Correlation of phytochemical content with antioxidant potential of various sweet potato (Ipomoea batatas) in West Java, Indonesia
Irda Fidrianny, Hendy Suhendy, Muhamad Insanu
Department of Pharmaceutical Biology, School of Pharmacy-Bandung Institute of Technology, Jl. Ganesa 10 Bandung-40132, Indonesia
1. Introduction
Phenolic and flavonoids compounds are found in many plants which have antioxidant and antibacterial activity[1,2]. Phenolic and flavonoid compounds might have antioxidant capacity[3-6]. They can prevent excessive free radical in oxidative stress that causes many degenerative diseases. Consumption of fruits and vegetables can prevent negative effect of oxidative stress, because they contain phenolic and flavonoid compounds which have antioxidant capacity[7,8]. Previous researches report that antioxidant activities can be influenced by phenolic and flavonoid content[9,10]. Many plants including sweet potato, tea, legumes and guava contain phenolic and flavonoid compounds[5,6,11,12].2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP) and 2,2’-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid (ABTS) methods could be used to determine antioxidant activity in many plants extracts[12]. The previous researches[8,10,12]present that antioxidant activity in fruits, vegetables and food can be evaluated by DPPH, ABTS and FRAP methods. Sweet potato leaves had higher antioxidant activity than sweet potato tubers[13].Researches by using ORAC, DPPH and ABTS methods show that tubers of sweet potato with varieties colors (white, cream, yellow,orange and purple) has antioxidant activity[14]. Antioxidant activities of leaves from six different places ofIpomoea batatas(I. batatas) in Malaysia and its correlation to total phenolic and flavonoid content are performed by DPPH method[15], but there is no information about color of tubers in this research.
There is no research regarding antioxidant activity of tubers from different varieties of sweet potato (I. batatas) which are extracted using increasing polarity solvents (n-hexane, ethyl acetate and ethanol) and tested by DPPH and FRAP assays.
The goals of this study are to observe antioxidant potential in various polarity extracts (n-hexane, ethyl acetate and ethanol) of tubers from different varieties of sweet potato grown in Tasikmalaya,West Java, Indonesia using DPPH and FRAP assays, and to explore correlations of total phenolic and flavonoid content with their antioxidant activities.
2. Materials and methods
2.1. Materials
DPPH, 2,4,6-tripyridyl-S-triazine (TPTZ), gallic acid, quercetin,were purchased from Sigma-Aldrich (MO, USA). All of other reagents were analytical grades.
2.2. Sample preparation
Tubers of four varieties of sweet potato (I. batatas) with their peelflesh color purple-purple were named as PP, purple-orange as PO,yellow-yellow purple as YYP and yellow-yellow orange as YYO.They were collected from Tasikmalaya, West Java, Indonesia, and were washed, sorted, cut, dried and grinded into powder.
2.3. Extraction
Powdered crude drug (300 g) was extracted by reflux. The first extraction usedn-hexane (three times). Ethyl acetate was used to extract the residue (three times). Then the residue was extracted three times using ethanol. Hence totally there were twelve extracts:n-hexane extracts (PP1, PO1, YYP1 and YYO1), ethyl acetate extracts (PP2, PO2, YYP2 and YYO2), and ethanolic extracts (PP3,PO3, YYP3 and YYO3).
2.4. Antioxidant activity by DPPH assay
Antioxidant activity by DPPH assay was determined by Blois’s method[16] with minor modifications. Each extract was prepared in various concentrations. Two mL of each concentration was added into 2 mL DPPH 50 μg/mL. After 30 min incubation, the absorbance was observed at λ 515 nm by UV-Vis spectrophotometer Hewlett Packard 8435. DPPH 50 μg/mL was used as control,methanol as a blank and ascorbic acid as standard. Analysis was performed in triplicate for each extract and standard. Antioxidant activity was determined by calculating percentage of reduction of DPPH absorbance. Based on its calibration curve the inhibitory concentration 50% (IC50) of DPPH scavenging activity of each extract could be calculated.
2.5. Antioxidant capacity by FRAP assay
FRAP solution was prepared using Benzi’s method[17], in acetate buffer with pH 3.6. Two mL of variation concentration of each extract was added into 50 μg/mL FRAP. After 30 min incubation,the absorbance was measured at λ 593 nm. Fifty μg/mL FRAP was used as control, acetate buffer as a blank, and ascorbic acid as standard. Analysis was carried out in triplicate for each extract and standard. Antioxidant capacity was determined based on increase in Fe (Ⅱ)-TPTZ absorbance by calculating percentage of antioxidant capacity. Exhibitory concentration 50% (EC50) of FRAP capacity of each extract could be determined using its calibration curve.
2.6. Total phenolic content (TPC)
Total phenolic content was determined using Folin-Ciolcalteu reagent[18]. The absorbance was observed at wavelength of 765 nm. Analysis was conducted in triplicate for each extract. Standard solution of gallic acid (80-200 μg/mL) was used to obtain a calibration curve. Total phenolic content was reported as gram gallic acid equivalent per 100 g extract (g GAE/100 g).
2.7. Total flavonoid content (TFC)
Total flavonoid content was measured using Chang’s method[19]with minor modifications. The absorbance was read at λ 415 nm.Three replications were performed for each extract. Quercetin solution (40-120 μg/mL) was used to obtain a calibration curve.Total flavonoid content was expressed as gram quercetin equivalent per 100 g extract (g QE/100 g).
2.8. Statistical analysis
Three replications were conducted for each sample. All of presented results were presented as means±standard deviation. Statistical analysis was performed by SPSS 16 for Windows using ANOVA and followed bypost-hocTukey. Correlation between the total phenolic,flavonoid, content and antioxidant activity, and correlation between two antioxidant testing methods were conducted using the Pearson’s method.
3. Results
3.1. Antioxidant activity by DPPH and FRAP assays
IC50of DPPH scavenging activities and EC50of FRAP capacities were determined in various extracts of sweet potato tubers with ascorbic acid as standard. The highest antioxidant activity expresses the lowest IC50or EC50. PO2 showed the lowest IC50DPPH (Table 1)and the lowest EC50FRAP (Table 2).
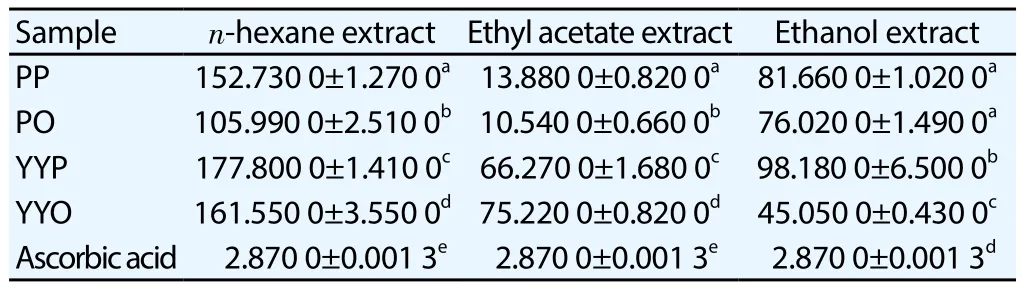
Table 1 Antioxidant activity of sweet potato tubers extracts by DPPH assay (μg/mL).
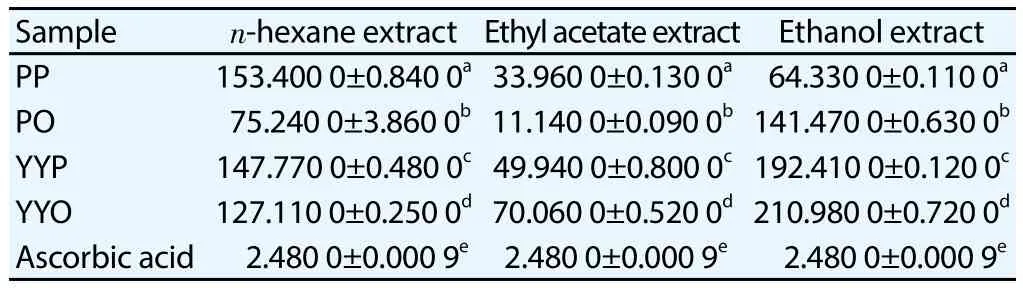
Table 2 Antioxidant capacity of sweet potato tubers extracts by FRAP assay (μg/mL).
3.2. TPC in sweet potato extracts
The linear regression equation of gallic acid (y=0.004 x-0.051,R2=0.998) was used to calculate TPC in various extracts of sweet potato tubers, hence the TPC was expressed as gallic acid equivalent.TPC in sweet potato tubers extracts showed different results in the range of 1.05-11.91 g GAE/100 g (Table 3). PO2 had the highesttotal phenolic content (11.91 g GAE/100 g), followed by PP2 9.44 gGAE/100 g, while PO3 gave the lowest TPC 1.05 g GAE/100 g.
3.3. TFC in sweet potato extracts
Quercetin was used as standard. TFC among various extracts of sweet potato was expressed as quercetin equivalent, using the linear regression equation of quercetin (y=0.009 x-0.155,R2=0.998). TFC in sweet potato tubers extracts varied from 0.60 to 17.83 g QE/100 g. The highest TFC (17.83 g QE/100 g) was presented by PO2,followed by YYP2 (13.72 g QE/100 g), and the lowest TFC (0.60 g QE/100 g) by PP3 (Table 4).
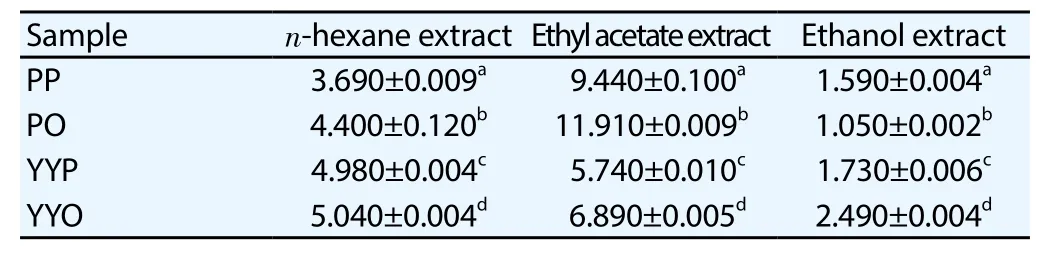
Table 3 TPC in sweet potato tubers extracts (g GAE/100 g).

Table 4 TFC in sweet potato tubers extracts (g QE/100 g).
3.4. Correlations between total phenolic, flavonoid content in sweet potato tubers extracts with their antioxidant activities
TPC in various tubers extracts of sample PO, YYP and YYO had significantly negative correlation with their EC50FRAP capacities(r=-0.974,r=-0.849,r=-1.000,P<0.01, respectively). TFC in tubers extracts of sample PO, YYP and YYO also showed negative and significant correlation with their EC50FRAP capacities (r=-0.999,r=-0.789,r=-0.943,P<0.01, respectively) (Table 5).
4. Discussion
The phytochemical content and antioxidant activities among the extracts can be compared if the density of the extracts are similar.Extract with high density may show higher phytochemical content and higher activity than low density extract. Therefore all extracts in the present study should be prepared in similar density.
DPPH free radicals dissolve in methanol and show absorption atwavelength 516 nm. Antioxidant will transfer the hydrogen to DPPH,which will be stable. Colors of DPPH would be changed from purple to yellow when the free radicals are scavenged by antioxidant.FRAP reagent is ferric (Ⅲ) chloride which is combined with TPTZ in acetate buffer of pH 3.6. Reduction potential of Fe (Ⅲ)/Fe (Ⅱ) is 0.77 V. Antioxidant with reduction potential lower than 0.77 V will reduce Fe (Ⅲ) to Fe (Ⅱ). Blue color complex of Fe (Ⅱ)-TPTZ shows absorption at λ 593 nm. The amount of Fe (Ⅲ) is reduced to Fe (Ⅱ) and then forms Fe (Ⅱ)-TPTZ, related to intensity of blue color.Sample with IC50or EC50lower than 50 μg/mL can be classified as very strong antioxidant, 50-100 μg/mL as strong, 101-150 μg/mL as medium and greater than 150 μg/mL as weak antioxidant[16].
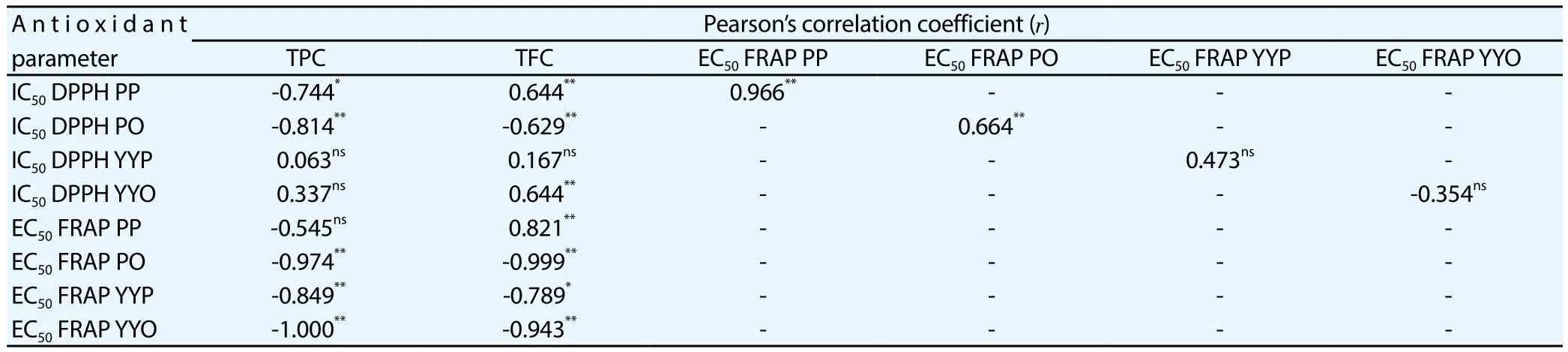
Table 5 Pearson’s correlation coefficient of total phenolic, flavonoid content with their antioxidant activities.
Determination of antioxidant activities by DPPH method is presented by IC50DPPH, the concentration of extract (antioxidant sample) that can scavenge free radical DPPH 50% and figured by decreasing absorbance of DPPH after adding extract. IC50DPPH can be determined using regression linear equation of calibration curve of each extract.
In the present research IC50DPPH scavenging activities were evaluated using tubers extracts from four varieties of sweet potato with different peel and flesh color. Sample PP (purple-purple) means sample with purple peel and purple flesh color. Ethyl acetate tubers extract of sample PO (PO2) showed the highest antioxidant activity by DPPH assay, which had the lowest IC50of DPPH scavenging activity (10.54 μg/mL) and can be categorized as very strong antioxidant. IC50of DPPH ascorbic acid standard was 2.88 μg/mL.It means that antioxidant potential of ascorbic acid is around four times antioxidant potential of PO2 by DPPH assay. Among ethanolic tubers extracts, sample YYO showed the highest antioxidant activity(IC50DPPH 45.05 μg/mL) compared to sample PP, PO and YYP. The previous study presented that methanolic leaves extract ofI. batatasvar. Indon (with purple tubers) had the highest antioxidant activity(IC50DPPH 372.4 μg/mL) compared toI. batatasvar. Batu Kelantan,I. batatasvar. Vitato,I. batatasvar. Batu Biasa andI. batatasvar.Oren[15]. Research by Teowet al[14] regarding nineteen sweet potato clones with varying flesh color indicated that methanolic extract of purple-flesh clones (NC415, 12-5 and 13-18) gave the highest antioxidant activity (> 1 μmol TE/g fresh weight) by DPPH assay.This research demonstrated that sweet potato tubers with purple flesh color had the highest antioxidant activity by DPPH assay,followed by orange, yellow and the lowest was given by white flesh color[14]. It was different from the present study which indicated that YYP3 showed lower antioxidant activity (IC50DPPH 98.18 μg/mL)than YYO3 (IC50DPPH 45.05 μg/mL).
More over antioxidant activities can be expressed by percentage of DPPH scavenging activity, by reacting 50 μg/mL DPPH and 50 μg/mL sample. The result was compared to percentage of DPPH scavenging activity of ascorbic acid, by adding 50 μg/mL DPPH and 50 μg/mL ascorbic acid. The DPPH scavenging activity of ascorbic acid did not achieve 100%, because there was still residual yellow color in solution after hydrogen atom was given to DPPH by antioxidant in sample extract[20]. The percentage of DPPH scavenging activity could not present the real antioxidant activities.Antioxidant activity can be expressed in percentage of DPPH scavenging activity, which is conducted normally by adding extract with concentration of 50 μg/mL only to 50 μg/mL DPPH solution(volume 1: 1). If the 50 μg/mL extract can scavenge 42% of the 50 μg/mL DPPH, it does not mean 60 μg/mL extract will always scavenge more than 42% DPPH. The 60 μg/mL extract may present the percentage of DPPH greater than 42% or lower than 42%. It’s due to extract consisted of many compounds and not all compounds in extract have antioxidant activities, some of which may act as antagonist of antioxidant. In 50 μg/mL extract, the compounds which can act as antagonist antioxidant have not achieved yet its effective concentration; therefore the antioxidant components can scavenge 42% DPPH. In 60 μg/mL extract, the antagonist of antioxidant components might have achieved its effective concentration, it will reduce the ability of antioxidant components, therefore 60 μg/mL extract will give percentage of DPPH lower than 42%.
Antioxidant activity can be stated as IC50. Many concentrations of extract should be used for calculating IC50, which show linear decreasing in absorbance of DPPH. Based on the results, the linear regression equation of calibration curve of each extract can be calculated. After determining linear regression equation, value of IC50DPPH can be observed. Based on the explanation above, it can be seen that the percentage of DPPH scavenging activities will not represent the real antioxidant activities though the real of antioxidant activities will be exposed by IC50DPPH value. Antioxidant activity can also be stated by using μmoL or mmoL trolox equivalent per g sample. Trolox is a standard that be used in antioxidant activity. The largest μmoL or mmoL trolox equivalent per g sample showed the highest antioxidant activity.
Antioxidant capacities by FRAP assay are expressed by EC50of FRAP capacity. EC50is exhibitory concentration 50%, concentration of antioxidant sample that can increase FRAP capacity 50% and expressed by increasing absorbance of complex of Fe (Ⅱ)-TPTZ after adding antioxidant sample. Linear regression equation of calibration curve of each extract can be used to calculate EC50FRAP.The present research shows that all of ethyl acetate tubers extracts of sweet potato (I. batatas) have EC50FRAP capacity in the range of 11.14-70.06 μg/mL and it means their antioxidant can be classified as very strong antioxidant (< than 50 μg/mL) by FRAP assay, except YYO2 (70.06 μg/mL) as strong antioxidant. PO2 has EC50FRAP of 11.14 μg/mL, while ascorbic acid standard has EC50FRAP of 2.49 μg/mL. It means antioxidant potential of ascorbic acid is around five times antioxidant potential of PO2 by FRAP assay. Reducing power of methanolic leaves extract ofI. batatasvar. Indon (with purple flesh color) had higher antioxidant activity than the other flesh color varieties[15]. Previous research[14] stated that methanolic fraction(hydrophilic fraction) of dark purple flesh sweet potato clones(NC415 and 13-18) had higher ORAC values (27 and 23 μmol TE/g fresh weight, respectively) compared to the other clones. It was similar to their antioxidant activity by ABTS method which showed NC 415 gave the highest antioxidant activity (1.6 mmol TE/g fresh weight).
Teowet al. reported that TPC of methanolic tubers extract of different flesh sweet potato varied from 0.003 mg chlorogenic acid equivalent (CAE)/g fresh weight to 0.949 mg CAE/g fresh weight.The highest TPC (0.949 mg CAE/g fresh weight) was given by sample 13-18 (with purple flesh color) and the lowest (0.003 mg CAE/ g fresh weight) showed by sample Xushu 18 (with white flesh color). Anthocyanin, one of the phenolic compounds in plant, was also determined in this research. Sample 13-18 gave the highest total anthocyanin (0.531 mg/g fresh weight) compared to the other sample[14]. Previous research revealed that methanolic leaves extract of different varieties of sweet potato presented that TPC ofI. batatasvar. Indon (with purple flesh color) showed the highest TPC (5.35 g GAE/100 g) compared to the other varieties[15]. It was similar to the present study which denoted that PP3 had higher TPC (1.59 g GAE/100 g) than PO3 (1.05 g GAE/100 g). The result was also similar to the previous research[21] which showed that ethanolic leaves extract of purple flesh sweet potato gave the highest TPC(19.64 g GAE/100 g). TFC of methanolic leaves extract ofI. batatasvar. Batu Biasa 263.5 μg catechin equivalent/g was higher than the other varieties[15]. It was similar to the present study which exhibited that TFC of PO3 (0.68 g QE/100 g) was higher than TFC of PP3.Previous research also figured that ethanolic leaves extract of yellow flesh sweet potato had higher TFC than the other ethanolic leaves extract[21].
The glycoside of flavonoid demonstrates lower antioxidant activity than its aglycone. Flavonoid which had ortho di OH at C-3’-C4’,OH at C-3, C-4 oxo, double bond at C-2 and C-3 showed high antioxidant activity[22]. The most significant influence of di OH C-3’-C-4’. TFC in YYP3 (2.45 g QE/100 g) was similar to TFC in YYO3 (2.53 g QE/100 g), but antioxidant activity of YYO3 by DPPH assay was higher than YYP3. YYO3 with IC50DPPH value of 45.05 μg/mL could be categorized as very strong antioxidant (≤ 50 μg/mL) and YYP3 with IC50DPPH value of 98.18 μg/mL as strong antioxidant (50-100 μg/mL). Based on this result it can be predicted that many flavonoids in YYO3 have high antioxidant activity, with ortho di OH at C3’-C4’, OH at C-3, oxo function at C-4, double bond at C-2 and C-3, while many flavonoids in YYP3 with other position have low antioxidant activity. TPC in YYO2 (6.89 g GAE/100 g) was higher than TPC in YYO3 (2.49 g GAE/100 g), but antioxidant activity of YYO3 by DPPH assay expressed by IC50DPPH value denoted that IC50DPPH of YYO3 45.05 μg/mL (very strong antioxidant) was lower than IC50DPPH of YYO2 75.22 μg/mL (strong antioxidant). The result figured that YYO3 contained most phenolic compounds with high antioxidant activity, while in YYO2 with low antioxidant activity. PP3 contained higher phenolic content with 1.59 g GAE/100 g than PO3 (1.05 g GAE/100 g). Anthocyanin compound was predicted in sample PP(purple peel and purple flesh color). Anthocyanin is phenolic group which is also included in flavonoid compound and is correlated with antioxidant activities and categorized as flavonoid glycoside. In the above statement, it can be seen that flavonoid aglycone has higher antioxidant activities than flavonoid glycoside. In addition, only fl avonoid which has certain OH position will give high antioxidant activities. Based on result of the present study, antioxidant activity of PP3 by DPPH method was lower than antioxidant activity of PO3.It suggests that many anthocyanin compounds in PP3 do not have ortho di OH at C3’-C4’ which have high antioxidant activity.
TPC in PP3 (1.59 g GAE/100 g) was similar to TPC in YYP3 (1.73 g GAE/100 g), but EC50FRAP of YYP3 (192.41 μg/mL) was higher than EC50FRAP of PP3 (64.33 μg/mL). The result showed that antioxidant activity of PP3 was higher than YYP3 by FRAP assay.It suggests that majority phenolic compounds in PP3 have lower reduction potential than Fe (Ⅲ)/Fe (Ⅱ) 0.77 V, meanwhile many phenolic compounds in YYP3 have reduction potential greater than 0.77 V. Sample will be oxidized. At the same time Fe (Ⅲ) reduces to Fe (Ⅱ) and then the Fe (Ⅱ) reacts with TPTZ, producing blue color complex.
The lowest IC50DPPH scavenging activity and EC50FRAP capacity will reveal the highest antioxidant activity. Coefficient of Pearson correlation was significantly negative if 0.61 ≤r≤ -0.97 and significantly positive if 0.61 ≤r≤ 0.97[12]. It means increase in TFC and TPC causes increase in antioxidant activities, which is stated by lower IC50DPPH and/or EC50FRAP. Therefore the good correlation between TPC and TFC with IC50DPPH or EC50FRAP was significant and negative[23]. The present study showed that TPC in sample PP had significant and negative correlation with their IC50DPPH (r=-0.744,P<0.05). It can be predicted that phenolic compounds are the main contributor in antioxidant activities of sample PP by DPPH method. TPC and TFC in sample PO had significantly negative correlation with their antioxidant activities by DPPH method (r=-0.814,P<0.01;r=-0.629,P<0.05, respectively).Antioxidant activity had similar result by FRAP method, the TPC and TFC in sample PO showed negative and significant correlation with their EC50FRAP (r=-0.974;r=-0.999,P<0.01, respectively).It suggests that phenolic and flavonoid compounds are the major contributors in their antioxidant activities by FRAP method.
The present study also revealed that IC50DPPH of sample PP and PO had significantly positive correlation with their EC50FRAP(r=0.966,P<0.01;r=0.664,P<0.05). It means antioxidant activity of sample PP and PO are linearly correlated in DPPH and FRAP methods. The previous research studied the correlation between two antioxidant testing methods by determining linear regression of calibration curve, while in the present study Pearson’s coefficient correlation was used. Antioxidant activities of nineteen sweet potato with varying flesh colors by DPPH method were linearly correlated to ABTS method (R2=0.822). The linear correlations were also given by hydrophilic ORAC and DPPH method (R2=0.859), as well as hydrophilic ORAC and DPPH method (R2=0.761)[14].
Determination of antioxidant activities by various methods may lead to different results. DPPH assay result shows that all different ethyl acetate and ethanolic tubers extracts of four sweet potato varieties vary from strong to very strong antioxidant activities. DPPH and FRAP assays indicate that phenolic and flavonoid compounds in sample PP and PO contribute together to their antioxidant activities.DPPH and FRAP methods gave linear result in antioxidant activities of sweet potato PP and PO. Tubers of four sweet potato varieties(PP, PO, YYP, and YYO) have many benefits to prevent oxidative stress and potential as sources of natural antioxidant for further exploitation.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The authors are grateful to the authorities of School of Pharmacy-Bandung Institute of Technology for providing the necessary facilities to perform this research.
[1] Zou Z, Xi W, Hu Y, Nie C, Zhou Z. Antioxidant activity ofCitrusfruits.Food Chem2016; 196: 885-896.
[2] Parashar S, Sharma H, Garg M. Antimicrobial and Antioxidant activities of fruits and vegetable peels: a review.J Pharmacogn Phytochem2014;3(1): 160-164.
[3] Othman A, Mukhtar NJ, Ismail NS, Chang SK. Phenolics, flavonoids content and antioxidant activities of 4 Malaysian herbal plants.Int Food Res J2014; 21(2): 759-766.
[4] Tharasena B, Lawan S. Phenolics, flavonoids and antioxidant activity of vegetables as Thai side dish.APCBEE Procedia2014; 8: 99-104.
[5] Sebei K, Gnouma A, Herchi W, Sakouhi F, Boukhchina S. Lipids, proteins,phenolic composition, antioxidant and antibacterial activities of seeds of peanuts (Arachis hypogaeaL.) cultivated in Tunisia.Biol Res2013; 46(3):257-263.
[6] Zielinski AAF, Haminiuk CWI, Alberti A, Nogueira A, Demiate IM,Granato D. A comparative study of the phenolic compounds and thein vitroantioxidant activity of different Brazilian teas using multivariate statistical techniques.Food Res Int2014; 60: 246-254.
[7] Yadav BS, Yadav R, Yadav RB, Garg M. Antioxidant activity of various extracts of selected gourd vegetables.J Food Sci Technol2016; 53(4):1823-1833.
[8] Venkatachalam K, Rangasamy R, Krishnan V. Total antioxidant activity and radical scavenging capacity of selected fruits and vegetables from South India.Int Food Res J2014; 21(3): 1039-1043.
[9] Somawathi KM, Rizliya V, Wijesinghe DGNG, Madhujith WMT.Antioxidant activity and total phenolic content of different skin coloured brinjal (Solanum melongena).Trop Agric Res2014; 26(1): 152-161.
[10] Raman ST, Ganeshan AKPG, Chen C, Jin C, Li SH, Chen HJ, et al.In vitroandin vivoantioxidant activity of flavonoid extracted from mulberry fruit (Morus albaL.).Pharmacogn Mag2016; 12(46): 128-133.
[11] Everette JD, Islam S. Effect of extraction procedures, genotypes and screening methods to measure the antioxidant potential and phenolic content of orange-fleshed sweet potatoes (Ipomoea batatasL.).Am J Food Technol2012; 7(2): 50-61.
[12] Thaipong K, Boonprakob U, Crosby K, Zevallos LC, Byrne DH.Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts.J Food Comp Anal2006;19: 669-675.
[13] Cook NC, Samman S. Flavonoids-chemistry, metabolism,cardioprotective effects, and dietary sources.Nutr Biochem1996; 7: 66-76.
[14] Teow CC, Truong VD, McFeeters RF, Thompson RL, Pecota KV, Yencho GC. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours.Food Chem2007; 103: 829-838.
[15] Hue SM, Boyce AN, Somasundram C. Antioxidant activity, phenolic and flavonoid contents in the leaves of different varieties of sweet potato(Ipomoea batatas).Aust J Crop Sci2012; 6: 375-380.
[16] Blois MS. Antioxidant determination by the use of stable free radicals.Nature1958; 181: 1199-2000.
[17] Benzi IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay.Anal Biochem1996;239: 70-76.
[18] Pourmorad F, Hosseinimehr SJ, Shahabimajd N. Antioxidant activity,phenol and flavonoid content of some selected Iranian medicinal plants.Afr J Biotechnol2006; 5(11): 1142-1145.
[19] Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods.J Food Drug Anal2002; 10: 178-182.
[20] Barreira JCM, Ferreira ICFR, Oliveira MBPP, Pereira JA. Effects of different phenols extraction conditions on antioxidant activity of almond(Prunus dulcis) fruits.J Food Biochem2009; 33: 763-776.
[21] Fidrianny I, Windyaswari AS, Wirasutisna KR. Antioxidant capacities of various leaves extract from five colors varieties of sweet potatoes tubers using ABTS, DPPH assays and correlation with total flavonoid, phenolic,carotenoid content.Res J Med Plant2013; 7(3): 130-140.
[22] Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants:chemistry, metabolism and structure-activity relationships.J Nutr Biochem2002; 13: 572-584.
[23] Fidrianny I, Johan Y, Sukrasno S. Antioxidant activities of different polarity extracts from three organs of makrut lime (Citrus hystrixDC)and correlation with total flavonoid, phenolic, carotenoid content.Asian J Pharm Clin Res2015; 8(4): 239-243.
 Asian Pacific Journal of Tropical Biomedicine2018年1期
Asian Pacific Journal of Tropical Biomedicine2018年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Prevalence of coronavirus from diarrheic calves in the Republic of Korea
- Effective Aeromonas specific monoclonal antibody for immunodiagnosis
- Pharmacodynamic profiling of optimal sulbactam regimens against carbapenemresistant Acinetobacter baumannii for critically ill patients
- Efficiency of combining pomegranate juice with low-doses of cisplatin and taxotere on A549 human lung adenocarcinoma cells
- Larvicidal activity of Xenorhabdus and Photorhabdus bacteria against Aedes aegypti and Aedes albopictus
- Proximate composition, nutritional values and phytochemical screening of Piper retrofractum vahl. fruits
