Proximate composition, nutritional values and phytochemical screening of Piper retrofractum vahl. fruits
Nurul Jadid, Byan Arasyi Arraniry, Dewi Hidayati, Kristanti Indah Purwani, Wiwi Wikanta, Sylviana Rosyda Hartanti, Rizka Yuanita Rachman
1Department of Biology, Institut Teknologi Sepuluh Nopember, Surabaya, Indonesia
2Department of Biology, Muhammadiyah University, Surabaya, Indonesia
1. Introduction
Tropical regions have been known as home of many fascinating medicinal plant species. This includes medicinal plants from the genusPiperthat covers around one thousand species[1]. In terms of morphological characteristics, all species in this genus possess three plant forms including creeping, climbing and branching stems.In addition, all plants have distinct leaves color and shape. Apart from its role in pharmaceutical domains, plants from the genusPiperalso used by traditional communities as supporting material for decorative arts, traditional ceremonies as well as food and beverages[2]. Among their various species,Piper retrofractum(P.retrofractum) vahl. is one of Piper species that are distributed mainly in the tropical region, including Thailand, Vietnam, Philipina and Indonesia.
Some Indonesian vernacular name ofP. retrofractumhave been reported including ‘Cabe Puyang’, ‘Cabe Jawa’, ‘Cabe Jamu’, and‘Cabai Jamu’ All these local names reflect its medicinal use. It is not surprising since this species contains various kind of phytochemical contents such as alkaloids/amides[3], which include more than 300 amide compounds that have been reported and thus become the prominent compounds found inP. retrofractum[4,5]. Furthermore,some terpenoid compounds have also been identified[6]. Recently,the presence of phenylpropanoids and alkylglycosides compound have been also reported and therefore the medicinal properties of this species were strengthened[7]. Many reports have demonstrated its biological and pharmaceutical functions, including antioxidant activity[8], potential bioresource for neurodegenerative diseases treatment[9], mast cell stabilization[10], mucolytic and expectorant agents[6] and potential regulation on human lipid metabolism[11], anti dengue[12] and larvacidal activity[13].
Based on the traditional use ofP. retrofractumas potential component for herbal medicine development, providing its pharmaceutical analysis is necessarily needed. Therefore, this present study aims to determine the proximate composition, nutritional values and to conduct preliminary study on phytochemical content ofP. retrofractumvahl. fruits.
2. Materials and methods
2.1. Collection and preparation of plant materials
P. retrofractumfruits were fresh-collected from the traditional market in Sumenep Region, Indonesia and were identified and authenticated by the Laboratory of Plant Biosciences and Technology, Department of Biology, Institut Teknologi Sepuluh Nopember, Surabaya, Indonesia. The voucher specimen was deposited in the form of herbarium in the laboratory for further references. The fresh-collected materials were then subjected to three times serial washing using tap water for eliminating residual soil particles and other unnecesary debris. Subsequently, the fruits were subjected to open-air and non-directed sunlight drying.
2.2. Determination of water content of P. retrofractum vahl.fruit
Water content was measured according to the Indonesian National Standard (SNI No. 01-2891-1992)[14]. Briefly, 2 g of freshP.retrofractumfruit was weighted (Wa) carefully and then subjected to an oven at 105 ℃ until a constant weight was reached (Wb). Finally,the percentage of water content was calculated using the following formula:
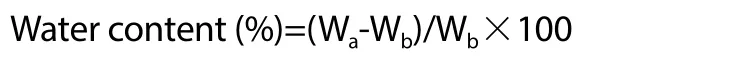
2.3. Determination of total ash content of P. retrofractum vahl. fruit
Total ash content was quantified according to the Indonesian National Standard (SNI No. 01-2891-1992)[14]. In this process, all volatile materials were vaporized and the organic matters would be perfectly burned, thanks to the presence of oxygen (O2) into carbondioxide (CO2), but not the organic materials. Firstly, an empty evaporating dish was pre-treated by heating at 600 ℃ in a muffle furnace and then directly cooled and weighed (W1). Subsequently, 2 g of sample (W) was placed into the evaporating dish and then was heated at 550 ℃ using muffle furnace until it was perfectly turned into grayish-white ash (W2). The later represents the total amount of minerals within theP. retrofractumfruits. The total ash content was then determined using the following formula:
Total ash content (%)=(W2-W1)/W×100
2.4. Crude protein analysis
Crude protein of theP. retrofractumvahl. was represented by measuring the nitrogen (N) content. This determination was done based on the Indonesian National Standard (SNI No. 01-2891-1992[14]. Briefly, 0.51 g of sample was placed in the 100 mL kjeldahl flask. Subsequently, 2 g of selenium mixture (2.5 g of SeO2; 100 g of K2SO4and 30 g of CuSO45H2O) and 25 mL of concentrated H2SO4were added into the kjeldahl flask. All the components were mixed thoroughly and heated until the mixture became clear and greenish.Distilled water was then added until the mixture reached 100 mL.Next, 5 mL of the mixture was pipetted and placed into distillation system and subsequently was added by 5 mL of 30% NaOH solution and few drops of mixed indicator solution (10 mL of 0.1 mL bromocresol green and 2 mL of methyl red). Distillation was done within 10 min and then the produced NH3was collected (in the form of NH4OH) in the conical flask supplemented with 10 mL of 2%boric acid solution and few drops of mixed indicator solution. Then,the distillate was subjected to titration against 0.01 N HCl. Finally,crude protein ofP. retrofractumvahl. fruit was calculated as:
Crude protein content (%)=6.25×[(V1-V2)×N ×0.014×f/W]×100
Where V1, V2, N, f and W are the sample titration reading, blank titration reading, HCl normality, sample dilution and sample weight,respectively. The 0.014 constant is the mili equivalent of nitrogen.
2.5. Analysis of total lipid content
Analysis of total lipid content within theP. retrofractumvahl. fruit was conducted using soxhlet extractor and based on the Indonesian National Standard (SNI No. 01-2891-1992)[14]. Briefly, 2 g of fruit sample was wrapped and dried using oven at 80 ℃ and then was placed into the lipid flask within the soxhlet extractor. The sample was then extracted using hexane solvent. The extract obtained was then distilled and subsequently dried using oven at 105 ℃. The later step was repeated until a constant weight was achieved. Total lipid content was calculated as follow:
Lipid content (%)=(Weight ofn-hexane extract)/(Weight of sample)×100
2.6. Determination of carbohydrate content and caloric value of P. retrofractum vahl. fruit
Carbohydrate content of theP. retrofractumfruit was determined by involving the addition of crude protein, lipid, water content, total ash content), as the equation below :
Carbohydrate (%)=100%-(Water content+Total ash+Total lipid+Crude protein )
Meanwhile, the caloric value of theP. retrofractumfruit was calculated as the sum of crude protein, carbohydrate and total lipid content (each was multiplied by a factor of 4.0, 3.5 and 9.0 kcal/100 g, respectively[15].
2.7. Determination of dietary fiber of P. retrofractum vahl.fruit
The analysis of dietary fiber was done by combining both enzymatic and gravimetric methods[16]. Briefly, samples were digested with sequential enzymatic reaction involving α-amylase,protease and amyloglucosidase followed by incubation at 60 ℃ for 30 min with constant agitation. Hereafter, 225 mL of 95 % ethanol was added to each digest product and then pre-heated to 60 ℃. The solution was precipitated at room temperature and then filtered. The residues were then washed twice using 15 mL of 78% ethanol, 95%ethanol and acetone. The washed residues were subsequently dried in oven at 105 ℃ until the constant weight was achieved. The residues were then cooled using dessicator and then weighed (W). All these analysis were done in duplicate, where the first sample was used to measure the sample protein residue (Pr) (using kjeldahl method) and the second sample was used to determine total ash residues (As).Dietary fiber was finaly determined using the following equation :Dietary fiber (%)=(W-Pr-As-blank)/(Weight of sample)×100
Where the value of blank was measured using:
Blank=Blank residue-Blank protein residue-Blank ash residue
2.8. Determination of mineral content of P. retrofractum vahl. fruit
Mineral contents were analyzed using inductively coupled plasmamass spectrometry. These minerals include calcium (Ca), copper(Cu), iron (Fe), magnesium (Mg), manganese (Mn), phosphor (P),potassium (K), sodium (Na) and zinc (Zn). In brief, a 0.3 g of sample was used for each tested mineral. In addition, all samples used were also spiked to the internal standard. Then, 6 mL of concentrated nitric acid (HNO3) was carefully added. Pre-digestion process was conducted around 10-15 min prior the digestion process. Hereafter,the sample was cooled and then diluted with deionized water. Finally,diluted samples were analyzed using inductively coupled plasmamass spectrometry. The value of each minerals were represented as mg/100 g DW (dry weight).
2.9. Phytochemical screening of P. retrofractum vahl. fruit
Prior conducting phytochemical screening, the samples were prepared for extraction. A maceration procedures using three different solvents including methanol, ethyl acetate andn-hexane(Brataco Chemical) were performed by procedure described previously[8]. Phytochemical screening ofP. retrofractumfruit was done firstly by qualitative analysis to test the presence of alkaloid,tannin and flavones, flavonoid, glycosides, sterols and quinone.These analysis were conducted using standard procedures described by the Indonesian Herb Pharmacopoeia and Harborne[17,18].
Quantitative analysis of total alkaloid ofP. retrofractumwas also conducted using Folin-Ciocalteu[19]. Briefly, 2.5 g of powdered samples were weighed and extracted using maceration with 5% of acetic acid for 4 h. Then, the mixture was filtered to remove cellular debris until the volume reached one quarter using water bath at 70 ℃.A concentrated ammonium hydroxide (NH4OH) was applied dropwise until pH reached 10 or the precipitate was complete. The solution was then centrifuged and the precipitate was washed using 1% of ammonium hydroxide and was de novo centrifuged. Finally, the obtained residu, which represented the total alkaloid, was weighed after previously being dried. The total alkaloid was represented as percentage using the following equation:
Total alkaloid (%)=Residue/(Weight of sample)×100
Total phenol content of methanol, ethyl acetate andn-hexane extraxts were also determined using Folin-Ciocalteu method. Briefly,1 mL of the extracts were mixed with 0.5 mL of Folin-Ciocalteu reagent. The mixture was left for 5 min. Subsequently, 2 mL of 10%(w/v) sodium carbonate. The mixture was then allowed to stand for 10 min in the dark and then the absorbance was measured using spectrophotometer at 770 nm. Total phenolic content was expressed as mg of gallic acid equivalent per g dry weight of the extract using a calibration curve generated with gallic acid.
Total flavonoid content was also measured. Firstly, 1 g of powdered samples was weighed and placed in a boiling flask round bottom.Then, 1 mL of 0.5% (w/v) hexamethylenetetramine, 20 mL of acetone and 2 mL of 25% (w/v) HCl were added. The mixture was then filtered and placed into a volumetric flask. Subsequently,100 mL of acetone was then added and homogenized. A total of 20 mL of filtrate obtained was added by 20 mL of distilled water.Hereafter, 15 mL of ethylactetate and then mixed thoroughly. The ethylacetate phase was separated and subjected to three additional 10 mL ethylacetate extraction. All the ethylacetate fractions were mixed together and then washed two times using 50 mL distilled water followed by filtration using cotton. Finally, filtrate was diluted using ethylacetate to 50 mL. Quantification of flavonoids was conducted based on Christ-Muller’s method. Briefly, 10 mL of ethylacetate fraction was mixed with AlCl3in a methanol-acetic acid.The absorbance was measured at 425 nm using spectrophotometer.The percentage of flavonoids were determined using the following formula :
Total flavonoids (%)=Absorbance of sample×1.25/(Weight of sample)×100
2.10. Statistical analysis
Statistical analysis were performed using Minitab 17 Statistical Software for Windows. Data were expressed as mean±standard deviation. Determination of mineral composition and phytochemical quantification were carried out in triplicate and duplicate,respectively. Comparison between extraction solvent used in total phenol quantification was analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s HSDpost hoctest.
3. Results
3.1. Proximate composition of P. retrofractum vahl. fruits
Water content ofP. retrofractumfruit was found to be 18%. High water content in this fruit support its function as supplemental herbal beverages. Some traditional and modern beverages derived from the plants also showed similar value in their water content[15,20].Meanwhile, total ash and fat of this fruit were 4.29% and 2.97%,respectively. Dietary fiber of the fruit was found to be 28.8%. This fiber represents polysaccharides as well as lignin and other plant fiber that are not easily digested by human’s digestive system. In addition, dietary fiber can also be an alternative source of energy.Nevertheless, we found high amount of carbohydrate in this fruit(63.4%). The crude protein was measured using Kjeldahl method by determining total nitrogen content within the fruit. Therefore, it includes nitrogen from the proteins and other nitrogen containing compounds. The value of crude protein reached 11.4%. Finally,the calorie was found to be 326 kcal/100 g. This means thatP.retrofractumfruits possesses an important value of energy and it could support the traditional uses of this fruit as supplement and medicinal beverages.
3.2. Mineral composition of P. retrofractum vahl. fruits
Determining the mineral content of the fruit or other source of food is pivotal for human health reasons. We observed high quantity of potassium (K), calcium (Ca), phosphor (P), and magnesium (Mg)which were (1 361.03±24.00); (414.97±5.85); (203.30±0.26) and(166.4±0.8) mg/100 g DW, respectively. Meanwhile, among the minerals observed in this study, the amount of manganese found in this fruit was found to be the lowest [(0.72±0.06) mg/100 g DW].The ratio level between zinc (Zn) and copper (Cu) was found in relatively balanced condition, where the concentration of Zn and Cu were (0.93±0.08) and (0.91±0.04) mg/100 g DW. Other trace elements such as iron (Fe) and sodium were found to be (5.12±0.06)and (9.41±0.32) mg/100 g DW, respectively.
3.3. Phytochemical screening of P. retrofractum vahl. fruit
The phytochemical screening ofP. retrofractumfruit was preliminary conducted using three different extracts, including methanol, ethylacetate andn-hexane extracts. Phytochemical parameters used in this study comprise quinone, sterols, glycosides,flavones, tanin and alkaloids. The results showed that methanol extract was positive for almost all parameters, except quinone.Meanwhile, ethylacetate extract ofP. retrofractumfruit was positive for quinone, sterols, glycosides, tanin and alkaloids but negative for flavones. Finally, we observed the presence of quinone, sterols,glycosides and alkaloids in then-hexane extract of the fruit. Our observation also showed thatP. retrofractumfruit contains low quantity of flavonoids and alkaloids, which were 0.060 0%±0.000 2%and 0.265%±0.008% (w/w), respectively. Furthermore, we did three extractions using different solvents including methanol, ethylacetate and n-hexane. Each type of extracts exhibited significant different amount of total phenols (P<0.05). Methanol extract showed the highest total phenol content [(16.31±0.05) ppm] followed by ethylacetate and n-hexane [(5.78±0.02) and (5.31±0.01) ppm respectively]. These indicate that the selection of solvent for an efficient extraction is important.
4. Discussion
Food and beverages industries apply a very strict regulation to ensure the quality of the product. The latter is necessarily required since this type of industry directly correlates with human health.Furthermore, medicinal based industry, especially natural-based drugs where some of the components are obtained from the living things, including plants, also possess similar regulation.Therefore, proximate analysis would give a preliminary data for determining the quality of the products as well as its nutritional value[21]. This dioceous climbers tree,P. retrofractumpossess a cylindrical berry fruits, where the apex part of the fruit is rounded.The color of the fruit is green and become reddish to brown when they reach its maturity. In term of traditional uses, theP.retrofractumfruit is commonly used as flavours in the folk cuisine preparation (i.e.curries). In addition, the Indonesian local wisdom knowledge of grinding, mashing and pounding the plant material into a valuable medicinal beverages, commonly called as ‘Jamu’has madeP. retrofractumas an economically important plants.Some litteratures have cited this plant for its biological function as antioxidant[8], hepatoprotective[22], anticancer[23], gastroprotective[24],antiobesity[11], potential mast cell stabilizer[10], antileishmanial[25],and antilarvacidal[13].
The water content analysis revealed thatP. retrofractumexhibited relatively high water content compared to otherPiperspecies includingPiper trichostachyonandPiper nigrum(P. nigrum) which possessed 4.31% and 4.55%, respectively[26]. The water content analysis helps to determine the usable and safety time of the product.High water content might provoke the presence and enhance the bacteria and mould growth, in some extent, this condition might reduce the quality of the product[27]. More than 14% of food water content is susceptible for microbial contamination[28]. Hence, good storage condition also plays a crucial role to prevent spoilage.Nevertheless, high water content is the best characteristic for plantbased beverages. Some reports showed that plant parts that are used for beverage production have high water content[14]. We observed the presence of high carbohydrate content (63.4%) within the fruit.This is could be the major contributor of energy, since crude protein and total fat contribute only in small portion. These values are lower than that observed in other Piper species, such asP. nigrum. The crude protein content and total fat ofP. nigrumhad been reported to be 25.45% and 5.34%, respectively. However, carbohydrate content ofP. retrofractumis higher thanP. nigrum, which is only 37.36%[29].The contribution of the three parameters (carbohydrate, crude protein and total fat) makeP. retrofractumas a valuable high energy plant source. This could be interesting since high calorie diet increases weight gain in anorexia nervosa incidence[30]. EventhoughP.retrofractumpossess high caloric value (326 kcal/100 g), this species contains piperidine alkaloids, which regulates lipid metabolism by activating the AMP-activated protein kinase. The biological function of the antiobesity constituent ofP. retrofractumis indicated by an increase of protein expression, which is responsible for fat burning and a decrease of protein expression involved in fat storage mechanism[11].
Dietary fiber has been always associated with human health,especially to certain diseases including cardiovascular, type Ⅱdiabetes, obesity and cancer[31]. Dietary fibers have been defined as non enzymatically digestible. The dieatry fiber was found to be relatively higher than other spices plants such asOcimum gratissimym,Penrgularia extensaandTetrapleura tetrapterawhich are ranging from 17.00%-20.24%[32]. In addition, our observation also showed that the dietary fiber contained inP. retrifractumis also higher thanP. nigrum(23.6%)[29]. These results also madeP.retrofractumas a potential dietary fiber source. Hence, it also supports the medicinal function of this spice plant. The minerals content of this species is also comparable to other Piper species. Calcium,magnesium and potassium were found higher that those observed inPiper guineense(8.50-12.38; 1.14-1.21; and 0.72-1.2 mg/100 g DW, respectively)[33] and inP. nigrum(195; 52 and 663 mg/100 g DW, respectively)[29]. Calcium functions not only for mediating the vascular contraction and vasodilatation, neural transmission and muscular contraction[34], but also plays an important role in bone health, including lowering the risk of osteoporosis[35]. Regarding to magnesium, this intracellular cation is also associated with calcium metabolism in the human bones and in some extent might be associated with hypertension, diabetes and cardiac perturbation[36].As an extracellular cation, potassium also plays a pivotal role in maintaining blood pressure. Therefore, high uptake of potassium could reduce the hypertension[37]. Our observation showed thatP.retrofractumposses high potassium and low level of sodium. This low Na/K ratio might also contribute to better dietary source[15].
A non significantly difference between copper and zinc content was observed in this study. The balance between those two minerals is essential for reducing the risk of coronary diseases by modulating the concentration of cholesterol within the plasma[38]. Furthermore,zinc and copper have been known for its function as co-factor of metalloproteins, including superoxide dismutase. Recent data showed that these two elements function to ensure and accelerate the oxidative refolding of metalloprotein. In other word, these elements play a role in preventing protein misfolding which is responsible for many serious diseases[39]. Our observation showed that zinc content ofP. retrofractumis higher than that ofPiper guineense(0.09-0.18 mg/100 g DW)[33]. Meanwhile, copper content ofP. retrofractumis lower than that observed inP. nigrum(1.3 mg/100 g DW)[29].
Compared to zinc, iron is abundantly found in earth and also plays an important role in human health. It involves in the formation of hemoglobin and ensures the oxygen transport through blood circulation. Low intake of iron can lead to the anemia disease and possibly can also cause neurodegenerative diseases[40]. Major source of iron is from meat, fishes and also from some vegetables. However,the absorption of iron from vegetables is reported to be lower than those absorbed from meat and fishes. This is might be due to other food components present in the vegetables including polyphenols,calcium and peptides[41]. In this study we found that iron is detected in comparable quantity compared to otherPiperspecies. It is lower than that observed inP. nigrumandPiper trichostachyon(25 mg/100 g DW and 10.84 mg/100 g DW, respectively)[26] and higher than iron observed inPiper guineense(0.62-0.85 mg/100 g DW)[33].Nonetheless,P. retrofractumcould be potential and alternative source of iron in human diet.
High phosphorous and low manganese content are found inP.retrofractum. Together with calcium, phosphorous involves and plays an important role in ensuring human bone health[42]. However,other report showed that high consumption of phosphorous is often associated with cardiovascular diseases[43]. Similar to the function of phosphorous and calcium, manganese is also essential micronutrient needed in supporting human bone formation. Additionally, it plays an important role as co-factor of some important enzymes involved in the neurotransmitter metabolism[44]. Our results showed that the quantity of phosphorous and manganese found inP. retrofractumfruits were higher thanP. nigrum(0.14 mg/100 g DW and 5.18 mg/100 g DW, respectively) and lower thanPiper trichostachyon(0.16 mg/100 g DW and 7.54 mg/100 g DW, respectively)[26].
Phytochemical screening ofP. retrofractumfruits extracted fromn-hexane, ethylacetate and methanol result in different profile of natural compounds ranging from quinone, sterols, glycosides,flavones, tannin and alkaloids. This demonstrates that the choice of solvent for extraction process is necessarily required and determines the type of phytochemicals. This is in accordance with the previous study which showed that different solvent mixture influenced the extracted compounds. Furthermore, the choice of solvent might also be species dependent[45]. Naturally occuring phytochemicals are also influenced by the genetic of the species, geographical factors,climate, ecological and plant part used for extraction methods[33,46].Regarding to the effect of different solvents used for extraction, it is also reflected in the results of total phenol quantification, which indicated that the use of different solvents resulted in different quantities of total phenol. Methanol is the best solvent for total phenol quantification since it gave the highest quantity compared to others. Our results also showed that total phenol contained in the fruit ofP. retrofractumthrough methanol extraction is silghtly lower than that obtained in theP. nigrumfruits (1.728 mg/g). Meanwhile,flavonoid content in theP. retrofractumis higher than that observed inP. nigrum(1.087 μg/g)[47]. Flavonoids have been known as a natural compounds that play an important role in some biological mechanisms of living thing. Previous reports showed that flavonoids obtained inPiper aduncumpossess anti-inflamatory effect[48].Moreover, flavonoid compounds found inPiper delineatumandPiper sarmentosumhave been reported to act as inhibitor of quorum sensing inhibitor[49] and cytoprotective against oxidative stress[50],respectively. Alkaloids are also major constituents in plant belongs to the genusPiperaceae[1]. Among them, piperine constitutes the main alkaloids found in this genus. Many reports have demonstrated the biological function of alkaloids including hepatoprotective,antidepressant and anti cancer[51]. Our results showed that total alkaloids found inP. retrofractumwas lower than alkaloid found inP. nigrumfruit (112.39 mg)[52]. Nonetheless, the overall results support the medicinal function ofP. retrofractumfruits.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgments
We thank to Erwin Kurniawan and Indah Prasetyowati who have supported this project. We are grateful to Directorate General of Resources for Science, Technology and Higher Education, Ministry of Research, Technology and Higher Education of the Republic of Indonesia who has financially supported this project through research grant No. 653/PKS/ITS/2017.
[1] Tseng YC, Xia N, Gilbert MG. Piperaceae C.Agardh. In: Wu ZY, Raven PH, editors.Flora of China.Volume 4 (Cycadaceae through Fagaceae).Beijing/St. Louis: Science Press/Missouri Botanical Garden Press; 1999.
[2] Chaveerach A, Mokkamul P, Sudmoon R, Tanee T. Ethnobotany of the genusPiper(Piperaceae) in Thailand.Ethnobot Res Appl2006; 4: 223-231.
[3] Jong-Woong A, Mi-Ja A, Ok-Pyo Z, Eun-Joo K, Sueg-Geun L, Hyung JK,et al. Piperidine alkaloids fromPiper retrofractumfruits.Phytochemistry1992; 31: 3609-3612.
[4] Muharini R, Liu Z, Lin W, Proksch P. New amides from the fruits ofPiper retrofractum.Tetrahedron Lett2015; 56: 2521-2525.
[5] Banerji A, Sarkar M, Datta R, Sengupta P, Abraham K. Amides from Piper brachystachyum andPiper retrofractum.Phytochemistry2002; 59:897-901.
[6] Tewtrakul S, Hase K, Kadota S, Namba T, Komatsu K, Tanaka K. Fruit oil composition ofPiper chabahunt,P. longumL. andP. nigrumL.J Essent Oil Res2000; 12: 603-608.
[7] Luyen BT, Tai BH, Thao NP, Yang SY, Cuong NM, Kwon YI, et al. A new phenylpropanoid and an alkylglycoside fromPiper retrofractumleaves with their antioxidant and α-glucosidase inhibitory activity.Bioorg Med Chem Lett2014; 24: 4120-4124.
[8] Jadid N, Hidayati D, Hartanti SR, Arraniry BA, Rachman RY, Wikanta W. Antioxidant activities of different solvent extracts ofPiper retrofractumVahl. using DPPH assay.AIP Conf Proc2017; 1854: 020019. Doi: http://dx.doi.org/10.1063/1.4985410.
[9] Kubo M, Ishii R, Ishino Y, Harada K, Matsui N, Akagi M, et al.Evaluation of constituents ofPiper retrofractumfruits on neurotrophic activity.J Nat Prod2013; 76: 769-773.
[10] Jadid N, Rachman RY, Hartanti SR, Abdulgani N, Wikanta W, Muslihatin W. Methanol extract ofPiper retrofractumvahl. potentially mediates mast cell stabilization.Int J Pharma Bio Sci2016; 7: 379-383.
[11] Kim KJ, Lee MS, Jo K, Hwang JK. Piperidine alkaloids fromPiper retrofractumvahl. protect against high-fat diet-induced obesity by regulating lipid metabolism and activating AMP-activated protein kinase.Biochem Biophys Res Commun2011; 411: 219-225.
[12] Abd Kadir SL, Yaakob H, Zulkifli RM. Potential anti-dengue medicinal plants: a review.J Nat Med2013; 67: 677-689.
[13] Chansang U, Zahiri NS, Bansiddhi J, Boonruad T, Thongsrirak P,Mingmuang J, et al. Mosquito larvicidal activity of aqueous extracts of long pepper (Piper retrofractumvahl.) from Thailand.J Vector Ecol2005;30: 195.
[14] Badan Standardisasi Nasional.Standar Nasional Indonesia, SNI 01-2891-1992, Food and beverages, test methods.Jakarta, Indonesia: Badan Standardisasi Nasional; 1992.
[15] Ooi DJ, Iqbal S, Ismail M. Proximate composition, nutritional attributes and mineral composition ofPeperomia pellucidaL. (Ketumpangan air)grown in Malaysia.Molecules2012; 17: 11139.
[16] AOAC.Official methods of analysis.17th edition. Gaithersburg, MD,USA: Association of Official Analytical Chemist Inc; 2003.
[17] Ministry of Health Republic of Indonesia.Farmakope herbal Indonesia.1st edition. Jakarta: Ministry of Health Republic of Indonesia; 2008.
[18] Harborne JB.Phytochemical methods a guide to modern techniques of plant analysis.3rd edition. London: Chapman and Hall; 1998.
[19] Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent.Method Enzymol1999; 299:152-178.
[20] Adedeji TO, Oluwalana IB. Development and quality evaluation of a non-alcoholic beverage from cocoyam (Xanthosoma sagittifoliumandColocasia esculenta).Niger Food J2014; 32: 10-20.
[21] Hassan AS, Kashlan NB, Al-mousa ZA, Shubber KM, Srivstava VP,Nawaz SP, et al. Proximate and mineral compositions of local Kuwaiti fast foods.Ecol Food Nutr1991; 26: 37-45.
[22] Matsuda H, Ninomiya K, Morikawa T, Yasuda D, Yamaguchi I,Yoshikawa M. Protective effects of amide constituents from the fruit ofPiper chabaon d-galactosamine/TNF-α-induced cell death in mouse hepatocytes.Bioorg Med Chem Lett2008; 18: 2038-2042.
[23] Jyothi D, Vanathi P, Mangala GP, Rama SRV, Madhusudana RJ, Sreedhar AS. Diferuloylmethane augments the cytotoxic effects of piplartine isolated fromPiper chaba.Toxicol In Vitro2009; 23: 1085-1091.
[24] Morikawa T, Matsuda H, Yamaguchi I, Pongpiriyadacha Y, Yoshikawa M. New amides and gastroprotective constituents from the fruit ofPiper chaba.Planta Med2004; 70: 152-159.
[25] Bodiwala HS, Singh G, Singh R, Dey CS, Sharma SS, Bhutani KK,et al. Antileishmanial amides and lignans fromPiper cubebaandPiper retrofractum.J Nat Med2007; 61: 418-421.
[26] Upadhya V, Pai SR, Ankad GM, Hegde HV. Pharmacognostic screening ofPiper trichostachyonfruits and its comparative analysis withPiper nigrumusing chromatographic techniques.Pharmacogn Mag2016; 12:S152-S158.
[27] Witthuhn RC, Engelbrecht S, Joubert E, Britz TJ. Microbial content of commercial South African high-moisture dried fruits.J Appl Microbiol2005; 98: 722-726.
[28] Ihekoronye AI, Ngoddy PO.Integrated food science and technology for the tropics.London: McMillan Publishers Ltd; 1985.
[29] Al-Jasass FM, Al-jasser MS. Chemical composition and fatty acid content of some spices and herbs under Saudi Arabia conditions.Sci World J2012; 2012: 5.
[30] Garber AK, Mauldin K, Michihata N, Buckelew SM, Shafer MA,Moscicki AB. Higher calorie diets increase rate of weight gain and shorten hospital stay in hospitalized adolescents with anorexia nervosa.J Adolesc Health2013; 53: 579-584.
[31] Slavin J. Fiber and prebiotics: mechanisms and health benefits.Nutrients2013; 5: 1417-1435.
[32] Okwu DE. The potentials ofOcimum gratissimum,Penrgularia extensaandTetrapleura tetrapteraas spice and flavouring agents.Nigeria Agric J2003; 34: 143-148.
[33] Isong EU, Essien IB. Nutrient and antinutrient composition of three varieties ofPiperspecies.Plant Food Hum Nutr1996; 49: 133-137.
[34] Straub DA. Calcium supplementation in clinical practice: a review of forms, doses, and indications.Nutr Clin Pract2007; 22: 286-296.
[35] Park HM, Heo J, Park Y. Calcium from plant sources is beneficial to lowering the risk of osteoporosis in postmenopausal Korean women.Nutr Res2011; 31: 27-32.
[36] Swaminathan R. Magnesium metabolism and its disorders.Clin Biochem Rev2003; 24: 47-66.
[37] Weaver CM. Potassium and health.Adv Nutr2013; 4: 368S-377S.
[38] Alissa EM, Bahijri SM, Lamb DJ, Ferns GAA. The effects of coadministration of dietary copper and zinc supplements on atherosclerosis, antioxidant enzymes and indices of lipid peroxidation in the cholesterol-fed rabbit.Int J Exp Pathol2004; 85: 265-275.
[39] Li HT, Jiao M, Chen J, Liang Y. Roles of zinc and copper in modulating the oxidative refolding of bovine copper, zinc superoxide dismutase.Acta Biochim Biophys Sin2010; 42: 183-194.
[40] Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health.J Res Med Sci2014; 19: 164-174.
[41] Hurrell R, Egli I. Iron bioavailability and dietary reference values.Am J Clin Nutr2010; 91: 1461S-1467S.
[42] Penido MGMG, Alon US. Phosphate homeostasis and its role in bone health.Pediatr Nephrol2012; 27: 2039-2048.
[43] Trautvetter U, Jahreis G, Kiehntopf M, Glei M. Consequences of a high phosphorus intake on mineral metabolism and bone remodeling in dependence of calcium intake in healthy subjects-a randomized placebocontrolled human intervention study.Nutr J2016; 15: 7.
[44] Bowman AB, Kwakye GF, Hernández EH, Aschner M. Role of manganese in neurodegenerative diseases.J Trace Elem MedBiol2011;25: 191-203.
[45] Boeing JS, Barizão ÉO, e Silva BC, Montanher PF, de Cinque Almeida V,Visentainer JV. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: application of principal component analysis.Chem Cent J2014; 8: 48.
[46] Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T,Wawrosch C, Uhrin P, et al. Discovery and resupply of pharmacologically active plant-derived natural products: a review.Biotechnol Adv2015; 33:1582-1614.
[47] Ahmad A, Husain A, Mujeeb M, Khan SA, Alhadrami HAA, Bhandari A.Quantification of total phenol, flavonoid content and pharmacognostical evaluation including HPTLC fingerprinting for the standardization ofPiper nigrumLinn fruits.Asian Pac J Trop Biomed2015; 5: 101-107.
[48] Thao NP, Luyen BT, Widowati W, Fauziah N, Maesaroh M, Herlina T,et al. Anti-inflammatory flavonoid C-glycosides fromPiper aduncumleaves.Planta Med2016; 82: 1475-1481.
[49] Martín-Rodríguez AJ, Ticona JC, Jiménez IA, Flores N, Fernández JJ,Bazzocchi IL. Flavonoids fromPiper delineatummodulate quorumsensing-regulated phenotypes inVibrio harveyi.Phytochemistry2015;117: 98-106.
[50] Ugusman A, Zakaria Z, Hui CK, Nordin NAMM, Mahdy ZA. Flavonoids ofPiper sarmentosumand its cytoprotective effects against oxidative stress.EXCLI J2012; 11: 705-714.
[51] Rosa Martha Perez G, Adriana Maria Neira G, Carlos HV. Alkaloids fromPiper: a review of its phytochemistry and pharmacology.Mini Rev Med Chem2013; 13: 163-193.
[52] Ajanal M, Gundkalle MB, Nayak SU. Estimation of total alkaloid in Chitrakadivati by UV-spectrophotometer.Anc Sci Life2012; 31: 198-201.
 Asian Pacific Journal of Tropical Biomedicine2018年1期
Asian Pacific Journal of Tropical Biomedicine2018年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Prevalence of coronavirus from diarrheic calves in the Republic of Korea
- Effective Aeromonas specific monoclonal antibody for immunodiagnosis
- Pharmacodynamic profiling of optimal sulbactam regimens against carbapenemresistant Acinetobacter baumannii for critically ill patients
- Efficiency of combining pomegranate juice with low-doses of cisplatin and taxotere on A549 human lung adenocarcinoma cells
- Correlation of phytochemical content with antioxidant potential of various sweet potato (Ipomoea batatas) in West Java, Indonesia
- Larvicidal activity of Xenorhabdus and Photorhabdus bacteria against Aedes aegypti and Aedes albopictus
