar MG,Dayal RK,Malhotra SN,Gill TPS.Evaluation of microstructure and electrochemical corrosion behavior of austenitic 316 stainless steel weld metals with varying chemical compositions.J Mater Eng Perform 2005;14:327-42.
[5]Lu BT,Chen ZK,Luo JL,Patchett BM,Xu ZH.Pitting and stress corrosion cracking behavior in welded austenitic stainless steel.Electrochim Acta 2005;50:1391-403.
[6]Anita T,Shaikh H,Khatak HS,Amarendra G.Effect of heat input on the stress corrosion cracking behavior of weld metal of nitrogen-added AISI type 316 stainless steel.Corrosion 2004;60:873-80.
[7]Flowers JW,Beck FH,Fontana M.Corrosion and age hardening studies of some cast stainless alloys containing ferrite.Corrosion 1963;19:186t-98t.
[8]Sherman DH,Duquette DJ,Savage WF.Stress corrosion cracking behavior of duplex stainless steel weldments in boiling MgCl2.Corrosion 1975;31:376-80.
[9]Gooch TG.Stress corrosion cracking of welded austenitic stainless steel.Weld World 1984;22:64-76.
[10]Stalder F,Duquette DJ.Slow strain rate stress corrosion cracking of type 304 stainless steels.Corrosion 1977;33:67-72.
[11]Ray SK,Shankar V,Balasubramaniam V,Sethi VK.Heat treatment of austenitic stainless steel components.In:Proceedings of the seminar materials R&D for PFBR;2003.p.215-31.
[12]Parvathavarthini N,Dayal RK,Khatak HS,Shankar V,Shanmugam V.Sensitisation behavior of modified 316 N and 316L stainless steel weld metals after complex annealing.J Nucl Mater 2006;355:68-82.
[13]Chandra K,Kain Vivekanand,Raja VS,Tewari R,Dey GK.Low temperature thermal ageing embrittlement of austenitic stainless steel welds and its electrochemical assessment.Corrosion Sci 2012;54:278-90.
[14]Suuatala N.Effect of solidification conditions on the solidification mode in austenitic stainless steels.Metall Mater Trans 1983;14:191-7.
[15]DeLong WT.Ferrite in austenitic stainless steel weld metal.Weld J 1974;53:273s-86s.
[16]Kotecki DJ,Siewert TA.WRC-1992 Constitution diagram for stainless steel weld metals:a modification of the WRC-1988 diagram.Weld J 1992;71:171s-8s.
[17]Elnura Gadirova,Comparison of corrosion resistance between 316 and different duplex stainless steel grades,Thesis by Faculty of Science and Technology,University of Stavanger.
[18]Potgieter JH,Olubambi PA,Cornish L,Machio CN.El-Sayed M.Sherif,Influence of nickel additions on the corrosion behavior of low nitrogen 22%Cr series duplex stainless steels.Corrosion Sci 2008;50:2572-9.
[19]Mathiesen T,Hansen JV.Consequences of sigma phase on pitting corrosion resistance of duplex stainless steel.In:Presented at duplex world 2010 conference beaune,France;October 2010.p.1-11.
[20]Escriba DM,Materna-Morris E,Plaut RL,Padilha AF.Chi-phase precipitation in a duplex stainless steel.Mater Charact 2009;60:1214-9.
[21]Kasper JS.The ordering ofatoms in the chi-phase ofthe iron chromium-molybdenum system.Acta Metall 1954;2:456-61.
[22]Hughes H,Llewelyn DT.χphase in the Fe-Cr-Ni-Ti system.J Iron Steel Inst 1959;192:170-8.
[23]Okafor ICI,Carlson ON.Equilibrium studies on a chi phase strengthened ferritic alloy.Metall Trans A 1978;9:1651-7.
[24]Lee EH,Maziasz PJ,Rowcliffe AF.The Structure and composition of phases occurring in austenitic stainless steels in thermal and irradiation environments.In:Proc.of the AIME symposium on irradiation phase stability,Pittsburgh,PA;1980.
[25]Ward AL.Thermal and irradiation effects on the tensile and creep rupture properties of weld deposited type 316 stainless steel.Nucl Technol 1974;24:201-15.
[26]Chopra OK,Chung HM.In:Theus GJ,Weeks JR,editors.Environmental degradation of materials in nuclear power systems-water reactors.Warrendale,Pa:T.M.S.-A.I.M.E.;1988.p.737-48.
[27]Vitek JM,David SA,Alexander DJ,Keiser JR,Nanstad RK.Low temperature aging behavior of type 308 stainless steel weld metal.Acta metal.Mater 1991;39:503-16.
[28]Shaikh H,Vinoy TV,Khatak HS.Correlation of microstructure and tensile properties of 316 stainless steels weld metal solution annealed at high temperatures.Mater Sci Technol 1998;14:129-35.
[29]Shashank Dutta B,Sasikala G,Shanthi G,Venugopal S,Nani Babu M,Kumar Parida Pradyumna,Bhaduri AK.Mechanical behaviour of SS 316(N)weld after long term exposure to service temperatures.Procedia Engineering 2011;10:2725-30.
[30]Majidi AP,Streicher MA.The double loop reactivation methods for detecting sensitization in AISI 304 stainless steels.Corrosion 1984;40:584-93.
[31]Pujar MG,Parvathavarthini N,Dayal RK.Influence of solution annealing and stress relieving on the pitting corrosion resistance of modified 316N SS weld metals:a study using EN technique.Mater Chem Phys 2010;123:407-16.
[32]Gill TPS,Vijayalakshmi M,Gnanamoorthy JB,Padmanabhan KA.Transformation of delta ferrite during the postweld heat treatment of type 316L stainless steel weld metal.Weld J 1986;66.122-s-128s.
[33]Nishimura R,Kudo K.Stress corrosion cracking of AISI 304 and AISI 316 austenitic stainless steels in HCl and H2SO4solutions-prediction of time to failure and criterion for assessment of SCC susceptibility.Corrosion Sci 1989;45:308-16.
[34]Kwok CT,Lo KH,K Chan W.Stress corrosion cracking of laser welded stainless steels.In:Proceedings of the 1st international joint symposium on joining and welding,Osaka,Japan;6-8 November 2013.
[35]Manning PE,Duquette DJ,Savage WF.The effect of retained ferrite on localized corrosion in duplex 304L stainless steel.Welding Research Supplement 1980:260s-2s.
[36]Cai JB,Yu C,Shiue RK,Tsay LW.Stress corrosion cracking of austenitic weld deposits in a salt spray environment.J Nucl Mater October 2015:774-83.
[37]Pujar MG,Kamachi Mudali U,Dayal RK,Gill TPS.Susceptibility of as-welded and thermally aged type 316LN weldments toward pitting and intergranular corrosion.Corrosion 1992;48:579-86.
[38]Toppo A,Pujar MG,Arivazhagan B,Vasudevan M,Mallika C,Kamachi Mudali U.Corrosion behaviour of 304LN activated tungsten inert gas and fluxcored arc weld metals.Corrosion Eng Sci Technol 2016:1-13.
[39]Poursaee Amir.Corrosion of steel bars in saturated Ca(OH)2and concrete pore solution.Concr Res Lett 2010;1(3):90-7.
[40]Legodi MA,de Waal D.The preparation of magnetite,goethite,hematite and maghemite of pigment quality from mill scale iron waste.Dyes Pigments 2007;74:161-8.
[41]Ohtsuka T,Kubo K,Sato N.Raman spectroscopy of thin corrosion films on iron at 100 to 150°C in air.Corrosion 1986;42:476-81.
[42]Oblonsky LJ,Devine TM.A surface enhanced Raman spectroscopic study of the passive films formed in borate buffer on iron,nickel,chromium and stainless steel.Corrosion Sci 1995;37:17-41.
[43]Adams FV.A comparison of the corrosion behaviour of 444 ferritic and 316 austenitic stainless steels in acidic chloride media.A dissertation submitted to the Faculty of Engineering and the Built Environment.Johannesburg:University of the Witwatersrand,in fulfilment of the requirements for the degree of Masters of Science in Engineering;2009.p.1-144.
[44]Gui J,Devine TM.A SERS investigation of the passive films formed on Iron in mildly alkaline solutions of carbonate/bicarbonate and nitrate.Corrosion Sci 1995;37:1177-89.
[45]Sousa PM,Silvestre AJ,Popovici N,Conde O.Morphological and structural characterization of CrO2/Cr2O3films grown by Laser-CVD.Appl Surf Sci 2005;247:423-8.
[46]Seguin L,Figlarz M,Cavagnat I,R,Lassegues J-C.Infrared and Raman spectra of MoO3,molybdenum trioxides and MoO3.xH2O molybdenum trioxide hydrates.Spectrochim Acta,Part A 1995;51.1323-134.
[47]Graedel TE,Frankenthal RP.Corrosion mechanism for iron and low alloy steels exposed to the atmosphere.J Electrochem Soc 1990;137:2385-94.
[48]Muraleedharan P,Khatak HS,Gnanamoorthy JB,Rodriguez P.Effect of cold work on stress corrosion cracking behavior of types 304 and 316 stainless steels.Metall Mater Trans 1985;16:285-9.
[49]Shaikh H,Khatak HS,Seshadri SK,Gnanamoorthy JB,Rodriguez P.Effect of ferrite transformation on the tensile and stress corrosion properties of type 316L stainless steel weld metal thermally aged at 873K.Metall Mater Trans 1995;26:1859-68.
- Defence Technology的其它文章
- Effects of ply orientation and material on the ballistic impact behavior of multilayer plain-weave aramid fabric targets
- Effect of magnesium on FOX-7 and its tautomers-A DFT treatment
- Influence of welding consumables on tensile and impact properties of multi-pass SMAW Armox 500T steel joints vis-a-vis base metal
- Effect of functional composite coating developed via sulphate and chloride process parameter on the UNS G10150 steel for structural and wear mitigation in defence application
- Optimizing submerged arc welding using response surface methodology,regression analysis,and genetic algorithm
- Virtual ballistic impact testing of Kevlar soft armor:Predictive and validated finite element modeling of the V0-V100probabilistic penetration response

