Point-of-care Ultrasound as a Useful Tool for Assessment of Undifferentiated Dyspnea in Rapid Response Team Consultation
Du, Jiang MD., Lin, Qiuhai MD., Xie, Hui MD., Zhou, Zhigang MD., Wang, Ruilan MD., PhD.*
Du, Jiang: First author, gowindj@163.com,
Background
Patients may have unexpected deterioration or even death during hospitalization, with an incidence of 2.9%-16.6%. Patients may deteriorate rapidly and leads to increased disability or even death. Such severe in-hospital deterioration may increase disease costs, prolong hospitalization and significantly affected the prognosis of patients[1-4]. Dyspnea is often one of the primary manifestations of patients with severe in-hospital deterioration; it is often accompanied by hemodynamic abnormalities and conscious changes. It is difficult to differentiate the diagnoses in such patients since the sudden onset and severe symptoms often preclude patients from undergoing detailed examinations such as CT scans. The application of point of care ultrasound may be able to quickly identify acute respiratory difficulties in a resource limited setting. Studies of POCUS have proven its value in identifying pulmonary diseases like pneumothorax, pulmonary edema and pneumonia[5-7]. Meanwhile in cardiovascular diseases such as myocardial infarction, congestive heart failure, pulmonary embolism, and shock, POCUS has also shown great diagnostic accuracy[8-9]. In recent years, utilization of ultrasound for the rapid identification of pathology is increasing due to improvements in the portability and cost of ultrasound equipment. These advancements are seen specifically in the emergency and intensive care settings[10-14]. We introduced the bedside ultrasound to RRT consultation in the in-hospital deterioration with severe respiratory difficulty to verify that whether the ultrasound will improve the diagnostic accuracy of such diseases.
Materials and Methods
StudyDesign
This was an observational study including patients with in-hospital deterioration manifested mainly by acute dyspnea. Ultrasound was first introduced as diagnostic tool in RRT consultant from March 2014. We documented those dyspneic patients who were consulted and POCUS screened by RRT from March 2014 through March 2016 in Shanghai General Hospital. Physicians taking care of those patients with in-hospital onset of dyspnea of unclear etiology consulted a rapid response team. All dyspneic patients consulted by RRT were screened with POCUS. The pre-POCUS diagnosis were given by the treating physicians before the ultrasound examination according to patients′medical history, symptoms, physical examination and lab testing. RRT physicians would also complete a physical exam and look at their lab tests. They would perform an ultrasound exam. After these patients dislocation to ICU, two medical directors would reach a final diagnosis based on ultrasound, CT and other examinations. The final diagnoses were treated as criterion diagnosis. In our hospital, intensivist from critical care department were in charge of the Rapid Response Team.
StudyPopulation
Patients were consecutively enrolled from March 2014 through March 2016. The Inclusion criteria was as follows[15]: (1) Age>18 years; (2) Noisy Breathing or stridor; (3) Airway obstruction; (4) Any difficulty breathing; (5) respiratory rate <8 per minute; (6) respiratory rate>30 per minute; (7) Oxygen saturation <90% despite high-flow oxygen; (8) Apnea; (9) Dyspnea with difficulty in differentiate etiologically: ① Dyspnea was difficult to be explained by the primary disease; ② serious condition with sudden onset within 24 hours; ③ Without recent recurrent history; ④ High risk of CT scan or even CT scan could not identify the diagnosis. In order to be included, patients had to meet one of criteria 1-8 and criterion 9.
Exclusion criteria were: (1)Younger than 18 years; (2)Clear history of recurrent episodes of dyspnea with known etiology; (3)Respiratory dyspnea secondary to a deteriorated primary disease or any end-stage disease; (4) Chronic onset, longer than 24 hours; (5) Known diagnosis.
Ultrasonographical Assessment
Lung ultrasound: Sonosite M-turbo from FUJIFILM (China, Investment Co, Ltd) was the POCUS tool we used and a 5-MHz curved (C60x) transducer for lung ultrasound which was based on BLUE protocol[6]. We examined each side of the chest wall in 5 examination zones: the second and fourth intercostal space in the midclavicular line, the same intercostal space at the mid-axillary line and the subscapularis area on the back. We focused on the following signs: (1) Sliding sign: the back and forth movement between the visceral and parietal layers of the pleura. Patients were diagnosed with pneumothorax if the sliding sign appeared and disappeared quickly in accordance with the pace of respiratory rate which may indicate a lung point sign; (2) A line: a horizontal line which was parallel to the bright pleura line. If located below the pleural line, less bright than pleural line; (3) B line: B-lines were defined as discrete laser-like vertical hyperechoic artifacts that arise from the pleural line and extended to the bottom of the image without fading. When the B lines appeared in more than three of the intercostal spaces, pulmonary edema or interstitial syndrome was considered. Echocardiography was then used to further differentiate the diagnosis[16]; (4) Consolidation sign: the liver tissue like appearance of the pulmonary parenchyma may indicate the consolidation of lung tissue caused by pneumonia; (5) According to the BLUE-protocol6, healthy lung tissue, pneumothorax, pulmonary edema, pneumonia and atelectasis could be identified by POCUS.
Echocardiography: The hearts were evaluated according to FATE protocol[17]: A 1- to 5-MHz phased array (P21x) transducer (Sonosite Inc, Shanghai, China) was used for echocardiography. The focused cardiac ultrasound included 3 views of the following: parasternal long axis view, parasternal short axis view and apical four chamber view with or without color Doppler. The parasternal long axis view was used to estimate the left ventricle systolic function visually or calculate the ejection fraction. Segmental ventricle wall dysfunction or paradoxical movement was also determined. At the parasternal short axis view, we evaluated the size of both ventricles. If there was a D sign, which indicates an enlarged right ventricle, acute pulmonary embolism was considered to be highly likely. Using the apical four chamber view, we evaluated for mitral valve regurgitation and stenosis. Presence and size of pericardial effusion can be identified in each of the views.
Inferior vena cava: The cardiac probe was placed at the sub-xiphoid or a laterally right view. The diameter of inferior vena cava was measured 2 cm proximal to the hepatic vein confluent point. Both the diameter and the collapse during inspiration were measured. Diameter≤2.1 cm and collapse>50% might indicate a normal CVP~3 mmHg(0~5 mmHg). When Diameter>2.1 cm, collapse<50% might indicate of high CVP~15 mmHg(10~20 mmHg). When Diameter≤2.1 cm collapse<50% or Diameter>2.1 cm collapse>50% might indicate an intermediate CVP of 8 mmHg (5~10 mmHg)[18].
Statistical Analysis
The final diagnosis was treated as the criterion standard. Here accurate pre-POCUS or post-POCUS diagnosis was defined as those with accordance with the final diagnoses. If the final diagnose failed to be reached, the pre and post-POCUS were also considered as failure and recorded as inaccurate. We divided the patients into two groups. Post-POCUS+ groups was those POCUS diagnosed accurately with accordance to the final diagnoses. Post-POCUS- group was those POCUS improperly diagnosed according to final diagnoses or POCUS fail to diagnose. Continuous data with normal distribution were analyzed by student t test between groups. Binary data like mortality, accuracy of diagnoses were tested by chi-square or fisher′ exact test. Also the pre and post POCUS diagnoses on each case were analyzed. Data were stratified according to the classification of disease. Diagnostic sensitivities and specificities to each stratification were analyzed and tested by mcnemar and kappa test. The total accuracy of the pre and post POCUS diagnosed were compared by McNema test. We used R 3.3.3 to do all the statistic test.
Result
During the study period, there were a total of 95,235 in-hospital patients discharged. There were 538 patients with critical care consults and 144 patients of them were dyspneic and required a RRT consultation. 53 patients needed further differentiation and PUCUS help. POCUS failed in further distinguishing the etiologies of dyspnea in 11 patients (Fig 1). The diagnoses and departments are listed in Table2. The highest incidence of in-hospital adverse deterioration happened in the ICUs and emergency department. Obstetrics was second followed by general surgery department and orthopedics. Our obstetrics department is a regional referral center in Shanghai Songjiang district so they have relatively higher risk patients. According to the final diagnosis, the most common adverse events were cardiogenic shock mainly caused by myocardial infarction. The incidence of valvular heart diseases was in the second place. The third most common cause was acute pulmonary embolism followed by hypovolemic shock. The relations between the pre-POCUS, post-POCUS and final diagnoses are listed in a cross table (Table 3). The Kappa value between pre-POCUS and final diagnosis was 0.516. The Kappa value between post-POCUS and final diagnosis was 0.91. The Kappa Value between pre and post PUCUS diagnosis was 0.58. Leveled data is listed in Table 3 according to different diseases. The incidences of acute congestive heart failure, pneumonia or atelectasis were not as high as imagined (Table 4). In the diagnosis of cardiac shock, valvular heart diseases and pulmonary embolism, there were increases of sensitivity and specificity but not significantly(P>0.05). We saw a statistical difference in the post-POCUS diagnosis of pneumonia. McNemar test were used to compare pre-POCUS and post-POCUS diagnosis accuracy. The accuracy increased from 37.7% (20/53) to 79.2% (42/53) in post-POCUS which is statistically significant (P<0.01), (Fig 2A). In post-POCUS+ group, we saw a significantly lower rate of mortality 26.2% (11/42) versus 63.6% (7/11) in post-POCUS- group (Fig 2B). The information about those 11 POCUS failures were listed in table5.
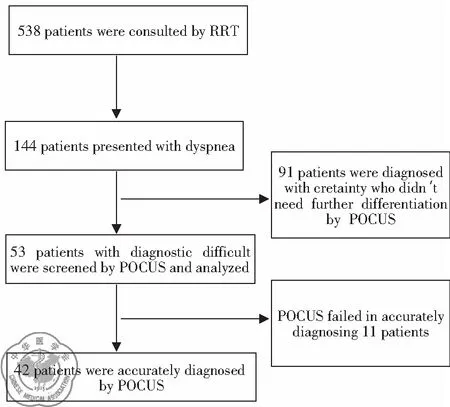
Fig 1 Flow chart of this study
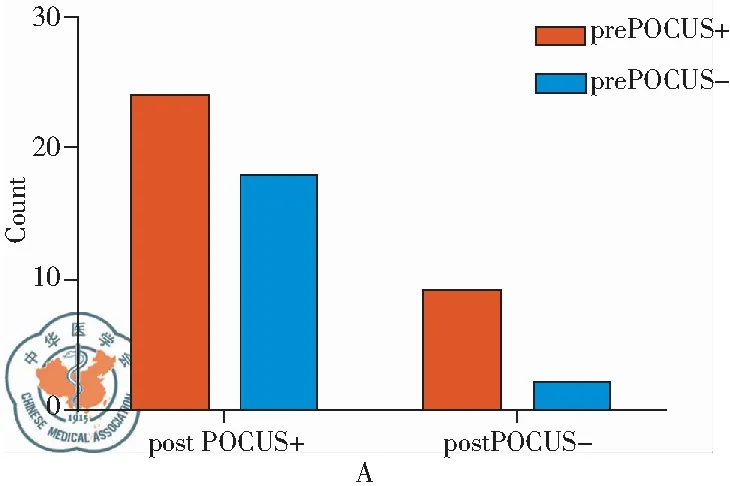
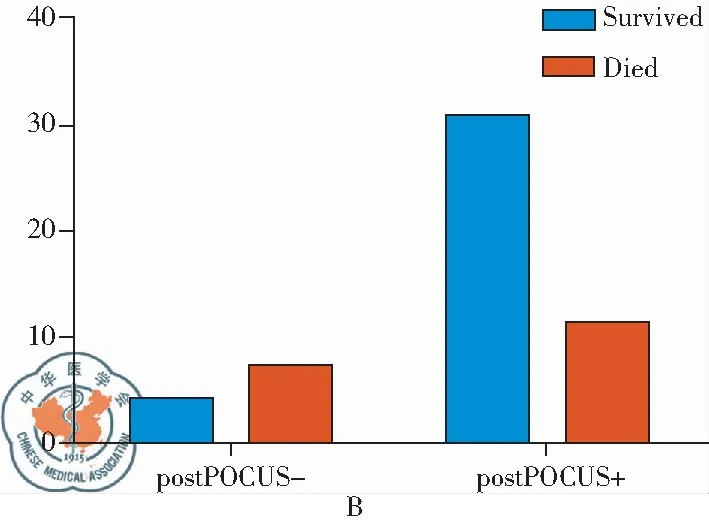
Fig 2 A:Mcnemar test of pre and post POCUS diagnoses,P<0.01; B:The Mortality in Two Groups,P<0.05; +: diagnoses were righ according to final diagnose; -: diagnoses were wrong or fail to diagnose
Discussion
Studies have shown that the incidence of sudden adverse events in the hospital to be 2.9%-16.6%[1-4]. The outcome of patients would be strongly affected. Researchers have attempted to reduce mortality by early detection of deteriorating patients and rapid intervention[19]. However, in a resource limited condition, it is difficult to differentiate the diagnoses as patients are often not stable enough to have CT scans done. Ultrasound may be an optimal diagnostic tool in such a resource limited setting. Different from other POCUS studies, here we introduced ultrasound as a diagnostic tool in RRT consultant to test its feasibility and accuracy.
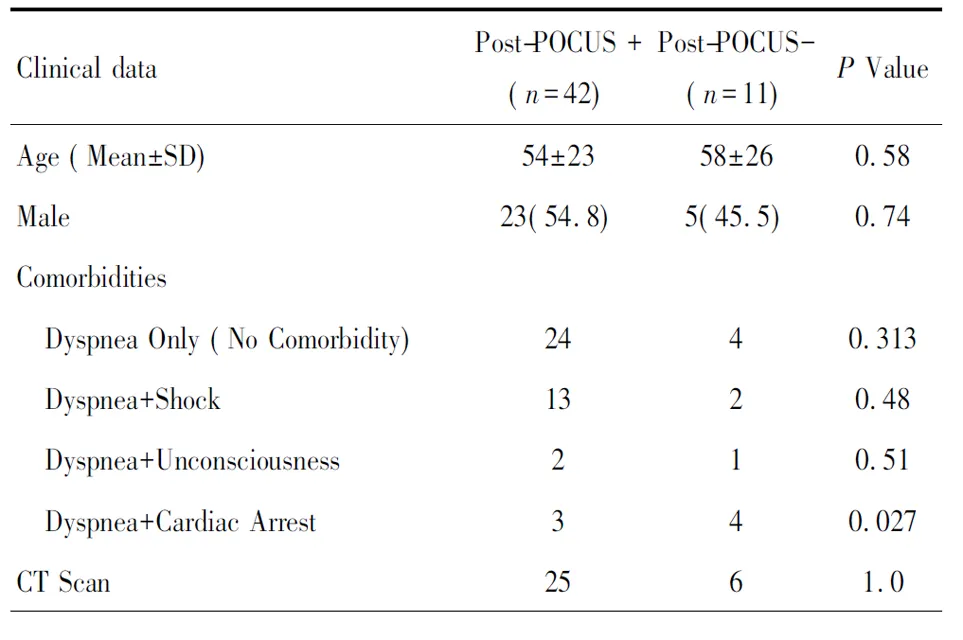
Table1 Baseline data in two groups(%)
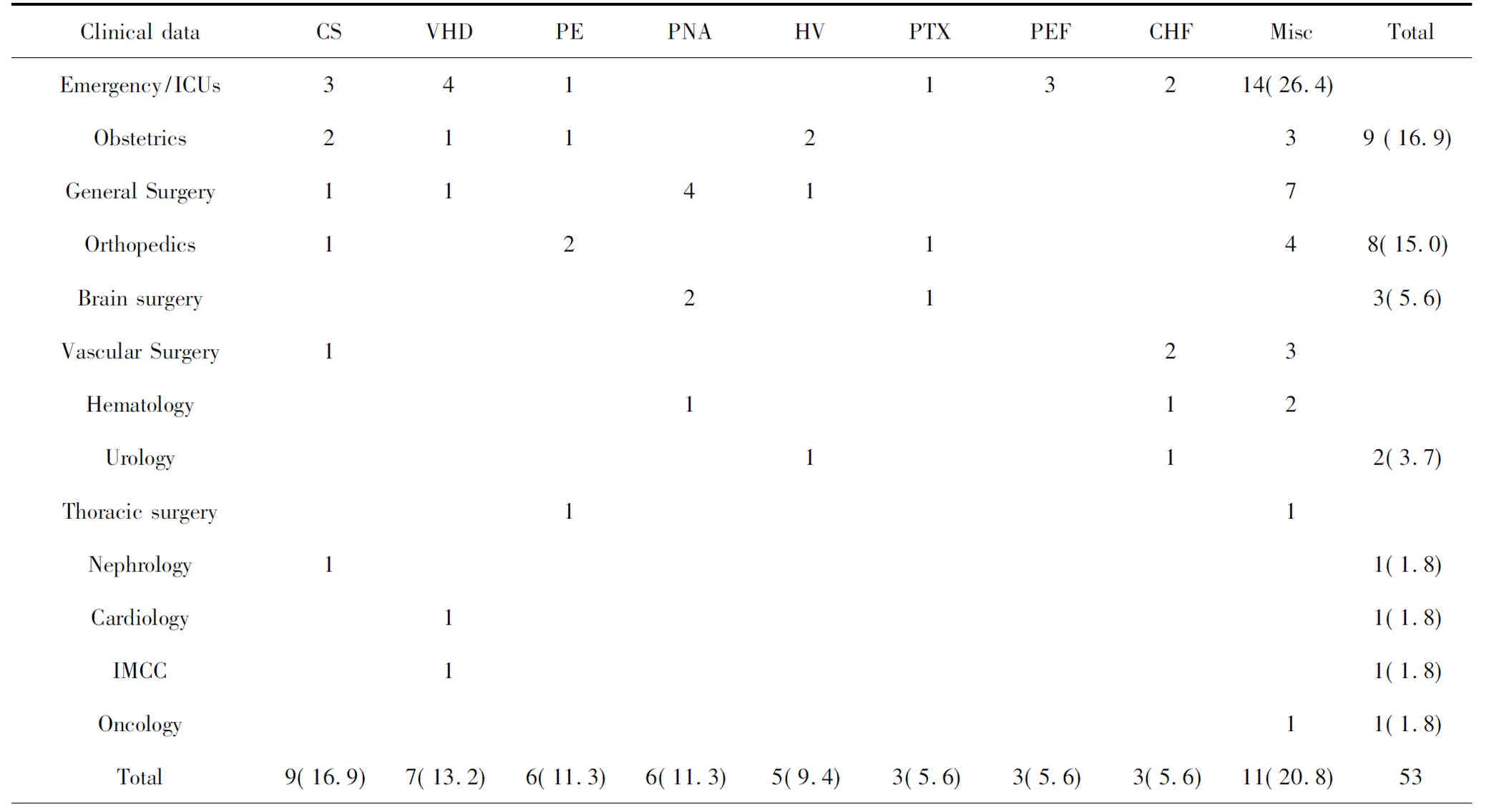
Table 2 The final diagnoses and departments(%)
Final Diagnoses: Misc: other diseases; CS: cardiogenic shock; VHD: valvular Heart disease; PE: Pulmonary Embolism; PNA: Pneumonia; HV: Hypovolemia; PTX: Pneumothorax; PEF: Pericardium Effusion; CHF: Congestive Heart Failure.
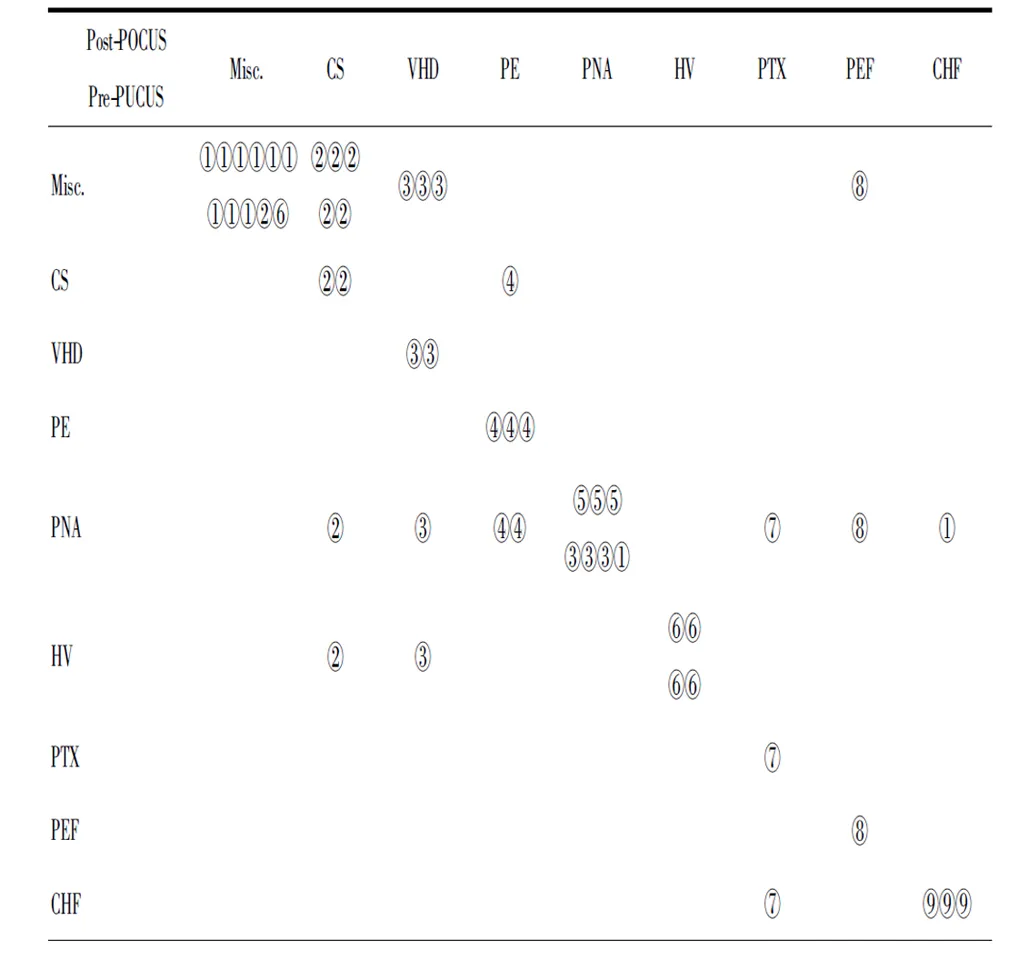
Table3 The Kappa value between pre-POCUS and final diagnosis is 0.516. The Kappa value between post-POCUS "picture_table_title">and final diagnosis is 0.91. The the Kappa Value between pre and post PUCUS diagnosis is 0.58.
Final Diagnoses: ①Misc.: other diseases; ②CS: cardiogenic shock; ③VHD: Valvar Heart disease; ④PE: Pulmonary Embolism; ⑤PNA: Pneumonia; ⑥HV: Hypovolemia; ⑦PTX: Pneumothorax; ⑧PEF: Pericardium Effusion; ⑨CHF: Congestive Heart Failure.
Ultrasound has now become an important point of care diagnostic tool especially for emergency and critical physicians to identify pneumonia, pneumothorax, and other acute lung diseases. In our study, three patients were identified with tension pneumothorax by POCUS after a sudden onset of desaturation in mechanical ventilation before the arrival of the chest X-ray. Studies have shown that the accuracy of ultrasound in diagnosis of pneumothorax (CT as gold standard)[7]. Also ultrasound has good diagnostic accuracy for pneumonia[20-21]. In ergent situation, it is sometimes difficult to distinguish bronchial asthma and cardiogenic asthma in clinic, which results in “double treatment”. However, lung ultrasound can easily identify these two diseases through B-line, to avoid “double treatment”. The BLUE6 protocol drafted by Lichtenstein is the most widely used pulmonary ultrasound criterion in diagnosing pulmonary diseases. However, The BLUE protocol only focuses on lung ultrasound. Dyspnea is too frequently presented in heart disease to be omitted[22-24]. Therefore, the heart and inferior vena cava should also be assessed by ultrasound.
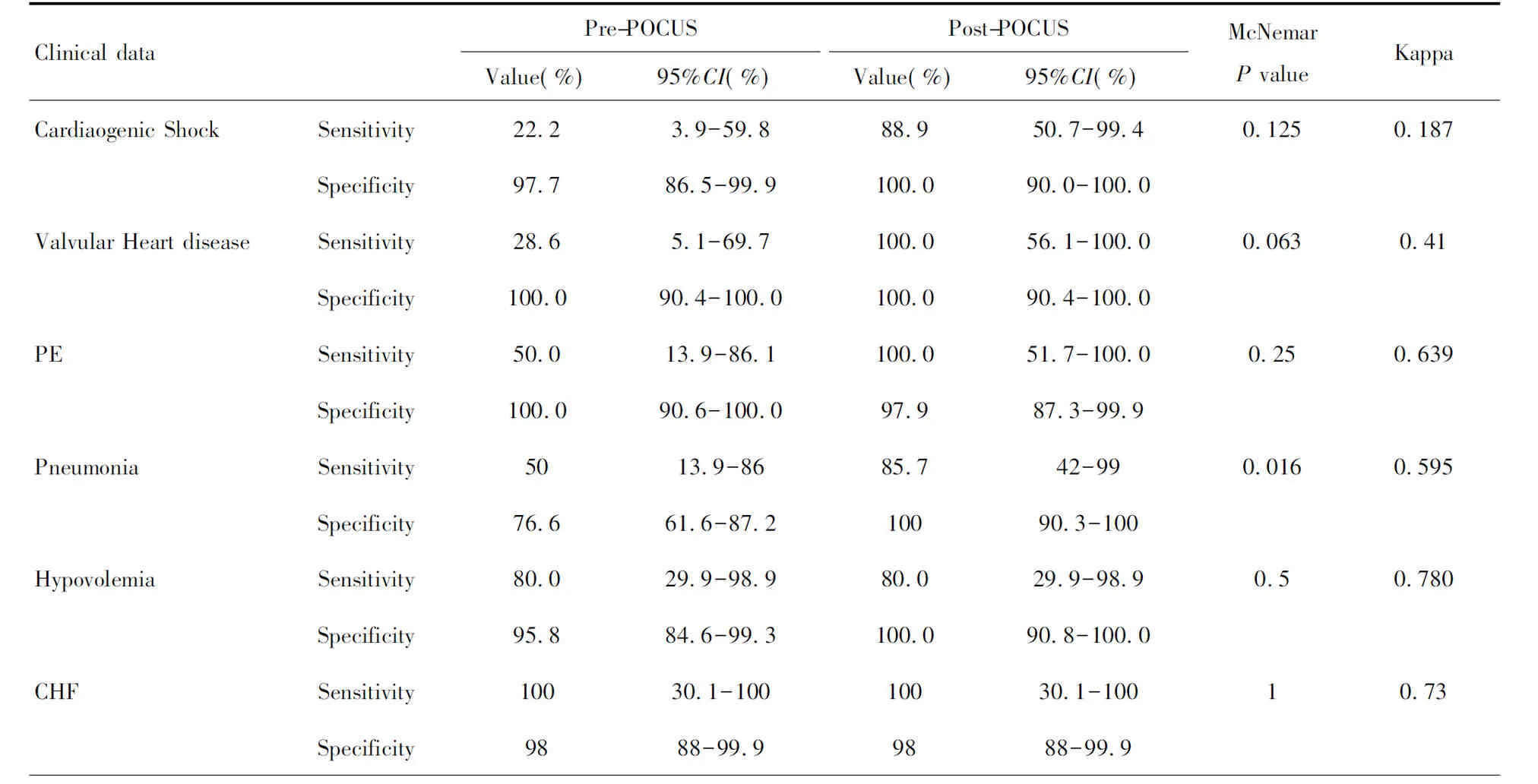
Table 4 Leveled data according to different diseases
In the diagnosis of cardiac shock, valvular heart diseases and pulmonary embolism, there were increase of sensitivity and specificity but no significant difference (P>0.05). Only in diagnosis of pneumonia we can see a significant difference in post-POCUS diagnosis.
The value of POCUS in diagnosing cardiogenic diseases and shock differentiation had received more and more attention. Heart can be assessed both morphologically and functionally by POCUS. Protocols like GDE[25], RUSH[26]and FATE[17]all indicated that ultrasound was a powerful tool for diagnosing pulmonary embolism, shock, and heart disease. In the study of Ghane, M. R, the implementation of RUSH protocol was helpful in identifying the etiological classification of shock[27]. SHOCK studies showed that 78.5% of cardiogenic shock originated from myocardial infarction[28]. In our study, 9 patients were diagnosed cardiogenic shock in which 7 cases were myocardial infarction. The most severe and difficult case was a 31-year-old woman in the process of delivery suffered a sudden onset of severe dyspnea complicated with hypotension. Originally she was diagnosed amniotic fluid embolism or pulmonary embolism. However, it was too risky to have a CTA for this patient in such setting. The RRT was consulted and a bedside POCUS excluded pulmonary embolism rapidly and found a segmental ventricle movement dysfunction by POCUS. She was diagnosed with myocardial infarction and her life was saved by the stent implantation. The higher incidence of heart valvar disease in this study was unexpected. Two hospitalized patients suffered acute myocardial infarction complicated with hemodynamic disorder and respiratory distress caused by rupture of chordae tendineae which were misdiagnosed by treating physician but found by RRT consultant with POCUS. One case presented with high fever and was diagnosed acute infective endocarditis combined with mitral valve perforation which caused acute hemodynamic changes and onset of respiratory distress. POCUS identified the mitral valve perforation bedside. Acute valvar regurgitation caused by endocarditis and myocardial infarction may cause acute and severe hemodynamic disorders that may lead to death. Such lethal situations cannot be missed when ultrasound examination[29-30]. The incidence of pulmonary embolism was high, especially in obstetrics and gynecology. Patients always suffer from difficulty of breathing combined with serious hemodynamic disorders. In BLUE protocol, the diagnosis of pulmonary embolism is only based on the pulmonary ultrasound without heart assessment. The sensitivity might be relatively low and misdiagnosis may easily happen5. Multi-organ involved POCUS including heart, lung and lower extremity vein POCUS can improve the diagnosis of acute large area pulmonary embolism accuracy[31].
Pulmonary and cardiac ultrasound should be combined for precise diagnosis. It is especially useful in distinguishing between ARDS and cardiogenic pulmonary edema[32,12-14]. The combined pulmonary and cardiac ultrasound is also useful in diagnosing pulmonary embolism, as pulmonary ultrasound alone can often lead to misdiagnosis. However, when combined with cardiac ultrasound, the diagnostic accuracy can be improved[31]. In the ICU-sound study[33], the patients in the ICU were evaluated after bedside cardiopulmonary ultrasound. 25.6% (32/125) of the patients′admission diagnosis were modified after POCUS, 58.4% (73/125) of the patients had admitted diagnosis consistent with ultrasound diagnosis; ultrasound does not work only accounted for 13.6% (17/125), missed diagnosis by ultrasound accounted for only 2.4% (3/125).
Our study is very different from those above. While the above studied patients in the ICU and ED, our patients were mostly on the general floor. Our patients′condition was also more emergent. Due to the condition of the patents, it was often too risky to have more imaging studies completed. In our study, patients were in the hospital with sudden deterioration and manifestation of dyspnea, the patients′primary problems were often other diseases such as pregnancy, trauma, fever or other non-cardiopulmonary disease when admission. Disease spectrum in our study was also very different. In the other studies, the ultrasound diagnosis of patients with dyspnea were mainly pulmonary edema by CHF, ARDS or pneumonia. However, our patients were mainly cardiogenic disease like shock, valvar disease, pulmonary embolism or cardiogenic pulmonary edema. This disease spectrum was more likely to be seen in patients with sudden onset of symptoms who were too critically ill to undergo more extensive diagnostic testing. It was often hard to differentiate the diagnose in such a setting. We propose that this is the setting where POCU can be most useful. It is a rapid and accurate bedside diagnostic tool tailor made for such a setting and patient population. In our study, nearly 20% of the patients could not be diagnosed even with the help of ultrasound. Although we saw a higher mortality in POCUS failure group, we are reluctant to say the ultrasound can improve the outcome of these critical patients. We would say that those patient might be more emergent and more complicated to disclosure the real problem. Ultrasound still requires the summation of other diagnostic tools, such as lab testing and physical examination. While the ultrasound did not confirm a diagnosis in 20% of our patients, we believe it still has utility in excluding life-threatening cardiopulmonary disorders[9].
There were several limitations in our study. The first is that this was an observational study instead of a RCT. Since we believe that ultrasound is beneficial to all patients, it was felt to be ethically unsound to withhold ultrasound evaluation in some patients. We also did not focus on outcomes. A future study could examine the outcomes in patients evaluated by POCUS. Another limitation is that the POCUS was so dependent on the dexterity of the consulting intensivist that the result may be biased on different physicians.
Conclusions
Ultrasound can be used as a rapid diagnostic tool for differentiating dyspnea in patients with in-hospital deterioration. It is helpful to improve diagnostic accuracy for a RRT consultant. Those patients with emergent pulmonary or cardiogenic dyspnea may benefit from POCUS examination. POCUS may change the diagnosis of some critical cases and change the strategy dramatically. The outcome of such patients might be influenced by POCUS. However, due to the fewer incidence of such emergent situation and patients, we might need really large size to achieve enough power to reveal ultrasound influence on patients′outcome. Current articles more focus on the diagnosis accuracy of ultrasound instead of its influence on outcome. How can POCUS improve the outcome of patients, there were no high evidence of proof[34]. More large and elegant designed researches are needed.
1 Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada[J]. CMAJ, 2004, 170(11): 1678-1686.
2 Davis P, Lay-Yee R, Briant R, et al. Adverse events in New Zealand public hospitals I: occurrence and impact[J]. N Z Med J, 2002 ,115(1167): U271.
3 Vincent C, Neale G, Woloshynowych M. Adverse events in British hospitals: preliminary retrospective record review[J]. BMJ, 2001, 322(7285): 517-519.
4 Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado[J]. Med Care, 2000, 38(3): 261-271.
5 Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol[J]. Chest, 2008, 134(1): 117-125.
6 Lichtenstein D. Lung ultrasound in acute respiratory failure an introduction to the BLUE-protocol[J]. Minerva Anestesiol, 2009, 75(5): 313-317.
7 Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis[J]. Chest, 2012,141(3): 703-708.
8 Perera P, Mailhot T, Riley D, et al. The RUSH exam: Rapid Ultrasound in SHock in the evaluation of the critically Ⅲ[J]. Emerg Med Clin North Am, 2010, 28(1): 29-56.
9 Volpicelli G, Lamorte A, Tullio M, et al. Point-of-care multiorgan ultrasonography for the evaluation of undifferentiated hypotension in the emergency department[J]. Intensive Care Med, 2013, 39(7): 1290-1298.
10 Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill[J]. Chest, 2015, 147(6): 1659-1670.
11 Wang X, Zhao H, Liu D, et al. The effects of Peking Union Medical College Hospital Critical Ultrasonic Management scheme on the etiological diagnosis of dyspnea and/or hemodynamic instability in ICU patients[J]. Zhonghua Nei Ke Za Zhi, 2014, 53(10): 793-798.
12 Shah SP, Shah SP, Fils-Aime R, et al. Focused cardiopulmonary ultrasound for assessment of dyspnea in a resource-limited setting[J]. Crit Ultrasound J, 2016, 8(1): 7.
13 Mantuani D, Frazee BW, Fahimi J, et al. Point-of-Care Multi-Organ Ultrasound Improves Diagnostic Accuracy in Adults Presenting to the Emergency Department with Acute Dyspnea[J]. West J Emerg Med, 2016, 17(1): 46-53.
14 Bataille B, Riu B, Ferre F, et al. Integrated use of bedside lung ultrasound and echocardiography in acute respiratory failure: a prospective observational study in ICU[J]. Chest, 2014, 146(6): 1586-1593.
15 Jones DA, DeVita MA, Bellomo R. Rapid-response teams[J]. N Engl J Med, 2011, 365(2): 139-146.
16 Lichtenstein D, Mézière G, Biderman P, et al. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome[J]. Am J Respir Crit Care Med, 1997, 156(5): 1640-1646.
17 Holm JH, Frederiksen CA, Juhl-Olsen P, et al. Perioperative use of focus assessed transthoracic echocardiography (FATE)[J]. Anesth Analg, 2012, 115(5): 1029-1032.
18 Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography[J]. J Am Soc Echocardiogr, 2010, 23(7): 685-713.
19 Maharaj R, Raffaele I, Wendon J. Rapid response systems: a systematic review and meta-analysis[J]. Crit Care, 2015, 19: 254.
20 Sperandeo M, Carnevale V, Muscarella S, et al. Clinical application of transthoracic ultrasonography in inpatients with pneumonia[J]. Eur J Clin Invest, 2011, 41(1): 1-7.
21 Cortellaro F, Colombo S, Coen D, et al. Lung ultrasound is an accurate diagnostic tool for the diagnosis of pneumonia in the emergency department[J]. Emerg Med J, 2012, 29(1): 19-23.
22 Konrad D, Jaderling G, Bell M, et al. Reducing in-hospital cardiac arrests and hospital mortality by introducing a medical emergency team[J]. Intensive Care Med, 2010, 36(1): 100-106.
23 Al-Qahtani S, Al-Dorzi HM, Tamim HM, et al. Impact of an intensivist-led multidisciplinary extended rapid response team on hospital-wide cardiopulmonary arrests and mortality[J]. Crit Care Med, 2013, 41(2): 506-517.
24 Beitler JR, Link N, Bails DB, et al. Reduction in hospital-wide mortality after implementation of a rapid response team: a long-term cohort study[J]. Crit Care, 2011, 15(6): R269.
25 Schmidt GA, Koenig S, Mayo PH. Shock: ultrasound to guide diagnosis and therapy[J]. Chest, 2012, 142(4): 1042-1048.
26 Seif D, Perera P, Mailhot T, et al. Bedside ultrasound in resuscitation and the rapid ultrasound in shock protocol[J]. Crit Care Res Pract, 2012, 2012: 503254.
27 Ghane MR, Gharib M, Ebrahimi A, et al. Accuracy of early rapid ultrasound in shock (RUSH) examination performed by emergency physician for diagnosis of shock etiology in critically ill patients[J]. J Emerg Trauma Shock, 2015, 8(1): 5-10.
28 Thompson CR, Buller CE, Sleeper LA, et al. Cardiogenic shock due to acute severe mitral regurgitation complicating acute myocardial infarction: a report from the SHOCK Trial Registry. SHould we use emergently revascularize Occluded Coronaries in cardiogenic shock? [J]. J Am Coll Cardiol, 2000, 36(3 Suppl A): 1104-1109.
29 Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes[J]. Circulation, 2008, 117(5): 686-697.
30 Vahanian A, Ducrocq G. Emergencies in valve disease[J]. Curr Opin Crit Care, 2008, 14(5): 555-560.
31 Nazerian P, Vanni S, Volpicelli G, et al. Accuracy of point-of-care multiorgan ultrasonography for the diagnosis of pulmonary embolism[J]. Chest, 2014, 145(5): 950-957.
32 Sekiguchi H, Schenck LA, Horie R, et al. Critical care ultrasonography differentiates ARDS, pulmonary edema, and other causes in the early course of acute hypoxemic respiratory failure[J]. Chest, 2015, 148(4): 912-918.
33 Manno E, Navarra M, Faccio L, et al. Deep impact of ultrasound in the intensive care unit: the “ICU-sound” protocol[J]. Anesthesiology, 2012, 117(4): 801-809.
34 Hew M, Tay TR. The efficacy of bedside chest ultrasound: from accuracy to outcomes[J]. Eur Respir Rev , 2016, 25(141): 230-246.

