Ethnobotanical survey of antimalarial plants in Awash-Fentale District of Afar Region of Ethiopia and in vivo evaluation of selected ones against Plasmodium berghei
Nega Alelign, Mirutse Giday, Tilahun Teklehaymanot, Abebe Animut
1Department of Biology, Faculty of Science, Addis Ababa University, P.O. Box 1176, Addis Ababa, Ethiopia
2Aklilu Lemma Institute of Pathobiology, Addis Ababa University, P.O. Box 1176, Addis Ababa, Ethiopia
1. Introduction
About 3.3 billion people are at risk of malaria globally. In 2013,there were 198 million cases and 584 000 deaths. Africa contributed to 90% of all malaria deaths, where children under five years old accounted for 78% of the deaths. However, there was a trend of significant reduction in malaria cases and deaths between 2001 and 2013. This reduction is attributed to expanded malaria funding,progress in vector control (insecticide net use and indoor residual spraying), scaled up diagnostic testing and antimalarial treatments[1].Ethiopia is also one of the sub-Saharan African countries where morbidity and mortality due to the disease is declining significantly.However, the emergence of resistantPlasmodiumparasites to most available drugs of choice posed a threat in the control of the disease.Plasmodium falciparumresistance to artimisinin has been detected in Cambodia, Lao People’s Democratic Republic, Myanmar, Thailand and Viet Nam. In many areas along the Cambodia-Thailand border,P. falciparumhas become resistant to most available antimalarial medicines. This is a threat to Ethiopia and other malarious areas of the world. Thus, it is important to search for new and effective antimalarial drugs.
Plants have been used in the treatment of malaria and several other illnesses since time immemorial[2]. The great majority of modern antimalarial drugs have been derived from medicinal plants or plant based lead compounds[2]. Among these, quinine and artemisinin are most notable antimalarial drugs. This entails a focus on plants in attempts of antimalarial research. In Ethiopia,medicinal plants have been widely used for the treatment of various ailments including malaria[3-6]. The country is rich for its plant species, culture, language and tradition which contribute to the diverse practices. However, the practices remain poorly documented and less accessible for modern research. Hence, the search for alternative antimalarial drugs of plant origin in the country requires a basic ethnobotanical survey in different localities and districts to document diverse knowledge owned by different ethnic groups and communities. So far no study has been carried out in the Afar Region in general and Awash-Fentale District in particular to solely document traditionally used antimalarial plants. This study was thus conducted to document medicinal plants used in traditional treatment of malaria by the Afar people in Awash Fentale District,Afar Region of Ethiopia, and evaluatedin vivoselected two plants[Aloe trichosantha(A. trichosantha) andCadaba rotundifolia(C.rotundifolia)] for their antimalarial activities.
2. Materials and methods
2.1. Study area
Ethnobotanical survey was undertaken in the Awash-Fentale District, Zone 3 of the Afar Regional State of Ethiopia, between January 2008 and May 2010. Awash Fentale District is located at about 200 km east of Addis Ababa and is bordered in the south by the Oromia Region, in the west by the Amhara Region, in the north and in the east by Dulcha and Amibara districts of the Afar Region,respectively.
2.2. Informants selection and ethnobotanical data collection
Twenty-four Afar informants (23 males and 1 female) that were considered knowledgeable were selected from the the study district using purposive sampling method with the help of local administrators and elders. The ages of the informants ranged from 30 to 78 years. Interviews were conducted in the Afar language with the help of local translator and ethnobotanical data were recorded in English. Additional data were also collected through field observation and local market surveys. Data collected, among others, included local name of the antimalarial plant, part used and procedures followed in remedy preparation. Voucher specimens were collected for all specimens and stored at the National Herbarium,Addis Ababa University, after identification by botanists.
2.3. Toxicity and antimalarial activity tests
2.3.1. Selection of plants
With the assumption that plants cited most frequently are more likely to be biologically active against the parasites[7],A. trichosanthaandC. rotundifoliawere selected from among the 17 plant species that were claimed by informants in the study District to have antimalarial activity.
2.3.2. Plant samples collection and extraction
The leaves ofA. trichosanthaandC. rotundifoliawere collected and dried under shade at room temperature and grounded to powder using mortar and pestle. The powders were extracted using distilled water and ethanol. Powder produced from each plant was mixed with each solvent in the proportion of 1: 10 (w/v) in separate Erlenmeyer flasks and placed on orbital shaker (GFL, Model 3020, Germany) at room temperature for 24 h. The extracts were then filtered through cotton and subsequently with Whatman filter paper (15.0 cm size).Ethanol was removed from the filtrate by rotary evaporator (Buchi RE 121, Switzerland) and the water extracts were freeze-dried by a centrifugal freeze drier. Each extract was placed in a labeled small screw cupped bottle and kept in refrigerator at -70 ℃ until use[8].
2.3.3. Toxic effect test of extracts
Acute toxic effects of the extracts were evaluated against white Swiss albino mice arranged into three groups (each group had 4 mice). Group one was treated orally with 500 mg/kg, group two with 1 000 mg/kg and group three with 1 500 mg/kg for four consecutive days. Signs of acute toxicity such as death, changes in physical appearance and behavioral changes were observed for ten days[9].
2.3.4. Antimalarial activity test
Plasmodium berghei(P. berghei) infected Swiss albino mice(5-7 weeks of age) obtained from Aklilu Lemma Institute of Pathobiology, Addis Ababa University were treated for four days by the extracts[10]. Blood was taken fromP. bergheiinfected donor mouse (with growing parasitaemia of 20%), diluted with 3% citrate and brought to a volume of 0.2 mL containing 1××106-1××107infected erythrocytes was injected intraperitonially on day 0.
The mice were put randomly into five groups each containing four mice. Group 1 was treated with 300 mg/kg, group 2 with 600 mg/kg,group 3 with 900 mg/kg of water and ethanol extracts of the leaves ofA. trichosanthaandC. rotundifolia. The negative control group(group 4) was given the vehicle (0.4 mL distilled water) and the positive control group (group 5) chloroquine (10 mg/kg). Treatment continued daily for four consecutive days starting 3 h after infection from day 0 to day 3. On day 4, thin smears of blood films were prepared for each mouse from the peripheral blood on the tail and percentage parasitaemia was recorded[11]. Packed cell volume was measured to predict the effectiveness of the extracts. The mice were fed with standard mice pellet ad libitum and given water.P. bergheiused in the test was subsequently maintained in the laboratory by serial blood passage from mouse to mouse.
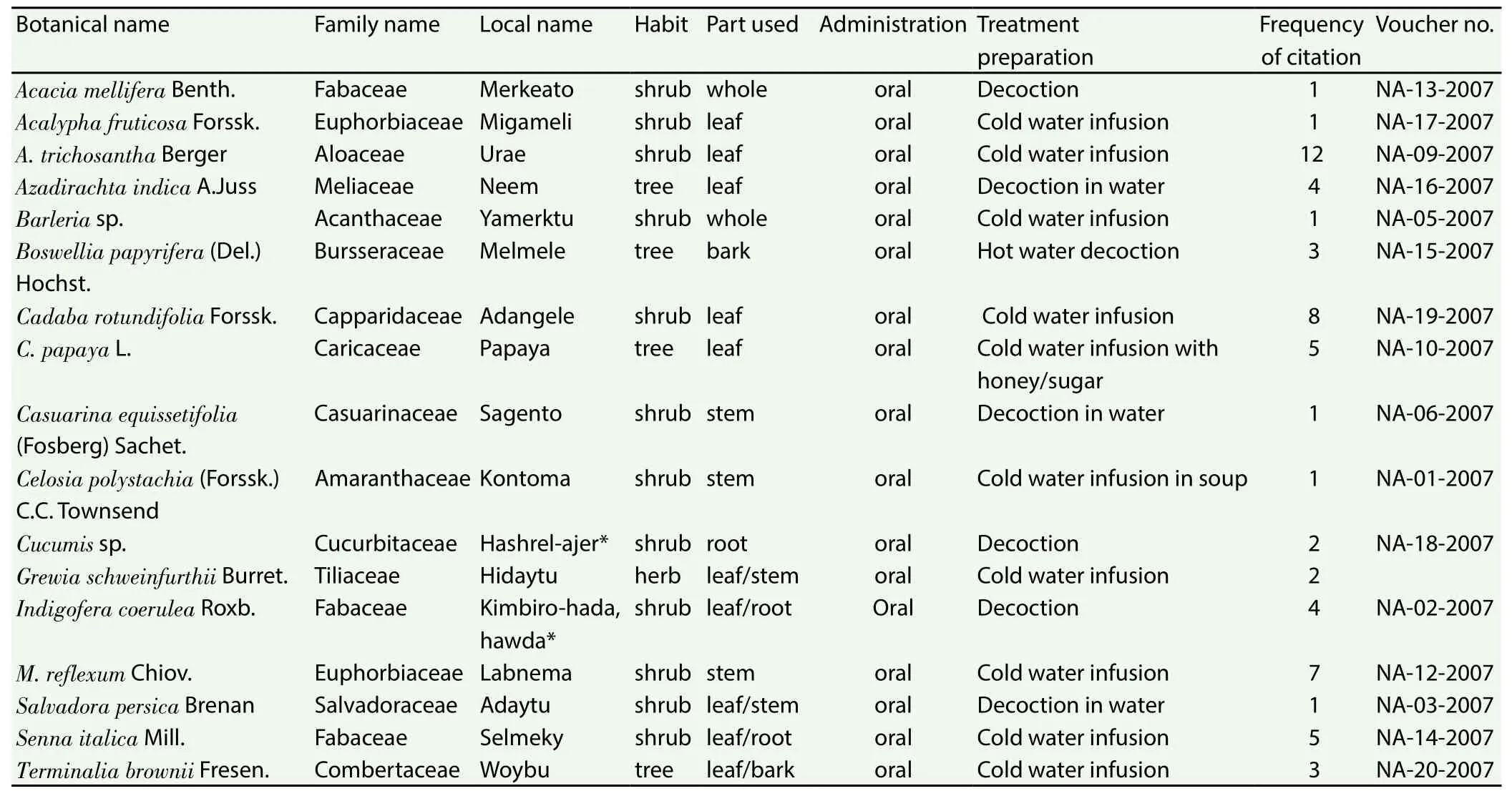
Table 1 Antimalarial plant species collected from Awash Fentale District of the Afar Region of Ethiopia.
2.4. Data analysis
Data was entered in to Microsoft Excel work sheet and frequencies were worked out. Day 4 parasitaemia was presented as mean plus or minus standard error (mean±SEM). Statistical significance was determined by one way analysis of variance using SPSS computer software. Students pairedt-test was used to compare parameters with in groups. For all the data values withP<0.05 were considered statistically significant. Percentage parasitaemia and percentage suppression were also determined[12]. Weight in grams and survival time in days were recorded for each mouse and the mean for each group calculated.
2.5. Ethical considerations
Approval letter was obtained from the Institutional Review Board of the Department of Biology, Faculty of Science, Addis Ababa University, before the actual conduct of the study. Oral informed consent was obtained from every informant participating in the ethnobotanical study. Study permission was also obtained from the Administration of the Awash-Fentale District.
3. Results
3.1. Ethnobotanical survey
3.1.1. Comparison of knowledge of informants on antimalarial plants
Analysis of knowledge between two age groups indicated that informants above the age of 40 years reported an average of 3 antimalarial plants, whereas, informants up to the age of 39 years,on average, cited 2.5 antimalarial plants. However, there was no significant difference between the two age groups (P>0.05) in the mean number of cited antimalarial plants.
3.1.2. Plants reported as antimalarials
A total of 17 species of antimalarial plants belonging to 14 families were documented as being used by the Afar people residing in Awash Fentale District, Afar Region of Ethiopia (Table 1). The families Fabaceae and Euphorbiaceae were represented by three and two antimalarial plants, respectively, and the rest by one antimalarial plan each. Most of the reported antimalarial plants were shrubs(63%), followed by trees (21%) and herbs (16%).
The most frequently cited antimalarial plant species wereA.trichosantha,C. rotundifolia,Monadenium reflexum(M. reflexum)andCarica papaya(C. papaya).A. trichosanthawas reported by 12 informants andC. rotundifoliaandM. reflexumby eight and seven informants, respectively (Table 1).
3.1.3. Plant parts used, mode of remedy preparation and route of administration
Leaf was the most frequently cited part used in the preparation of remedies accounting for 44% of the reported antimalarial plants,followed by stem (22%), root (17%), whole plant (13%) and bark(4%). The remedies were prepared in the form of decoctions and cold infusions using water as a solvent. In some cases, honey, sugar or crushed Allium sativum is added to preparations to make them more palatable and/or improve their effectiveness. All antimalarial preparations were administered orally.
3.1.4. Abundance of antimalarial plants
According to reports of informants and field observation, two of the antimalarial plants,M. reflexumandA. trichosantha(Aloaceae) are becoming rare in the nearby areas and as a result collectors needed to travel longer distances to harvest them.
3.2. In vivo acute toxicity and antimalarial activity tests
3.2.1. Acute toxicity of the extracts in mice
Water extracts of the leaves ofC. rotundifoliaandA. trichosanthashowed no lethal effect on mice up to a dose of 1 500 mg/kg. The mice did not show sign of toxicity for a week. No urination and muscle weakness. There was no significant change (P>0.05) in body weight of mice between day 0 and day 4 (Table 2).
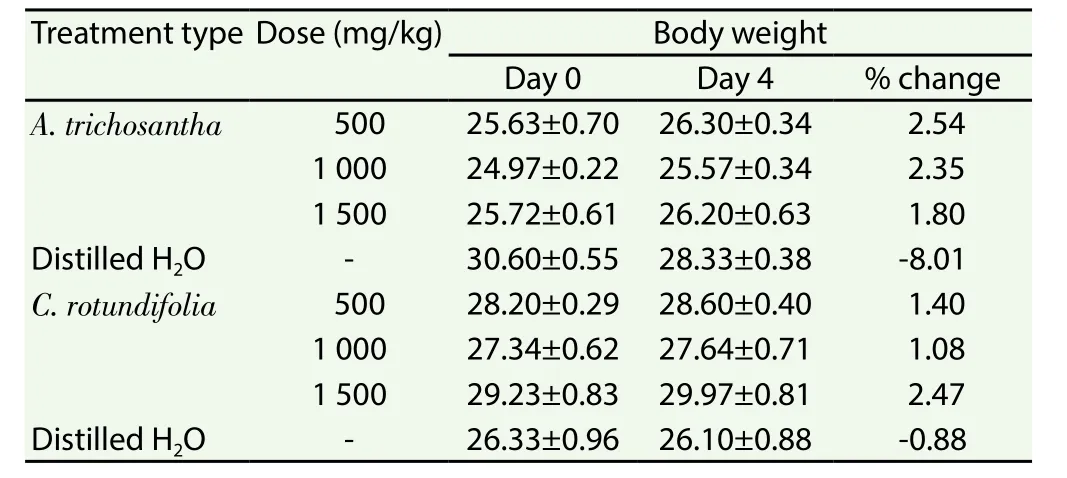
Table 2 Body weight of mice after administration of aqueous extracts of leaves of A.trichosantha and C. rotundifolia on non-infected mice.
Similarly, ethanol extracts of the leaves of the two plants showed no lethal effect up to the dose of 1 500 mg/kg. Mice treated with ethanol extracts of the leaves ofA. trichosanthadid not show sign of toxicity. Gross behavioral and physical observations revealed no urination, no muscle weakness and no significant (P>0.05) change in body weight was recorded. Oral administration of ethanol extracts ofC. rotundifolialeaves revealed no toxicity signs. The mice were physically active, no body convolution, and active to feed. However,some body weight loss was observed, although not significant(P>0.05) on the fifth day in mice given a dose of 500 mg/kg. Mice given 1 000 mg/kg and 1 500 mg/kg of the ethanol extract ofC.rotundifoliashowed significant (P>0.05) body weight change and were physically active (Table 3).
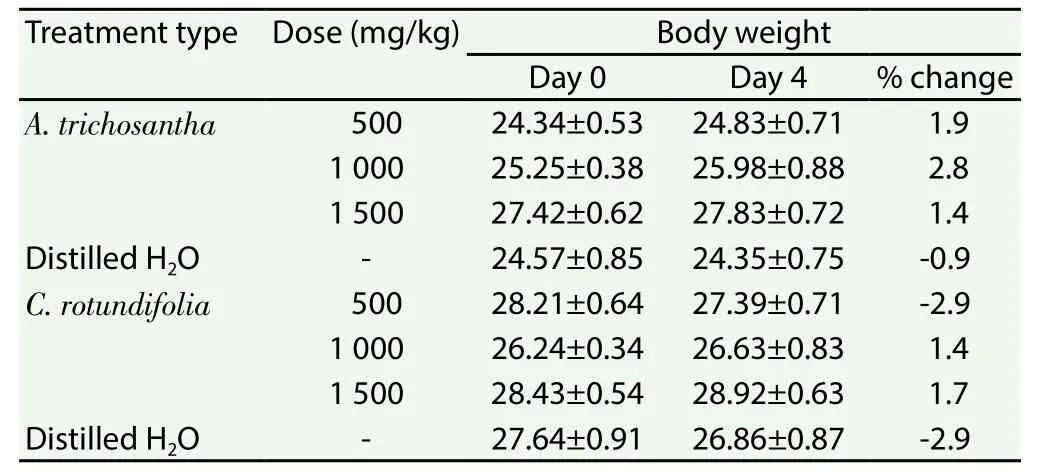
Table 3 Body weight of mice after administration of ethanol extracts of leaves of A.trichosantha and C. rotundifolia on non-infected mice.
3.2.2. Efficacy of the extracts against P. berghei in mice
Treatment with crude aqueous and ethanol extracts ofA.trichosanthaandC. rotundifoliaresulted in lowerP. bergheiparasitaemia in mice compared to their respective negative controls(Table 4). However, parasitaemia was not cleared in the experimental groups. The highestP. bergheisuppressive value (53.73%) was recorded for ethanol extract of the leaves ofC. rotundifoliaat a dose of 900 mg/kg and the lowest (3.85%) for the aqueous extract of the same plant at a dose of 300 mg/kg. Mice treated with 600 mg/kg and 900 mg/kg of ethanol extracts of the two plants (A. trichosanthaandC. rotundifolia) significantly (P<0.05) lowered parasitaemia.But treatment at the dose of 300 mg/kg of ethanol extracts of both plants did not show significant effect against parasitaemia compared to the negative control. Mice treated with 900 mg/kg of aqueous extracts of both plants significantly (P<0.05) lowered parasitaemia.But treatment at the doses of 300 mg/kg and 600 mg/kg of aqueous extracts of both plants did not show significant effect against the parasitaemia compared to the negative control.
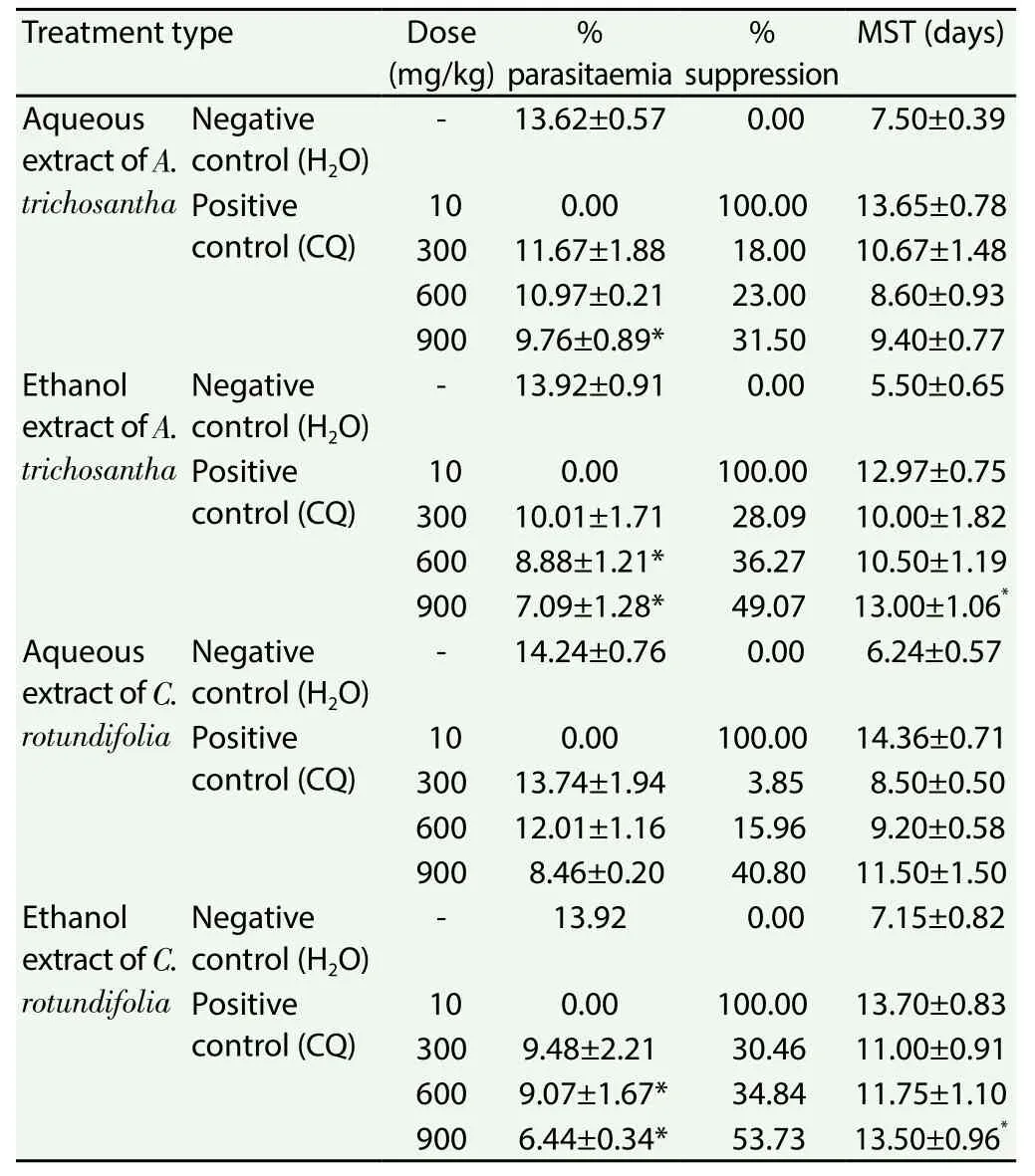
Table 4 Antiplasomodial activity of crude extracts of leaves of A. trichosantha and C.rotundifolia against P. berghei in mice.
The mean survival times of the mice treated with 600 mg/kg and 900 mg/kg ethanol extract ofA. trichosanthaleaves were(10.50±1.19) and (13.00±1.06) days, respectively (Table 4), whereas,mice in the negative control group lived for (5.50±0.65) days. On the other hand, mean survival times of mice treated with water extract of the leaves ofA. trichosanthaat a dose of 600 mg/kg and 900 mg/kg were (8.6±0.93) and (9.4±0.77) days, respectively.
Mean survival times of the mice treated with 600 mg/kg and 900 mg/kg ethanol extract of leaves ofC. rotundifoliawere (11.75±1.10)and (13.5±0.96) days, respectively (Table 4), whereas that of mice in the negative control group was (7.50±0.15) days. On the other hand, mean survival times of water extract of the leaves ofC.rotundifoliaat a dose of 600 mg/kg and 900 mg/kg were (9.20±0.58)and (11.5±1.5) days, respectively, which is better even though not significant as compared to the negative control group of (6.24±0.57)days.
Extracts of the leaves ofA. trichosanthadid not cause reduction of body weight in the infected mice even with increasing parasitaemia(Table 5). Analysis of packed cell volume on day 4 indicated that water and ethanol extracts of leaves of the two plants (A. trichosanthaandC. rotundifolia) showed insignificant effect on packed cell volume values (Table 5).
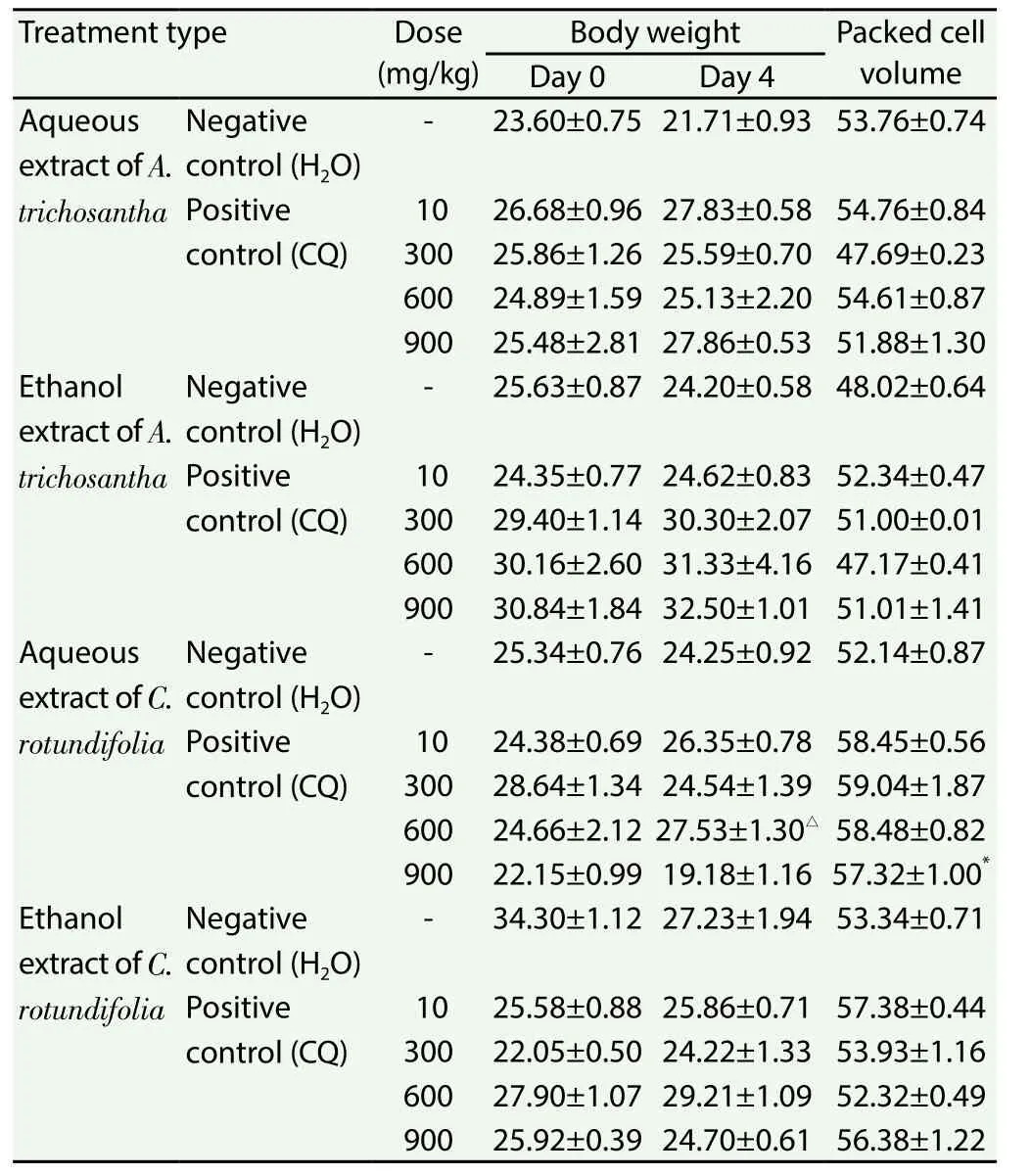
Table 5 Effect of crude extracts of leaves of A. trichosantha and C. rotundifolia on body weight and packed cell volume of P. berghei infected mice.
4. Discussion
Some of the antimalarial plants reported during the current study were found to be also used in Ethiopia and elsewhere in Africa for the same purpose. These includedC. papaya,Azadirachta indica,Aloespp.,Senna italica,M. reflexumandAcalypha fruticosa[2,5,6,13-16].The fact that the plantsA. trichosantha,C. rotundifolia,M. reflexumandC. papayawere the most frequently cited antimalarial plants in the study area may also indicate their effectiveness.
The most widely used antimalarial plants in the study area were trees and shrubs, and this may be related to their accessibility throughout the year tolerating harsh environmental conditions. Leaf was the most commonly used plant part in the area, the harvest of which does not normally causes significant harm to survival of individuals as compared to other parts such as the root, stem and bark. Different communities in Ethiopia commonly use leaves in the preparation of remedies[17-21].
Antimalarial remedies in the study area were prepared in the forms of decoctions and cold water infusions, which in agreement with results of a study conducted elsewhere[2]. The reason behind the choice of these two preparation forms might be related to their relatively better efficiency of extracting active compounds.All antimalarial preparations were administered orally. A study conducted in Shinile District of the Somali Region of Ethiopia also reported that the majority of antimalarial preparations were taken orally.
Knowledge of medicinal plants in the study area is largely transferred orally and thus there is a great danger of losing it.Medicinal plants in the study area are harvested from an area which is facing great pressure due to habitat destruction and over utilization of plant resources. As a result, some antimalarial plants may disappear from the area before appropriate conservation measures are taken.
There was no observed sign of acute toxicity in mice provided with water and ethanol extracts of the leaves of two plants (A. trichosanthaandC. rotundifolia). Administration of the extracts of both plants did not bring significant change in body weight of the mice. The result could justify the local use of the two plants in the study area to treat malaria. The traditional healers or practitioners in the study area primarily used water as a solvent. But thein vivostudy showed that ethanol extracts were of better antimalarial activity. This may be due to the better solubility of the active components in organic solvents.The observed antimalarial activity of extracts of the two plants is consistent with their traditional use to treat malaria in Awash Fentale District as well as elsewhere in Ethiopia[6,22]. The suppression effect caused by extracts of the two plants might be associated with the presence of chemical ingredients that have antimalarial properties. DifferentCadabaspecies were reported to contain alkaloids and sesquiterpene lactones. Cadabicine and cadabicine acetate spermidine alkaloids were isolated from the stem bark ofCadaba farinosa[23-25]. A new flavonol triglycoside, rhamnocitrin-3-O-neohesperoside-4-O-glucoside was also isolated from the ethanol extract ofCadabaglandulosa together with two known diglycosides rhamnocitrin-3-O-neohesperoside and rhamnetin-3-neohesperoside[8].
The ethnobotanical study conducted in Awash Fentale District of the Afar National Regional State of Ethiopia documented 17 plant species that were traditionally used by the Afar community for the management of malaria. Two plants (A. trichosanthaandC. rotundifolia) that had the highest number of informant citations were screened for theirin vivoantiplasmodial activity and found to demonstrate appreciable suppressive effects. Despite the presence of rich knowledge and use of antimalarial medicinal plants in the study area, the ongoing habitat destruction and overexploitation of plant resources is posing a great danger to the continuation of traditional medical practice. Thus, appropriate conservation measures are required to save the medicinal plants from further destruction.The antimalarial activity ofA. trichosanthaandC. rotundifoliamay partly explain and support the traditional use of the plants for malaria treatment. However, further activity tests and phytochemical investigations need to be carried out to isolate and identify active principles that may serve as potential source of antimalarial drugs.
Conflict of interest statement
Authors declare that they have no conflict of interests.
Acknowledgments
We are grateful to the School of Graduate Studies, Addis Ababa University, for financially supporting the research work (grant number GSR/1702/99). We also thank Engdawork Mulleta for his unreserved assistance in plant material extraction, W/o Kokebe G/Michael and W/o Baysasaw G/Medhin for their help inin vivoantiplasmodial activity test and Girma Kebede for his enthusiastic help in preserving the experimental mice.
[1] WHO.World malaria report.Geneva: World Health Organization; 2014.
[2] Muthaura CN, Rukunga GM, Chhabra SC, Omar SA, Guanti AN,Gathirawaj W, et al. Antimalarial activity of some plants traditionally used in treatment of malaria in Kewale district of Kenya.Sci Dir2007; 112:545-551.
[3] Belayneh A, Bussa NF. Ethnomedicinal plants used to treat human ailments in prehistoric place of Harla and Dengego valleys, eastern Ethiopia.J Ethnobiol Ethnomed2014; 10: 18.
[4] Chekole G. Ethnobotanical study of medicinal plants used against human ailments in Gubalafto District, Northern Ethiopia.J Ethnobiol Ethnomed2017; 13: 55.
[5] Teklehaymanot T, Giday M, Medhin G, Mekonnen Y. Knowledge and use of medicinal plants by people around Debre Libanos monastery in Ethiopia.J Ethnopharmacol2007; 111: 271-283.
[6] Giday M, Teklehaymanot T, Animut A, Mekonnen Y. Medicinal plants of the Shinasha, Agew-awi and Amhara people in northwest Ethiopia.J Ethnopharmacol2007; 110: 516-525.
[7] Trotter RT, Logan MH. Informants consensus: a new approach for identifying potentially effective medicinal plants. In: Etkin NL, editor.Plants in indigenous medicine and diet.Bedford Hill, NY: Redgrave Publishing Company; 1986, p. 91-112.
[8] Ahmed AG. Flavonol glycosides fromCadaba glandulosa.Z Naturforsch2002; 57: 216-220.
[9] Asnake S, Teklehaymanot T, Hymete A, Erko B, Giday M. Evaluation of the antiplasmodial properties of selected plants in southern Ethiopia.BMC Complement Altern Med2015; 15: 448.
[10] Peters W, Portus JH, Robinson BL. The chemotherapy of rodent malaria.ⅩⅫ. The value of drug-resistant strains ofP. bergheiin screening for blood schizonticidal activity.Ann Trop Med Parasitol1975; 69: 155-171.
[11] Nardos A, Makonnen E.In vivoantiplasmodial activity and toxicological assessment of hydroethanolic crude extract of Ajuga remota.Malar J2017; 16: 25.
[12] Muluye AB, Melese E, Adinew GM. Antimalarial activity of 80%methanoloc extract ofBrassica nigra(L.) Koch. (Brassicaceae) seeds againstPlasmodium bergheiinfection in mice.BMC Complement Altern Med2015; 15: 367.
[13] Asnake S, Teklehaymanot T, Hymete A, Erko B, Giday, M. Survey of medicinal plants used to treat malaria by Sidama people of Boricha District, Sidama Zone, South region of Ethiopia.Evid Based Complement Altern Med2016: 2016. Doi: http://dx.doi.org/10.1155/2016/9690164.
[14] Lagnika L, Djehoue R, Yedomonhan H, Sanni A. Ethnobotanical survey of medicinal plants used in malaria management in South Benin.J Med Plants Res2016; 10: 748-756.
[15] Systematic review of traditional medicinal plants used for treatment of malaria in Ethiopia: trends and perspectives.Malar J2017; 16: 307.
[16] Bora U, Sahu A, Sikia PA, Raykala KV, Goswami P. Medicinal plants used by the people of north east India for curing malaria.Phytother Res2007; Doi: 1002/ptr.2178.
[17] Getaneh S, Girma Z. An ethnobotanical study of medicinal plants in Debre Libanos Wereda, Central Ethiopia.Afr J Plant Sci2014; 8: 366-379.
[18] Mekonnen N, Abebe E. Ethnobotanical knowledge and practices of traditional healers in Harar, Haramaya, Bati and Garamuleta, Eastern Ethopia.Ethiop Vet J2017; 21: 40-61.
[19] Yineger H, Yewhalaw D, Teketay D. Ethnomedicinal plant knowledge and practice of the Oromo ethnic group in southwestern Ethiopia.J Ethnobiol Ethnomed2008; 4: 11.
[20] Giday M. Asfaw Z, Woldu Z. Ethnomedicinal study of plants used by Sheko ethnic group of Ethiopia.J Ethnopharmacol2010; 132: 75-85.
[21] Adefa MS, Abraha TB. Ethnobotanical survey of traditional medicinal plants in Tehuledere District, South Wollo, Ethiopia.J Med Plant Res2011; 5: 6233-6242.
[22] Teklehaymanot T. An ethnobotanical survey of medicinal and edible plants of yalo Woreda in Afar Regional State, Ethiopia.J Ethnobiol Ethnomed2017; 13: 40.
[23] Viqar UA, Anwar B, Atta-Ur-Rahman. Identification and C-13 NMR spectrum of stachydreine fromCadaba fruticosa.Phytochemist1975; 14:292-293.
[24] Viqar UA, Aziaur RA, Shoib AC, Marie HM, Cladry J.Cadabacine, an alkaloid fromCadaba farinosa.Phytochemist1985; 24: 2709-2711.
[25] Viqar UA, Kaniz F, Aziaur RA, Shoib A. Cadabacine and Cadabacine diacet-ate fromCrataeva nurvalaandCadaba farinosa.J Nat Prod1987;50: 1186.
 Asian Pacific Journal of Tropical Biomedicine2018年1期
Asian Pacific Journal of Tropical Biomedicine2018年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Prevalence of coronavirus from diarrheic calves in the Republic of Korea
- Effective Aeromonas specific monoclonal antibody for immunodiagnosis
- Pharmacodynamic profiling of optimal sulbactam regimens against carbapenemresistant Acinetobacter baumannii for critically ill patients
- Efficiency of combining pomegranate juice with low-doses of cisplatin and taxotere on A549 human lung adenocarcinoma cells
- Correlation of phytochemical content with antioxidant potential of various sweet potato (Ipomoea batatas) in West Java, Indonesia
- Larvicidal activity of Xenorhabdus and Photorhabdus bacteria against Aedes aegypti and Aedes albopictus
