Efficiency of combining pomegranate juice with low-doses of cisplatin and taxotere on A549 human lung adenocarcinoma cells
Nasser Mohamad, Hijazi Akram✉, Sayed Ahmad Bouchra,2✉, Jamal Eddine Zeinab, Ibrahim Sajida, Rammal Hassan, Al Rekaby Abd-El-Ameer, Nasser Mouhamad
1Doctoral School of Science and Technology, Research Platform for Environmental Science (PRASE), Lebanese University, Lebanon
2Université Fédérale de Toulouse Midi-Pyrénées, INP-ENSIACET, Laboratoire de Chimie Agro-industrielle, Toulouse, France
3Al Mustansiriya University, College of Science, Department of Biology, Iraq
4American University of Beirut, Beirut, Lebanon
1. Introduction
Lung cancer is one of the most common cancers and it is the most leading cause of preventable death. It is the leading cause of cancer related death in men and women in the United States accounting for approximately 28% of total cancer deaths in 2010 despite comprising only 15% of new cancer cases[1]. Lung cancers are aggressive tumors that are classified into two main types which are the non-small cell lung cancer (NSCLC) representing (80%-85%)and small cell lung cancer (SCLC) representing (15%-20%) based on histological, clinical and neuroendocrine characteristics[2,3].NSCLC are subdivided into: adenocarcinomas, squamous cell, large cell carcinoma, bronchoalveolar lung cancer, and mixed histological types.
SCLC arises from neuroendocrine cells ‘kulchitsky cells’ of the lungs[4]. These cells make polypeptide hormones and are characterized by dense core neurosecretory granules[5], while the NSCLC arises from the alveolar cells[6,7]. Adenocarcinomas come from basal bronchial cells and type Ⅱ pneumocytes that appear in the periphery of the lung, and the squamous type arises from the bronchial epithelial cells located more centrally[8]. Concerning the treatment, it depends on the cancer stage, in which surgical treatment is reserved for early stages Lebanese University for the financial support of this work (Project M. Nasser UL2017). (Ⅰ andⅡ) however such intervention seemed harmful for advanced stages(ⅢB and Ⅳ) and treated with chemotherapeutic drugs[9]. There is no specific agent for cancer treatment which is the focal point of the cancer research. Nowadays, the traditional and complementary/alternative medicine was the insight for cancer researchers[10],and we choose the pomegranate in our study as a hope to treat the advanced stages adenocarcinoma A549 cells.
Epidemiological studies have shown that diets rich in phytochemicals and antioxidants play a major role in combating diseases and maintaining health[11]. One of the most important fruits that has been studied is the pomegranate. Besides its use as a fruit,its medicinal properties have attracted the interest of researchers of many countries. Pomegranate fruit has medicinal properties such as anti-inflammatory and antibacterial activities. The pomegranate seed oil has inhibitory effect on skin and breast cancers. The pomegranate seed oil has phytoestrogenic compounds and the fruit is rich in phenolic compounds with strong antioxidant activity. Ellagic acid is one of the main components of pomegranate with phenolic structure and antioxidant activity.
Cisplatin (CDDP) was known to be a chemotherapy drug for all types of cancer, but it was found to induce cytotoxic side effects that will harm normal cells. It is used alone or combined with other chemotherapeutic drugs, such as taxotere, which is an antimicrotubule agent. However, these conventional therapies have failed to make a major impact on the survival of NSCLC patients highlighting the need for new approaches.
In our present work, it is aimed to evaluate the effect of the combination of pomegranate juice with low dose of chemotherapeutic drugs (taxotere and cisplatin) on A549, human lung adenocarcinoma cells.
2. Materials and methods
2.1. Preparation of pomegranate
Lebanese pomegranates were peeled and the arils were mixed and then centrifuged. The supernatant was frozen at –80 ℃ and then lyophilized and stored in a desiccator at room temperature.Lyophilized pomegranate was dissolved in complete medium prepared at different concentrations (50, 100, 150, and 200 μg/mL).
2.2. Chemical tests
2.2.1. Antioxidant test (DPPH radical scavenging method)
2,2-diphenyl-1-picrylhydrazyl (DDPH) is a dark-colored crystalline powder composed of stable free radical molecules with antioxidant activity[12]. In this study, we prepared two solutions of DPPH(0.003 g): the first one in 50 mL of methanol and the second in 50 mL of ethanol to compare the activity of the best extract, and we prepared 0.003 g of DPPH and dissolved them in 20 mL of ethanol to monitor the change of the activity. Then we prepared our extract by measuring 0.04 g of the pomegranate powder and dissolved them in 20 mL of distilled water and we vortexed them to obtain a homogeneous solution. After that, we prepared 5 different concentrations (0.5, 0.4, 0.3, 0.2, 0.1 mg/mL) from our solution.
2.2.2. Phytochemical screening tests
Qualitative tests were performed on the juice to determine its content in compounds involved in some of the biological properties antioxidants The presence of phenolic acid, terpenoid, flavonoid,quinone, alkaloid, tannin, resin and saponin was analysed using the methods of Farhanet al. (2012)[13,14].
2.2.3. Infrared spectroscopy (IR)
Pomegranate powder was mixed with potassium bromide powder,a material having no IR-active vibrations in the mid-IR range. The mixture was pressed into a transparent disk and inserted in the beam path. In this case, a sum of vibrational properties were recorded,and the functional groups would be determined. This procedure was performed to evaluate the presence of hydroxyl group which is the main functional group for antioxidant activity.
2.3. Cell culture and treatment
2.3.1. A549 cells
The A549 human lung adenocarcinoma cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin in an atmosphere of 5% CO2at 37 ℃.
2.3.2. Normal cells (control)
Mouse mesangial cells (MCs) were cultured in DMEM supplemented with 0.1 mM non-essential amino acids, 10% foetal bovine serum, 100 units/mL penicillin and 0.1 mg/mL streptomycin at 37 ℃ in a humidified incubator containing 5% CO2.
2.3.3. Treatment of cells
Lyophilized pomegranate was dissolved using vortex in the culture medium specific for each cell type, as a concentrated stock solution(10 mg/mL) and then filtered by a filter diameter of 0.2 μm, and used for the preparation of different concentrations for the treatment of cells. A total of 50% to 60% confluent cells were treated with the pomegranate for 48 h in complete medium, seeded in a 24-well plate in a concentration of 10 000 cells/well.
The evaluation of the anticancer activity was performed by measuring the viability of the A549 cell line and normal MCs(control) after a treatment for 48 h with increasing concentrations of cisplatin (4, 8, 12 and 16 μg/mL) and pomegranate (50, 100,150, 200 μg/mL). Similarly, the A549 cells were treated with the combination of the pomegranate (200 μg/mL) and cisplatin (4 μg/mL) with or without taxotere (5 μg/mL).
2.4. Viability test
2.4.1. Neutral red uptake assay
Neutral red assay was performed to measure the cell viability. It was used to evaluate the cytotoxicity of pomegranate and cisplatin on the A549 adenocarcinoma cells.
Neutral red uptake assay was carried out following the protocol[15].Briefly, after 48 h treatment, the medium was aspirated and cells were washed twice with PBS and incubated for 3 h in a medium supplemented with neutral red (50 μg/mL). The medium were taken out, and it was washed twice again with PBS. Cells were subjected to further incubation for 10 min at room temperature in a mixture of acetic acid (1%) and ethanol (50%) to extract the dye. Then, the medium was transferred (100 μL/well) to 96-well plates. The plates were read at 490 nm using multi-well microplate reader.
2.4.2. Clonogenic survival assay
A549 Cells were seeded (100 cells/well) into a 6-well plate and treated with pomegranate of concentrations (50, 100, 150, 200, 400 and 1 200 μg/mL) for 24 and 48 h. After that, they were kept for 10 d in culture. After 10 d of incubation, the cells were washed twice with PBS, and then the crystal violet was added to each well and incubated for half an hour at 37 ℃.Thirty minutes later, they were washed by distilled water and the colony of survival cells that were colored by crystal violet was counted.
2.4.3. XTT assay
XTT colometric-assay (Sigma, St. Lois, MO) was used to evaluate the cellular viability of A549 cells through measuring the activity of enzymes that reduce the water soluble XTT [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] reagent to an orange formazan by actively respiring cells. After treatment of A549 cells with cisplatin at concentrations (4 and 8 μg/mL), pomegranate(150 μg/mL), and a combination of both cisplatin and pomegranate of concentration (4+150) μg/mL for 48 h in 96-well mixing 5 mL of XTT with 100 μL of activator. From this mixture 50 μL was added to each well plate, and then the 96-well plate was incubated for half an hour at 37 ℃ and 5% CO2. After that the absorbance was measured at an optical density of 450 nm[16].
2.4.4. Cell migration
Nearly confluent monolayers of cells were maintained in complete medium, and a track was scraped across these cells by a plastic tip.The cells were allowed to migrate through the edges of the wound at 37 ℃ for 24 h after treatment with or without pomegranate.The cells that have migrated through the wounded edges were photographed using a computer imaging system and the distance (in cm) was subsequently measured[17]. For migration assays, a uniform cell-free area was created by scratching confluent monolayers with a plastic micropipette tip as previously described. Briefly, the wound area was inspected at different time intervals (0, 24 hour) to determine the distance migrated by the cells. The closer the gap, the faster the cell migrated. At each time point four photographs were taken and the widths of the wound space were evaluated by multiple measurements.
2.5. Statistical analysis
All statistical analyses were carried out with PRISM (GraphPad,San Diego), andPvalues <0.05 or 0.001 were considered significant. The one way Anova was used to estimate cell viability,and differences were analyzed by the Bonferroni test.
3. Results
3.1. Antioxidant test (DPPH radical scavenging method)
It showed that the optical density was decreased as the concentration of pomegranate juice was increased. In addition, the antioxidant activity of DPPH was more efficient with methanol than with ethanol. This antioxidant activity represents 70% with methanol compared to 33% of ethanol, and this was also observed by the transformation of violet color to yellow color as the concentration of DPPH was increased from 0.1 to 0.5 mg/mL. This indicates that 0.5 mg/mL of methanolic extract has higher antioxidant activity than that of ethanol. This finding represents that methanol has the strongest antioxidant activity in pomegranate extract[18].
3.2. Phytochemical screening test
The phytochemical screening tests were performed to evaluate the presence of secondary metabolites responsible of the antioxidants property. The obtained results (Figure 1) confirmed the existence of alkaloid (orange), flavonoid (yellow), phenolic acid (blue to green), terpenoid (violet), and quinone, which was observed by the formation of orange, yellow blue to green, violet and red color respectively, in addition to resin that was observed by the presence of turbidity.
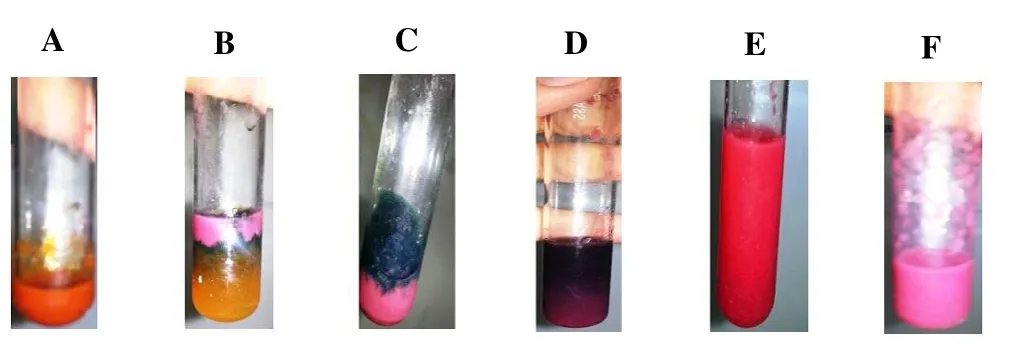
Figure 1. Phytochemical screening tests.
3.3. Infrared spectroscopy results
Figure 2a showed the presence of broad spectrum band at 3 424.96 cm-1, indicating clearly the existence of hydroxyl group that played a major role in achieving the anti-oxidant activity.
3.4. Viability tests results
3.4.1. Neutral red
The optical density decreased as a function of concentration and time of treatment. Indeed, only living cells incorporated neutral red solution in their lysosomes. The decrease in optical density reflected that the number of living cells began to decrease compared to the control which was not treated.
3.4.2. Cisplatin treatment decrease cell viability in a dose dependent manner
Treatment with increased concentrations of cisplatin (4, 8, 12 and 16 μg/mL) showed a significant decrease in A549 cells viability when compared to the control cells (Figure 2b). A significant difference was seen between cells treated with (4 and 8 μg/mL) and another group cells treated. No significant difference was observed in cell viability between cisplatin 12 μg/mL and 16 μg/mL.
3.4.3. Pomegranate treatment decrease cell viability in a dose dependent manner
Results showed a significant decrease in cell viability by comparison to the control (non-treated cells). On the other hand,no significant difference was observed between the two highest concentrations of cisplatin (300 and 400 μg/mL). Consequently, 300 μg/mL of pomegranate was considered as a high concentration for the treatment of the A549 cell lines (Figure 2c).
3.4.4. Effect of combination of pomegranate and cisplatin in cell viability
The neutral red results showed a significant decrease in A549 cells viability with increased concentrations of either pomegranate or cisplatin, while there was a same inhibition for pomegranate (150 μg/mL) alone as that for cisplatin (4 μg/mL) alone. However, when we combined pomegranate (150 μg/mL) with cisplatin (4 μg/mL), there was a significant increase in A549 cells death. A finding that became more significant as we increased the concentration of pomegranate to 300 μg/mL. Furthermore, by increasing the cisplatin concentration to 8 μg/mL more cell death was observed in comparison to low dose cisplatin (4 μg/mL). In order to minimize the side effects of cisplatin by decreasing its concentration without impairing its cytotoxity efficacy, we considered that combination of cisplatin (4 μg/mL) with pomegranate (300 μg/mL) would be the ideal concentration to face A459 cancer cells and indeed decreased cisplatin toxicity (Figures 2d and 2e).
3.4.5. Effect of the combination of pomegranate, cisplatin and taxotere on cell viability of A549
The combination of CDDP with taxotere decreases the cell viability of A549 up to 65% compared with the control cells, whereas in the presence of the pomegranate 200 μg/mL decreased the cell viability seems to be more important compared to CDDP and taxotere alone(Figure 2f).
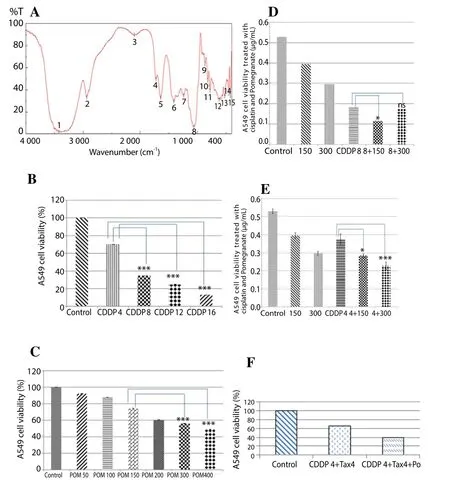
Figure 2. Infrared spectroscopy result.
3.4.6. Effect of combination of pomegranate and cisplatin on normal cells
Results showed no significant difference for cells treated with either pomegranate, cisplatin alone or a combination of these treatment compared to those untreated control cells (Figure 3a).
3.4.7. Pomegranate reduced the migration abilities of A549 cells
Pictures taken after 24 h of treatment showed a significant decrease in migration by comparing the treated groups with the untreated control. Indeed comparison between treatment groups showed that combination chemotherapy with pomegranate decreased the migration more than that of chemotherapy alone (Figure 3a, 3b).
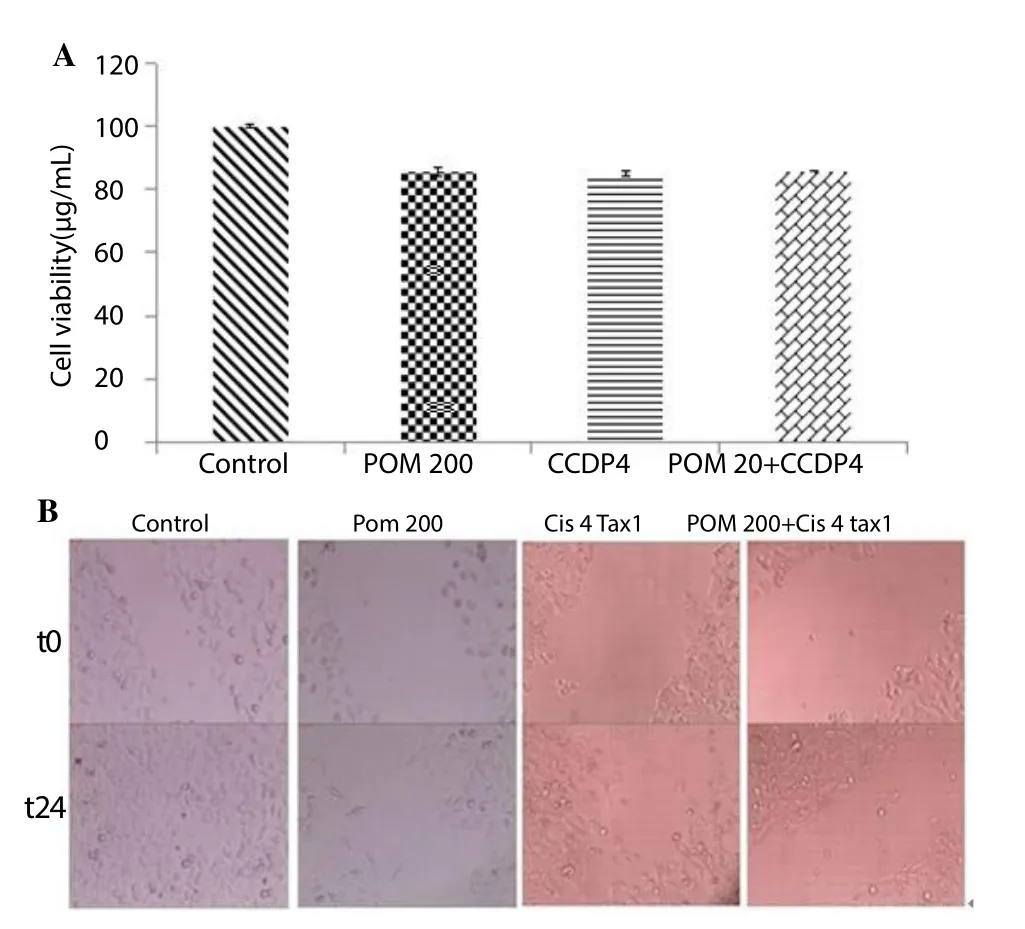
Figure 3. Effect of combination of pomegranate and cisplatin on normal cells.
3.5. Clonogenic survival assay
The results showed a slight decrease in the number of A549 cells with increased concentration of pomegranate from 50 to 100 μg/mL at 24 and 48 h. On the other hand, increasing the concentration of pomegranate to 200, 400, and 1 200 μg/mL resulted in higher A549 cells death when compared to the control at 24 and 48 h (Figure 4).
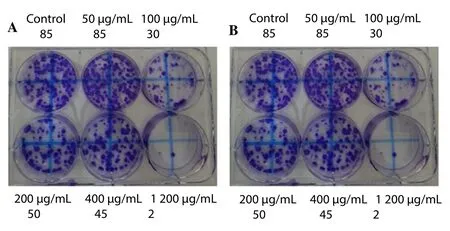
Figure 4. Clonogenic survival assay.
3.6. XTT assay
XTT assay showed a significant decrease in cell viability with increased concentration of cisplatin (Figure 5).
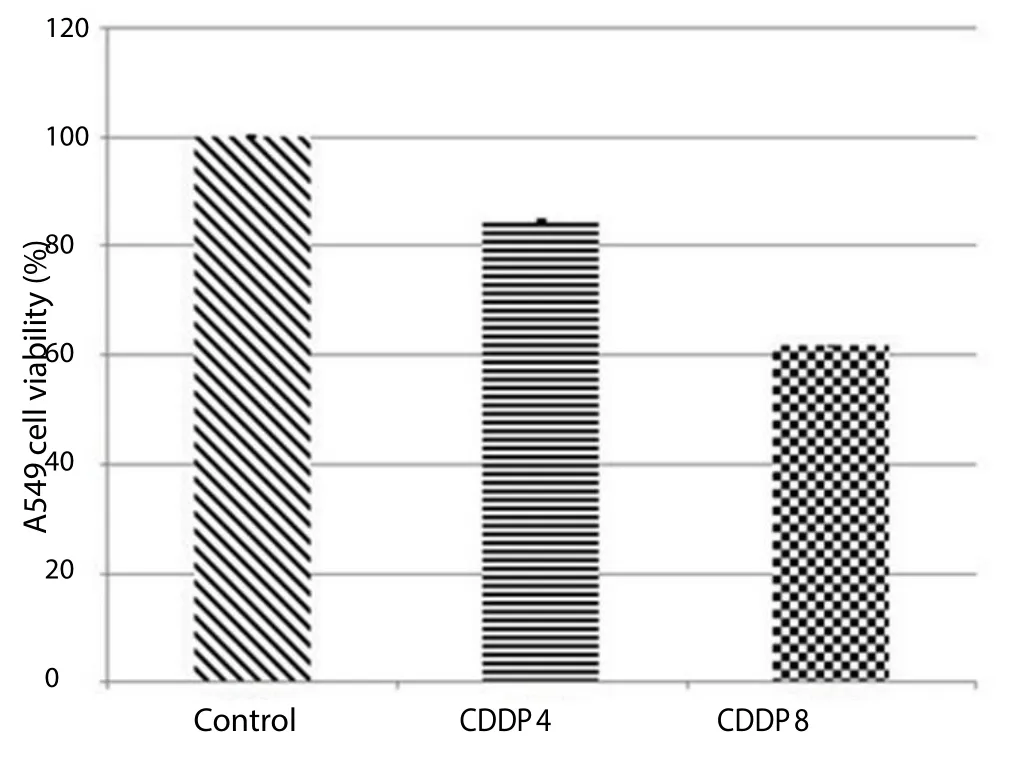
Figure 5. XTT cytotoxicity assay showing more cell death in cisplatin (8 μg/mL) than with cisplatin (4 μg/mL).
4. Discussion
Lung cancer was considered as a rare cancer in the 19th century.However in the 20th and 21st century, lung cancer became the 2nd common cancer especially in developing countries.
A dramatic change in lung cancer incidence has occurred due to several factors including smoking, pollution, radiation, ionization,and certain infections. Among these factors, tobacco smoke has been considered the main culprit of this deadly cancer. But despite the different strategies that take place to overcome this disease,lung cancer is still the most leading cause of cancer death. Multiple therapeutic modalities are now used to treat lung cancer like chemotherapy, radiotherapy, surgery, and recently genetic targeted therapy. Yet, chemotherapy, the gold standard modality for advanced stages, is associated with multiple side effects especially with platinum based chemotherapy. Combination chemotherapy regimen has been used to minimize the chemotherapy related side effects but the mortality is not decreased significantly.
In this study, we evaluated the response of human cells from nonsmall cell lung cancer (A549) against the pomegranate combined with a low dose of two chemotherapeutic drugs: cisplatin and taxotere. The lyophilized pomegranate has an antineoplastic effect represented by a decrease in viability of A549 cells in a dosedependent manner.
The preliminary phytochemical screening of pomegranate showed the presence of phenolic acids, flavonoids, terpenoids,quinones, alkaloids, tannines and resins. In addition, infrared analysis confirmed the presence of compounds having the -OH group which exerts the antioxidant effect. These results suggest that these compounds are responsible for the cytotoxic effect of the pomegranate on A549 cells.
In vivostudies have confirmed this antineoplastic effect of pomegranate, patients treated with cisplatin alone or in combination with other chemotherapeutic drugs, suffer from side effects that are not observed in the case of treatment with pomegranate. Hence,the idea to minimize the dose of chemotherapeutic drugs and compensates it by combination with the pomegranate, and then minimizing their side effects.
When combined pomegranate with a low dose of cisplatin or with low doses of cisplatin and taxotere, results showed a higher decrease in the viability of A549 cells compared to the groups treated with chemotherapy or pomegranate alone where pomegranate enhances the anticancer effect of a low dose of cisplatin[19].
It is difficult to cure lung cancer because of its ability to metastasize to distant vital organs. The ability of cancer cells to metastasize to vital organs is the most life-threatening stage of cancer, therefore inhibiting the migration abilities of cancer cells is critical for any chemotherapeutic drug. Thus, the pomegranate showed an inhibitory effect on the mobility of NSCLC A549 cells, after a 24-hour treatment and an improvement in the inhibitory effect of the chemotherapeutic combination of cisplatin and taxotere at low dose. Our results from the wound-healing assay showed an important decrease in the ability of NSCLC A549 cells to migrate after combining low dose of chemotherapeutic drugs with Lebanese pomegranate, suggesting a role in inhibiting metastasis.
In conclusion, this study has shown that the pomegranate has an inhibitory effect on the viability of A549 cells. In addition, the combination of pomegranate with a low dose of chemotherapeutic drugs exhibits a synergistic inhibitory effect against cell viability,and cell migration and then against the metastatic properties of NSCLC. Our results highlight a new therapeutic concept that offers combining pomegranate with low dose of chemotherapeutic drugs to treat lung adenocarcinoma.
Conflict of interest statement
The authors declare no conflict of statement.
Acknowledgments
The authors are thankful to Lebanese University for the financial support of this work (Project M. Nasser UL2017).
[1] Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Rick AR, et al.Cancer treatment and survivorship statistics, 2016.CA Cancer J Clin2016;66(4): 271-289.
[2] Gazdar AF. Should we continue to use the term non-small-cell lung cancer?J Eur Soc Med Oncol ESMO2010; 21(7): 225-229.
[3] Reck M, Rabe KF. Precision diagnosis and treatment for advanced non–small-cell lung cancer.N Engl J Med2017; 377(9): 849-861.
[4] Samanidis G, Paliouras D, Samanidou E, Tzimorota Z, Asteriou C, Xirou P, et al. Facial skin metastasis due to small cell lung cancer: a case report.J Med2009; 3: 32.
[5] Linnoila RI, Tsokos MG, Hitchcock CL, Cutler GB, Nieman L,Chrousos G, et al. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural,immunohistochemical, and flow cytometric study of 35 cases.Am J Surg Pathol1991; 15(6): 529-553.
[6] Muscat JE, Thompson S, Hoffmann D, Wynder EL, Stellman SD. Risk of squamous cell carcinoma and adenocarcinoma of the lung in relation to lifetime filter cigarette smoking.Cancer1997; 80(3): 382-388.
[7] Tsakonas G, Petris LD, Ekman S. Management of brain metastasized non-small cell lung cancer (NSCLC)-from local treatment to new systemic therapies.Cancer Treat Rev2017; 54: 122-131.
[8] Schiller JH, Gazdar AF, Sun S. Lung cancer in never smokers-a different disease.Nat Rev Cancer2007; 7(10): 778-790.
[9] Valley AW. New treatment options for managing chemotherapy-induced neutropenia.Am J Health-Syst Pharm2002; 59(15): 11-17.
[10] Rheingans JI. A systematic review of nonpharmacologic adjunctive therapies for symptom management in children with cancer.J Pediatr Oncol Nurs2007; 24(2): 81-94.
[11] Lampe JW. Health effects of vegetables and fruit: assessing mechanisms of action in human experimental studies.Am J Clin Nutr1999; 70(3):475-490.
[12] Cuvelier ME, Berset C, Brand-Williams W. Use of a free radical method to evaluate antioxidant activity.J Food Sci Technol1995; 28(1): 25-30.
[13] Farhan H, Rammal H, Hijazi A, Hamad H, Daher A, Reda M, et al.In vitroantioxidant activity of ethanolic and aqueous extracts from crudeMalva parvifloraL. grown in Lebanon.Asian J Pharm Clin Res2012;5(3): 234-238.
[14] Sayed-ahmad B, Hijazi A, Fayyad KH, Rammal H, Kobeissy A, Badran B, et al. Extraction, phytochemical screening, chemical quantification and identification of bioactive compounds from Lebanese Urtica dioica.Am J Pharm Tech Res2014; 4(2): 591-604.
[15] Kashyap MP, Kumar V, Al-Khedhairy AA, Musarrat J, Pant AB, Siddiqui MA. Protective potential of trans-resveratrol against 4-hydroxynonenal induced damage in PC12 cells.Toxicol In Vitro2010; 24(6): 1592-1598
[16] Castrillón JA, Palma A, Leal SM, Escobar P, Bahsas A, Gómez-Ayala S.Synthesis, structural elucidation andin vitro antiparasitic activity against Trypanosoma cruzi and Leishmania chagasi parasites of novel tetrahydro-1-benzazepine derivatives.Bioorg Med Chem2010; 18: 4721-4739.
[17] Lu QY, Jin YS, Pantuck A, Zhang JF, Heber D, Belldegrun A, et al. Green tea extract modulates actin remodeling via Rho activity in an in vitro multistep carcinogenic model.Clin Cancer Res2005; 11(4): 1675-1683.
[18] Sayed AB, Thierry TT, Saad Z, Hijazi A, Cerny M, Kanaan H, et al.Fennel oil and by-products seed characterization and their potential applications.Industrial Crops & Products2018; 111: 92-98.
[19] Hijazi A, Sabbah A, As-Sadi F, Zeiter S, Rammal H, Nasser M.Antioxidant, antiproliferative properties and chemical composition of the ethanolic extract from leaves and stems of Lebanese Anacyclus nigellifolius Boiss.JAMPS2017; 11(4): 1-9.
 Asian Pacific Journal of Tropical Biomedicine2018年1期
Asian Pacific Journal of Tropical Biomedicine2018年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Prevalence of coronavirus from diarrheic calves in the Republic of Korea
- Effective Aeromonas specific monoclonal antibody for immunodiagnosis
- Pharmacodynamic profiling of optimal sulbactam regimens against carbapenemresistant Acinetobacter baumannii for critically ill patients
- Correlation of phytochemical content with antioxidant potential of various sweet potato (Ipomoea batatas) in West Java, Indonesia
- Larvicidal activity of Xenorhabdus and Photorhabdus bacteria against Aedes aegypti and Aedes albopictus
- Proximate composition, nutritional values and phytochemical screening of Piper retrofractum vahl. fruits
