Larvicidal activity of Neem oil and three plant essential oils from Senegal against Chrysodeixis chalcites (Esper, 1789)
Saliou Ngom, Raimundo Cabrera Perez, Ma Anta Mbow, Rokhaya Fall, Saliou Niassy, Andreea Cosoveanu,Serigne Mbacké Diop, El Hadji Barka Ndiaye, Moussoukhoye Diop, Georges Lognay
1Institut Sénégalais de Recherches Agricoles, B.P. 3120 Dakar, Sénégal
2Unidad de Fitopatologia, Facultad de Biología, Universidad de la Laguna, 38206 San Cristobal de la Laguna, Tenerife, Islas Canarias, España
3Département de chimie, Faculté des Sciences et Techniques, Université Cheikh Anta Diop de Dakar, B.P. 5005 Fann, Sénégal
4Gembloux Agro-Bio Tech, Université de Liège, Chimie Analytique Department AgroBioTech, 2 Passage des Déportés-5030 Gembloux
5Development, University of Pretoria, Private Bag X20, Hatfield 0028, South Africa
1. Introduction
the main means for pest control. Pesticides are used uncontrollably by the producers who ignore their consequences[1], which created
In West Africa, horticulture is an important economic activity. It supplies major urban centres with fruits and vegetables and exports part of its production to Europe and Asia. However, the production is affected by high pest attacks. The use of chemical pesticides is serious health and ecological problems. Soil and groundwater are strongly contaminated by pesticide residues[2], therefore, it is urgent to find an alternative to limit the damage.
Biocides plants are important sources of natural substances that could be used as alternatives[3,4]. In some countries of Asian, Eastern Europe and South America, extracts from plants are approved and currently used as insecticides for crop protection[5]. On the other hand, in Senegal and in the West African region, plant resources with insecticidal effects are enormous[6,7], but they are limitedly used by the populations. Farmers are aware of their potential, but lack of scientific knowledge has established their phytosanitary properties.Neem oil (Azadirachta indica) currently remains the only product used as a biopesticide by most producers. Yet the Senegalese flora is remarkably rich with diverse species of plants containing substances with interesting biocidal properties such as essential oils. Due to their specific and complex mechanisms of action, they can be used on their own repetitively without the potential risk of causing any form of resistance in pests[8]. In addition, it has been shown that essential oils generally have broad spectrum effectiveness. However,they were extensively studied in view of potential use in agriculture.Their use remains empirical and is not based on scientific basis.
Optimizing techniques for using these plants requires proven scientific knowledge on their physicochemical properties and biological effectiveness. The study was carried out to determine the chemical composition of the essential oils of three aromatic plants [Callistemon viminalis(C. viminalis),Melaleuca leucadendron(M. leucadendron) andHyptis suavolens (H. suavolens)] and to compare their biological activity with that of neem oil against larvaeChrysodeixis chalcites(C. chalcites) (serious pest for several vegetable crops).
2. Materials and methods
2.1. Plant material
The aerial parts of the plants (C. viminalis,M. leucadendronandH.suaveolens) were harvested between October and December 2013 at the Botanical garden of the Faculty of Science and Technology of UCAD, at the Forestry Park of Hann and within the walls of the ISRA-LNERV institution. Representative copies were deposited in the Herbarium of the plant biology Department of FST/UCAD.In the Index Seminal (1985) of the botanical garden of the Faculty of Science and Technology, the plants are registered under the following numbers:C. viminalis329;M. leucadendron334 andH.suaveolens250.
The collected samples were dried at room temperature on the benches, out of direct sunlight for 6 d.
2.2. Breeding of insects
Mass animal husbandry was conducted in the laboratory in plastic jars deposited at room temperature and the tests were performed on pepper (Capsicum annuum) plants grown in pots in the laboratory.
2.3. Extraction and analysis of essential oils
Essential oils were obtained by steam distillation using a Clevenger-type mounting tool for 2 h, then dried with anhydrous sodium sulfate. The extraction of essential oils was carried out in the laboratory of natural products at UCAD. Neemland-Senegal (Thies)provided us with neem oil samples.
The characterization of essential oils was performed using chromatograph gas coupled with a flame ionization detector and by gas chromatograph coupled with mass spectrometry.
The gas coupled with a flame ionization detector was equipped with a capillary column of type 5% phenyl-dimethylpolysiloxane(30 m×0.25 mm ID) with film thickness of 0.25 μm. The carrier gas used is helium (He) at a flow rate of 1.5 mL/min. The oven temperature was from 40 (5 min hold) to 280 ℃ at 8 ℃/min with a final hold at 280 ℃ for 5 min. The injector was in splitless mode at 290 ℃. The detector was set at 290 ℃ (air and hydrogen with respective flow rates of 350 mL/min and 35 mL/min); make up gas(N2) at 30 mL/min.
The gas chromatograph coupled with mass spectrometry was equipped with a capillary column and was used under conditions identical to those of gas coupled with a flame ionization detector.The CPG was coupled to a mass spectrometer (FINNIGAN TRACE MS). Fragmentation was done by electron impact (70 eV) and the mass range was comprised between 35 and 300 amu.
The identification of the compounds was made using the spectral library (Wiley 275L) connected to the gas chromatograph coupled with mass spectrometry and by calculating the retention indices, they were compared to those in the literature[9,10].
2.4. Insect and bioassays
Bioassays were performed in the laboratory of plant pathology at the University of Luguna in Tenerife in the Canary Islands.Toxic, anti-repulsive and anti-nutritional effects of the extracts of plants were tested onC. chalcites(Lepidoptera, Noctuidae) larvae according to different applications: contact, inhalation and treatment on the entire plant and leaves cut into disks (disk-Leaf Bioassay) in a controlled environment. Only adult larvaes of the same age group were used for the tests after being starved for one hour. For this purpose, mass animal husbandry was conducted in the laboratory.The tests were performed on pepper plants grown in pots in the laboratory. Concentrations at 0.4 μg/mL, 2 μg/mL and 4 μg/mL of essential oils dissolved in a solution of ethanol were tested in comparison with those of neem oil and the control sample.
To perform the contact efficacy tests, four larvae were placed in a Petri dish containing the food treated with various concentrations with a total of 5 repetitions (5 petri dishes/concentration). The number of dead larvae was counted during the time of exposure.For the efficacy testing by inhalation, the larvae were placed in 250 mL glass jars with food and an open capsule containing cotton where essential oils were deposited with different doses (0.4 μg/L, 2 μg/L and 4 μg/L air). The number of dead larvae was counted during and after treatment.
For the repulsive and antinutritional tests, two experiments were conducted. For the first one, the plant leaves were cut into disc with a diameter of 1 cm. Four leaf discs were set equidistant on a plastic substrate in a petri dish. Then 2 μL of each of the prepared solutions was uniformly spread on the discs with choice (two discs treated with the extracts while the other two of the same box have only received the same volume of ethanol as control sample) and without selection (four discs in the same box treated with extracts, and as control sample four discs in the same box treated with ethanol).After the treatments, four larvae were locked in each device with five repetitions per device. For the second experiment, the leaves of a whole plant were marked and treated with the same concentrations.After 24 h of exposure the discs and the leaves of the plants were scanned and the area consumed by the larvae was calculated using the software (image J).
2.5. Statistical analysis
For both tests, the mortality rate was calculated according to Abbott(1):
Mc=(Mo-Mt)/(100-Mt)×100 (Mo=mortality in the treated groups,Mt=mortality in the control group and Mc=calculated mortality).
Data was analyzed using SPSS IBM statistical software 21. The Newman-Keuls test was used to analyze non-parametric data. The difference is considered statistically significant when P<0.05.
3. Results
3.1. Chemical characteristics of essential oils of the plants used
Table 1 represents the main constituents of the essential oils of the three analyzed plants. Only compounds whose concentrations were above 1.86% (minor one) were registered.
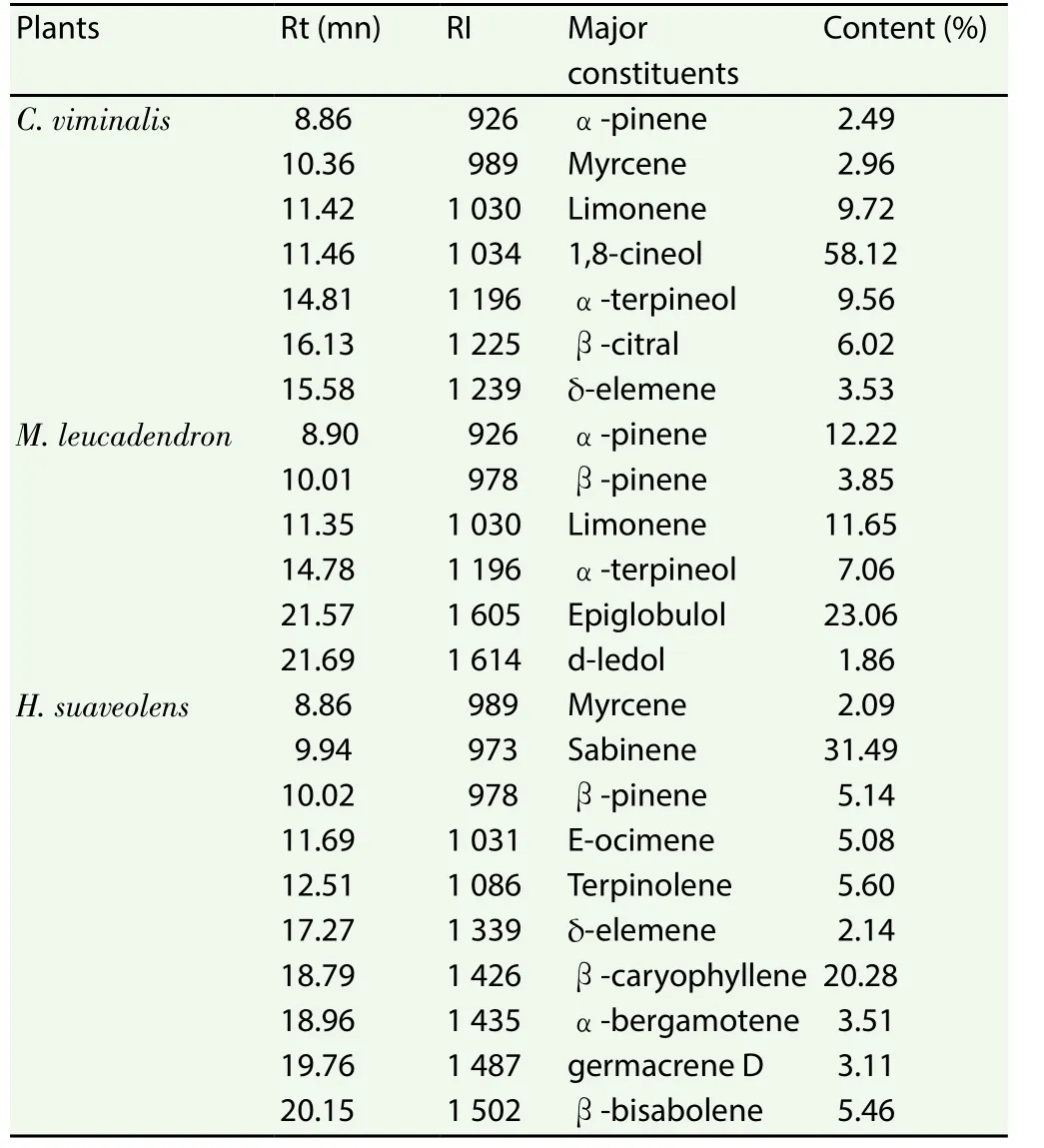
Table 1 Main constituents in essential oils of plants.
ForC. viminalis, 34 compounds representing 99.07% of the essential oil were identified. The main compounds were 1.8 cineol(58.12%), limonene (9.72%), α-terpineol (9.56%) and β-citral (β-geranial) (6.02%).
InM. leucadendron, 44 compounds representing nearly 99.22%of the essential oil were identified. The main compounds were 1,8-cineol (28.87%), epiglobulol (23.06%), α-pinene (12.22%),limonene (11.65%), α-terpineol (7.06%), β-pinene (3.85%) and d-ledol (1.86%).
For the essential oil ofH. suaveolens, 36 compounds representing 98.61% of the oil have been identified. It is essentially composed of: sabinene (31.49%), β-caryophyllene (20.28%), terpinolene,(5.60%), β-bisabolene (5.46%), β-pinene (5.14%), E-ocimene(5.08%), α bergamotène (3.51%) and germacrene d (3.11%).
3.2. Insecticidal properties of plant extracts
Table 2 represents the insecticidal effect of essential oils ofC.viminalis,M. leucadendron, andH. suaveolenson the larvae ofC.chalcites, compared to that ofAzadirachta indica,neem oil.
The results showed that all the essential oils studied were more toxic than the neem formulation used in this study. At a concentration of 4 μg/mL ethanol, only 50% larval mortality was recorded on the neem oil after 24 h whereas, all essential oils have resulted in at least a 90% mortality using the same concentration after 24 h. However,oil ofC. viminaliswas more effective by contrast with a mortality of 100% of the larvae at 4 μg/mL in an hour. The concentration of 2 μg/mL of this same oil has revealed a mortality of 90% after 2 h of application and 100% after 24 h. The essential oils ofM.leucadendronandH. suaveolenshave led to a mortality of 90% for larvae with the concentration of 4 μg/mL, in 2 h. However, essential oil ofM. leucadendronat 4 μg/mL, killed all larvae after 24 h and 10% at 2 μg/mL.

Table 2 Effects larvicides extracts of plants in function of time and dose per contact(%).
By inhalation, the highest toxicity level was observed with the essential oil ofM. leucadendron. After 24 h exposure, the essential oil ofM. leucadendronshowed a larval mortality rate of 40%, 60%and 80% for concentrations at 0.4, 2.0 and 4.0 μg/L air, respectively.ForH. suaveolens, 50% larval mortality rate was observed with the concentration of 2.0 and 4.0 μg/L air, and 10% larval mortality rate was observed with the concentration of 0.4 μg/L airafter 24 h exposure. On the other hand, forC. viminalisthe mortality rate was 10% at concentration of 2.0 and 4.0 μg/L air after 24 h of treatment.Table 3 and Table 4 showed that all plant extracts had deterrent and repulsive activity against the larvae ofC. viminalis. The percentage of consumption of treated leaf-disk with the extracts of the four plants (with choice) was lower than that observed for the control.Compared to the essential oils, neem oil (Azadirachta indica)revealed a significantly higher anti-feeding activity with a significant difference between the percentage of consumption of leaves treated with the neem oil and control (Mann-Whitney test): 4.4% for the treated leaves against 41.6% for the control on leaf-disk, with choice 3.8% against 54.3% on leaf-disk non-choice, and 1.8% against 19.9% for tests on whole plant.
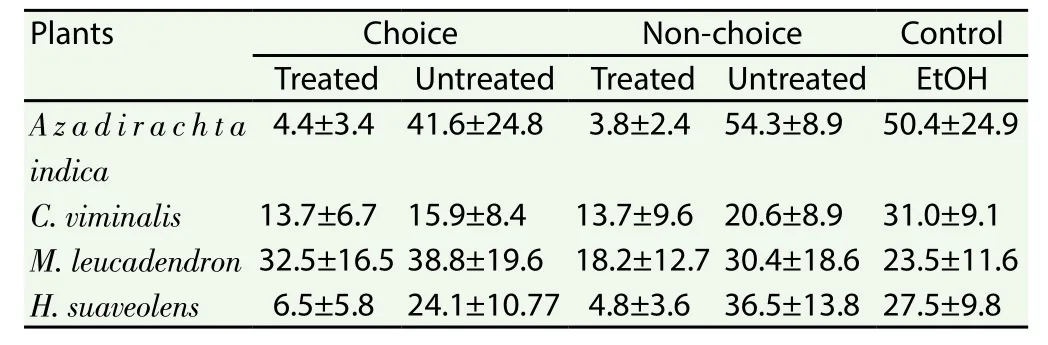
Table 3 Leaf surface consumed (%) on leaf disk after 24 h of application of extracts of plants.

Table 4 Leaf surface consumed on whole plant after 24 h of application of plant extracts (%).
4. Discussion
Several scientific studies have reported the chemical composition of the essential oils ofC. viminalisfrom different areas[11,12]. This chemical profile ofC. viminalisessential oil collected in Dakar is comparable to that described in Cameroon, Egypt and India[11-13].Main compounds are very similar except the Brazilian species. 1,8-cineol (58.12%-71.77%) is the predominant constituent of the oil.However, the Indian and Egyptian species showed higher rates of α-pinene (20.43%-24.20%) as compared to the species in Cameroon and Senegal with 0.38% and 2.49% respectively. We also detected Δ3-carene and menthyl acetate only in Camerounian and Indian species in considerable rates (8.6% and 5.3%, respectively). For the Brazilian species, the principal compounds of the essential oils in the leaves were eucalyptol (84.60%) and α-pinene (10.28%)[14].
For the essential oil ofM. leucadendron, the chemical composition of the species and the species studied by Faraget alin Cairo (Egypt)[15] showed slight differences with the absence of epigblobulol. The egyptian species contains more 1,8-cineol (64.3% against 28.87%)and α-terpineol (11.02% against 7.06%). Inversely, the oil essential collected in Senegal onM. leucadendronin Dakar showed higher rates of α-pinene, limonene and β-pinene (12.22%, 11.65% and 3.85%, as compared to the specie in Egypt with 4.24%, 6.70% and 1.67% respectively)[16]. 1,8-cineol (0.1%) and epigblobulol were found in traces in Pakistani variety ofM. leucadendron. According to Saimaet al[17], eugenol methyl ether (95.4%) was the major component in the essential oil ofM. leucadendronspecies grown in Pakistan.
ForH. suaveolens, results obtained on the chemical profile of the essential oil were compared with those reported in the literature. The biochemical profile obtained for the essential oil ofH. suaveolensis different from that found in Benin[18]. In addition to the main compounds identified, the latter contains other relevant compounds at higher rates such as 1.8-cineol (14.0%-24.6%), β-phéllandrène(10.2%) and fenchone (4.1%-8.1%). However, the extract ofH.suaveolensstudied in Burkina Faso by Djibo[19] presents a similar chemical profile to our study with dominant compounds sabinene(36%), β-caryophyllene (17%) and terpinolene (7.3%) in the Burkinabe species.
Insecticidal activity of the essential oil againstC. chalciteswere determined by the bioassays in the laboratory conditions and compared with neem oilAzadirachta indica. All the essential oils showed a larvicidal activity. However, only the essential oil ofC.chalcitesshowed a larvicidal activity at the lowest concentration(0.4 μg/mL). The high larvicidal activities ofC. chalcitesandM.leucadendroncould be explained by their important compounds such as the 1-8-cineole forC. chalcitesand the epiglobulol forM.leucadendron. Indeed, a strong biological activity of essential oils rich in compounds with alcohol function was reported by several authors[20,21]. 1-8-cineole has been reported to possess insecticidal activity against several insects includingTribolium castaneum,Callosobruchiis maculatus,Rhyzopertha dominica, andSitopliilus oryzaeL.[22,23]. In addition, these two plants also contain other compounds such as α- and β-pinene, limonene, α-terpineol at considerable amounts and their insecticidal properties have been demonstrated. The larvicidal activity of essential oils with similar compounds has been proved by several authors[24-26].
For the essential oils,H. suavolens seems to show a stronger antifeeding and repulsive activity with a consumption of 6.45% on treated leaves against 24.08% for the control leaf-disk with choice,4.80% against 36.47% on leaf-disk non-choice and 4.80% against 14.65% for tests on whole plant. Although the essential oils ofC.chalcitesandM. leucadendronwere considered repulsive according to Lachance[27], our results have revealed that this property is low.However, this low repulsive and anti-feeding activity observed with the essential oils could be explained by the concentration and the mode of treatment used for the tests as this did not allow a strong adherence of the product on the leaves.
To overcome this limitation on the mode of application we notify that in the established formulas of biopesticides, essential oils are diluted in the neem oil. In addition, the essential oils will induce a toxic effect associated with the activity anti-feeding repulsive effect of neem.
In Senegal and other countries of the West African sub-region,the use of insecticides against pests of cultures is not based on any scientific evidence. This study is therefore an important contribution to the knowledge of the chemical profile of the essential oils of the plants (C. viminalis,M. leucadendron, andH. suaveolens) as well as their insecticidal potential. The chemical characterization showed that the essential oils ofC. viminalisandM. leucadendronare rich in oxygenated compounds (monoterpene oxides, monoterpenic alcohols sesquiterpene).H. suaveolensis essentially composed of monoterpenes and hydrocarbon sesquiterpenes. By contrast, the essential oils of the three plants showed a remarkable biological activity on larvae ofChrysodeixis. Neem treatment showed a repulsive and anti-feeding activity. On the basis of these results, it suggests that the association of essential oils with the neem oil is efficient in the management of lepidopterans particularlyC. chalcites.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
Authors are grateful to the support from WAAPP/CERAAS/SENEGAL.
[1] Ngom S, Traore S, Thiam MB, Manga A. Contamination of agricultural products and groundwater by pesticides in the Niayes area in Senegal.Rev Sci Technol Synthèse2012; 25: 119-130.
[2] Ngom S, Manga A, Diop M, Thiam MB, Rousseau J, Cissé I, Traoré S.Study of the evolution of pesticide residues in horticultural products of high consumption in Senegal.Rev Ivoir Sci Technol2013; 21&22: 31-44.
[3] Abtew A, Subramanian S, Cheseto X, Kreiter S, Garzia GT, Martin T. Repellency of plant extracts against the legume flower thripsMegalurothrips sjostedti(Thysanoptera: Thripidae);Insects2015; 6(3):608-625.
[4] Sutthanont N, Choochote W, Tuetun B, Junkum A, Jitpakdi A, Chaithong U, et al. Chemical composition and larvicidal activity of edible plantderived essential oils against the pyrethroid-susceptible and resistant strains ofAedes aegypti(Diptera: Culicidae).J Vector Ecol2010; 35(1):106-115.
[5] Vera Sergeeva. Influence of plant extracts and essential oils in modern plant protection. The 29th International Horticultural Congress 17-22 August 2014, Brisbane, Australia Symposium: Plants, as factories of natural substances, edible and essential oils.
[6] Berhaut J.Illustrated flora of Senegal. Dicotyledones. Government of Senegal-Ministry of Rural Development and Hydraulics, Directorate of Water and Forests, Dakar; 1979, p. 634.
[7] Kerharo J, Adam JG.Medicinal and toxic plants. Senegalese traditional pharmacopoeia.Paris: Edition Vigot Brothers; 1974, p. 1011.
[8] Chiasson H., Beloin N. Essential oils, biopesticides new kind.Bull Entomol Soc Quebec 2007; 14(1): 3-6.
[9] Joulain D, König WA. The atlas of spectral data ofSesquiterpene hydrocarbons, EB-Verlag, Hamburg; 1998.
[10] Adams RP.Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy.USA: Allured Publishing Corporation, Carol Stream III; 2001, p. 455.
[11] Salem MZM, EL-Hefny M, Nasser RA, Hayssam MA, El-Shanhorey NA, Elansary HO. Medicinal and biological values ofCallistemon viminalisextracts: History, current situation and prospects.Asian Pac J Trop Med2016; 3: 229-237.
[12] Badawy MEI, Abdelgaleil SAM. Composition and antimicrobial activity of essential oils isolated from Egyptian plants against plant pathogenic bacteria and fungi.Ind Crop Prod2014; 52(1): 776-782.
[13] Gohar AA, Maatooq GT, Gadara SR, Walaa SA. The profile and antimicrobial activity of the essential oil fromCallistemon viminalis(Sol.Ex Gaertner) G.Don Ex Loudon leaves.J Biotechnol Pharm Res2014;5(1): 7-11.
[14] Sales TA, Cardoso MDG, Guimarães LGDL, Camargo KC, Rezende DADC, Brandão RM, et al. Essential oils from the leaves and flowers ofCallistemon viminalis: chemical characterization and evaluation of the insecticide and antifungal activities.Am J Plant Sci2017; 8: 2516-2529.
[15] Farag RS, Shalaby AS, El-Baroty GA, Ibrahim NA, Ali MA, Hassan EM. Chemical and biological evaluation of the essential oils of differentMelaleucaspecies.Phytother Res2004; 18: 30-35.
[16] Fall R, Ngom S, Samb A, Sembène MB. Fumicide insecticidal activity of the essential oils ofCallistemon viminalis,Melaleuca leucadendronandHyptis suavolenagainst Sitophilus spp., pest of corn.J Soc Ouest-Afr Chim2017; 43: 31-36.
[17] Saima S, Zahida P, Firdaus B, Sania. Chemical composition, antibacterial and antioxidant activities of essential oils from leaves of threeMelaleucaspecies of Pakistani flora.Arab J Chem2017; Doi: https://doi.org/10.1016/j.arabjc.2017.01.018.
[18] Noudogbessi JP, Agbangnan P, Yehouenou B, Adjalian E, Nonviho G,Osseni MA, et al. Physico-chemical properties ofHyptis suaveolensessential oil.Int J Med Arom Plant2013; 3(2): 191-199.
[19] Djibo AK. Analysis of the essential oils of some Burkina Faso plants belonging to the Lamiaceae family [Hyptis spicigeraLam.,Hyptis suaveolensPoit.,Ocimum americanumL.) and Poaceae (Cymbogon schoenanthusL Spreng,Cymbogon gigenteusChiov andCymbogon citratus(DC) Stapf). [PhD Thesis], University of Ouagadougou; 2000.
[20] Zoubiri S, Baaliouamer A, Seba N, Chamouni N. Chemical composition and larvicidal activity of AlgerianFoeniculum vulgareseed essential oil.Arab J Chem2014; 7: 480-485.
[21] Seo SM, Kim J, Lee SG, Shin CH, Shin SC, Park IK. Fumigant antitermitic activity of plant essential oils and components from Ajowan(Trachyspermum ammi), Allspice (Pimenta dioica), caraway (Carum carvi), dill (Anethum graveolens), Geranium (Pelargonium graveolens),and Litsea (Litsea cubeba) oils against Japanese termite (Reticulitermes speratusKolbe).J Agr Food Chem2009; 57(15): 6596-6602.
[22] Aggarwal KK, TripathiAK, Prajapati V, Kumar S. Toxicity of 1,8-cineole towards three species of stored product coleopterans.Int J Trop Insect Sci2001; 21(2): 155-160.
[23] Hind Suhail A. Fumigant toxicity ofCallistemon viminalisessential leaves oils against vinegar fly,Drosophila melanogaster.Asian J Biol Life Sci2016; 5(2): 196-200.
[24] Samira G, Habib A. Chemical composition and insecticidal effects of the essential oil of cardamom,Elettaria cardamomumonthe tomato leaf miner, Tuta absoluta.Toxin Rev2017; 36(1): 12-17.
[25] Lucia A, Audino GA, Seccacini E, Licastro S, Zerba E, Masuh H.Larvicidal effect of Eucalyptus grandisessential oil and turpentine and their major components onAedes aegyptilarvae.J Am Mosq Contr Assoc2007; 3: 299-303.
[26] Amer A, Mehlhorn H. Larvicidal effects of various essential oils againstAedes,Anopheles, andCulexlarvae (Diptera: Culicidae).Parasitol Res2006; 99: 466-472.
[27] Lachance S, Grange G. Repellent effectiveness of seven plant essential oils, sunflower oil and natural insecticides against horn flies on pastured dairy cows and heifers.Med Vet Entomol2014; 28(2): 193–200.
 Asian Pacific Journal of Tropical Biomedicine2018年1期
Asian Pacific Journal of Tropical Biomedicine2018年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Prevalence of coronavirus from diarrheic calves in the Republic of Korea
- Effective Aeromonas specific monoclonal antibody for immunodiagnosis
- Pharmacodynamic profiling of optimal sulbactam regimens against carbapenemresistant Acinetobacter baumannii for critically ill patients
- Efficiency of combining pomegranate juice with low-doses of cisplatin and taxotere on A549 human lung adenocarcinoma cells
- Correlation of phytochemical content with antioxidant potential of various sweet potato (Ipomoea batatas) in West Java, Indonesia
- Larvicidal activity of Xenorhabdus and Photorhabdus bacteria against Aedes aegypti and Aedes albopictus
