Protective effect of ashwagandha (Withania somnifera) against neurotoxicity induced by aluminum chloride in rats
Mohamed E. Elhadidy, Hussein G. Sawie, Nagwa A. Meguid, Yasser A. Khadrawy✉
1Department of Research on Children with Special Needs, Medical Research Division, National Research Centre, Giza, Egypt
2Department of Medical Physiology, Medical Research Division, National Research Centre, Giza, Egypt
1. Introduction
Aluminum (Al) is widely distributed in the environment representing the third prevalent element in the earth’s crust[1].The massive daily life use of Al permits easy exposure to human beings from compounds used in utensils, food additives, water purifiers and pharmaceuticals[2]. In addition, the contamination of the environment with compounds containing aluminum such as particulate matter resulting from cement factories expose the population residing near them to occupational airborne Al particulates[3,4]. Moreover, exploitation of bauxite mines and acid rain can cause the flux of large amounts of Al salts from insoluble minerals, increasing the risk of human contact with Al[5]. Therefore,further research on the mechanism of Al-induced neurotoxicity is necessary to avoid the hazardous effects of Al.
Al is categorized as a neurotoxin and has hazardous effects on the development of the brain, prenatally or postnatally, in humans and experimental animals[6,7]. It has been reported that the excessive Al intake for long time causes neuroinflammation and deficits in cognitive functions. Neuroinflammation alters dendritic spines density, which, in turn, influences cognitive function[8].
Severalin vitroandin vivostudies indicated that Al induces oxidative stress in the brain[9,10] and the generation of reactive oxygen species (ROS) that exhaust the antioxidant mechanisms and induce lipid peroxidation, DNA damage, protein modification and other effects[11]. Gonzalez-Munozet al. reported that aluminum trichloride (AlCl3) causes a significant increase in malondialdehyde(MDA) and a significant decrease in glutathione (GSH) content[12].Other factors associated with Al neurotoxicity involve an increase in nitric oxide, ROS-induced damage and alterations in neurotransmission[13,14].
Aluminum can cross the blood brain barrier and accumulate in brain tissues[15,16] with the highest concentrations in the hippocampus[17].A relationship between learning and memory deficits and Al exposure has been demonstrated by Exley[18]. It has been observed that AlCl3enhances pro-inflammatory cytokines production (TNF-α,IL-6) and nitric oxide in rat brain, probably by both astrocytes and microglia[19] and stimulates iNOS expression by immune-competent and phagocytes in the brain[20].
Although the causes of Alzheimer’s disease are not well understood,many studies propose Al as one of the potential contributing causes[21,22]. This was supported by the reported functional deficits in spatial memory discrimination after chronic exposure of rats to Al in dietary supplements corresponding to the range of Al in urban American diets[23]. Moreover, experimental and human studies showed that Al caused an impairment in long-term memory[24] and hippocampal long-term potentiation[25].
Ashwagandha [Withania somnifera(W. somnifera)] has many therapeutic uses in the ayurvedic system of traditional medicine.Many toxicological studies demonstrated that ashwagandha is a safe and edible herb[26]. Several pharmacological effects of ashwagandha have been reported including antioxidant, antibacterial, adaptogen,liver tonic, aphrodisiac and anti-inflammatory effects[27]. In addition,the well-described pharmacological effects of ashwagandha include restoration of physiological and metabolic parameters, improvement of cognitive function in geriatric states, anti-arthritic, anti-aging, and recovery from neurodegenerative diseases[28]. Active constituents ofW. somniferainclude sitoindosides Ⅶ-Ⅹ and withaferin A which have antioxidative activities as evident from the activation of endogenous superoxide dismutase and catalase, the increase in vitamin C, and decrease in lipid peroxidation[29].
Ashwagandha roots were used as tonic for the nervous system and in preventive health and were described as Medhya Rasayana (i.e.beneficial to the brain) in ayurveda literature[30]. The root extract ofW. somniferaenhances cognitive function and augments the capacity of mental retention associated with diabetes, and the memory loss induced by amyloid beta (Aβ) and scopolamine[31,32]. Recently,it has been reported that supplementation of withanolide enriched extract ofW. somniferaroot prevented the depletion of brain reduced glutathione and free radical scavenging enzymes and memory impairment induced by hypoxia by modulating brain corticosterone level through the nitric oxide-cyclooxygenase-prostaglandin pathways[33].
It has been proposed that ashwagandha may improve cholinergic activity and this may underly its therapeutic potency against amnesia and neuroplasticity disorders constantly affected by age and stress[34]. The root extract ofW. somniferais also known to enhance the hippocampal cholinergic activity[31].
Therefore the current study aimed to evaluate the neuroprotective efficacy of alcoholic ashwagandha extract against the neurotoxic effects of Al. This was carried out by studying the action of alcoholic ashwagandha extracts against the changes in levels of reduced glutathione, nitric oxide, lipid peroxidation and tumor necrosis factor-α induced by AlCl3in the cortex, hippocampus and striatum.In addition, the effect of alcoholic ashwagandha extract in stead extracts on the changes in acetylcholinesterase and Na+, K+, ATPase in the selected brain regions of Al-intoxicated rats was assessed.
2. Material and methods
2.1. Experimental animals
Twenty two male Wistar rats, weighing 230-250 g, were used in the present study. They were supplied by the Animal House of the National Research Centre, Cairo. The animals were maintained under temperature- and light-controlled conditions (normal 12-h light/dark cycle) and provided with standard laboratory rodent chow and water provided ad libitum. All procedures were approved by the Ethics Committee of the National Research Centre which follows the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (publication No. 85-23, revised in 1985).
2.2. Chemicals
AlCl3was obtained from Merck. Ashwagadha root powder was purchased from Imtinan Company, Egypt.
2.3. Preparation of alcoholic extract of ashwgandha
For preparation of ethanol extract 100 g of root powder was dissolved in 1 000 mL of ethanol and kept at 27 ℃ for 24 h under continuous stirring and filtered using Whatmann No. 1 filter paper.The filtrate was then allowed to evaporate to get concentrated filtrate which was again reconstituted in small volumes of distilled water to obtain the used dose of ashwagandha (200 mg/kg).
2.4. Experimental design
The rats were divided randomly at the beginning of the experiment into three groups. The first group (n=7) is the control that received daily oral saline solution for 30 days. The second group (n=7) is the AlCl3-intoxicated group that was treated daily with AlCl3(100 mg/kg orally) for 30 days. The third group (n=6) represents the AlCl3intoxicated rats protected with ashwagandha extract. This group was treated daily with ashwagandha extract (200 mg/kg, orally) and AlCl3(100 mg/kg, orally) for 30 days. The time interval between ashwagandha and AlCl3administration was 60 min. On the 31th day the rats were sacrificed and the brain of each rat was dissected into cortex, hippocampus and striatum. Each brain region was weighed and kept frozen at -20 ℃ till carrying out the following analyses.
2.5. Neurochemical measurements
2.5.1. Lipid peroxidation
The measurement of lipid peroxidation depends on the estimation of MDA in the cerebral tissues[35]. Malondialdehyde was estimated by determination of thiobarbituric acid reactive species. The principal of this method depends on the reaction between one molecule of MDA and two molecules of thiobarbituric acid in an acidic pH and a temperature of 95 ℃ for 20 min to form thiobarbituric acid reactive substances. The absorbance of the pink colored product was measured at a wavelength of 532 nm in a UV-visible spectrophotometer (model UV-2401 PC, Shimadzu, Japan).
2.5.2. Nitric oxide
The spectrophotometric method described by Montgomery and Dymock was used to measure nitric oxide (NO) in the different brain areas[36]. Endogenous nitrite concentration was used as an indicator for nitric oxide levels. Griess reagent converted nitrite into an azo compound with a deep purple color whose absorbance was measured at 540 nm.
2.5.3. Reduced glutathione
Reduced GSH level was determined in the selected brain areas according to the method of Beutleret al[37]. The reduction of‘Ellman’s reagent’ [5, 5’ dithiobis-(2-nitrobenzoic acid) or 5,5’-dithiobis-(2-nitrobenzoic acid)] with GSH produced a yellow compound whose absorbance was read spectrophotometrically at 412 nm. The obtained chromogen was directly proportional to GSH levels.
2.5.4. Acetylcholinesterase activity
Acetylcholinesterase (AchE) activity was determined by the modified method of Ellmanet al[38]. The hydrolysis of acetylthiocholine iodide by acetycholinesterase yields thiocholine,which reacted with 5,5’-dithiobis-(2-nitrobenzoic acid), reducing it to thionitrobenzoic acid, whose yellow color was read at 412 nm.
2.5.5. Na+/ K+-ATPase activity
The spectrophotometric method of Tsakiriset al. was used to measure Na+/ K+-ATPase activity[39]. The difference between total activity of ATPase (Na+/ K+-ATPase and Mg-ATPase activity) and Mg-ATPase activity gives the activity of Na+/ K+-ATPase.
2.5.6. Tumor necrosis factor-alpha (TNF-α)
TNF-α level was estimated in the selected brain areas using rat TNF-α ELISA Kit supplied by Assaypro LLC (3400 Harry S Truman Blvd, St. Charles, MO 63301-4046, USA). The concentration of TNF-α was expressed in pg/g brain tissue.
2.6. Statistical analysis
The present data were represented as mean±SEM. One-way analysis of variance was used to test the statistical significance between the different groups by Statistical Package for Social Sciences program. Duncan was applied aspost hoctest to compare the significance between groups. Statistical significance was set atP-value <0.05.
3. Results
The present findings revealed that the daily treatment with AlCl3resulted in a significant increase in the cortical and hippocampal AchE activity by 137.974% and 167.360%, respectively, above the control values. However, striatal AchE activity showed nonsignificant changes. The parallel treatment of rats with ashwagandha and AlCl3prevented the increase in AchE activity induced by AlCl3.Similarly, the activity of Na+, K+, ATPase increased significantly in the cortex, hippocampus and striatum of AlCl3-intoxicated animals recording 23.456%, 27.755% and 22.459%, respectively, above the control value. In animals treated with ashwagandha and AlCl3, nonsignificant changes were recorded in Na+, K+, ATPase activity in the three studied brain regions (Table 1).
Table 2 showed a significant increase in the level of cortical and hippocampal TNF-α in AlCl3-treated rats recording 17.36%and 48.95% more than the control values. The pretreatment with ashwagandha extract prevented the increased TNF-α induced by AlCl3.
In the present study one-way analysis of variance revealed a significantly increased value of MDA in the cortex (45.72%),hippocampus (384.44%) and striatum (461.56%) of AlCl3-intoxicated rats. AlCl3treatment also elevated significantly NO level in the cortex (165.22 %,), hippocampus (67.86%) and striatum(46.58%). In addition, GSH level declined significantly in the hippocampus (-27.60%) and striatum (-13.79%) and was elevated significantly in the cortex (12.48%) of rats treated with AlCl3when compared to control values (Table 3).
The pretreatment of rats with ashwagandha extract restored thesignificant increase in MDA and NO level caused by AlCl3in the three studied brain regions to non significant changes as matched with the control value. Furthermore, ashwagandha extract restored the changes in GSH level induced by AlCl3to non significant changes (Table 3).
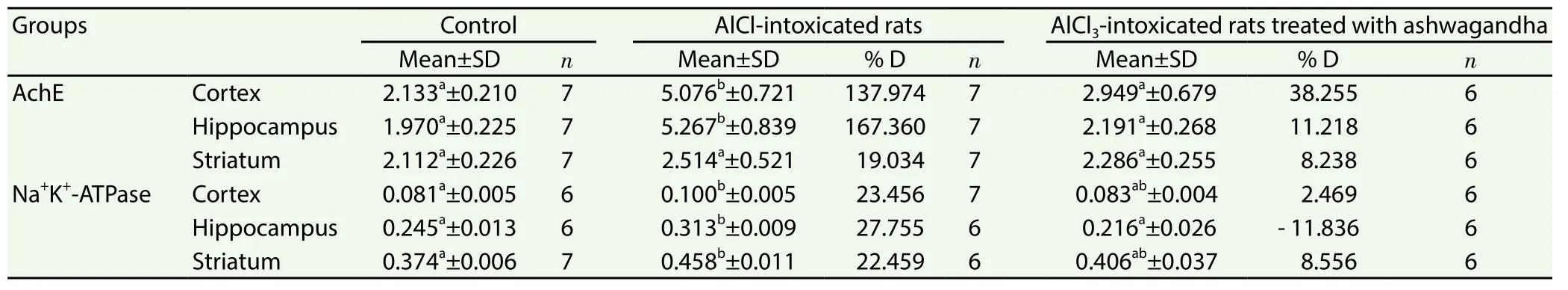
Table 1 Effect of ashwagandha extract on the activities of AchE (μmol SH/g/min) and Na+K+-ATPase (μmol Pi/min/g) in cortex, hippocampus and striatum of AlCl3 intoxicated rats.
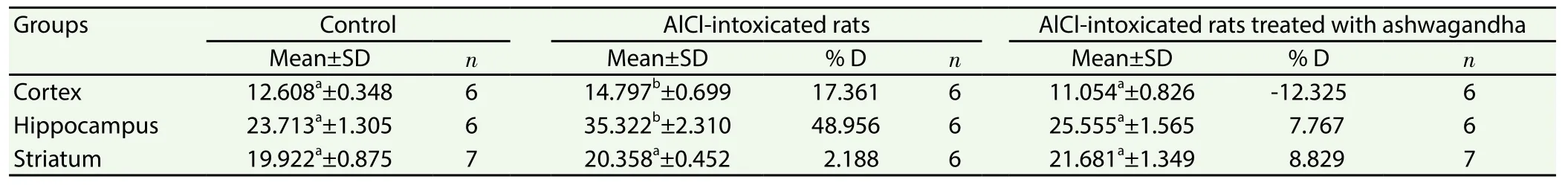
Table 2 Effect of ashwagandha extract on the level of TNF-α (μg/g) in cortex, hippocampus and striatum of AlCl3 intoxicated rats.
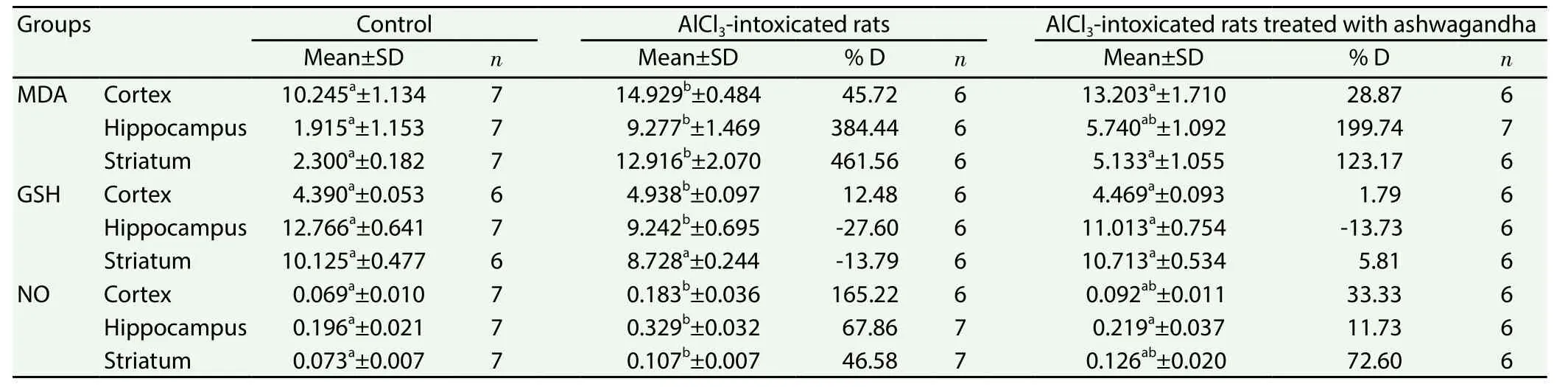
Table 3 Effect of ashwagandha extract on MDA (nmol/g), GSH (mmol/g) and NO (μmol/g) in cortex, hippocampus and striatum of AlCl3 intoxicated rats.
4. Discussion
Aluminum chloride has been established as a neurotoxic agent.It accumulates gradually in the cortex and limbic structures of susceptible patients’ brains initiating progressive hippocampal lesions that consist of dysfunctional microtubules, and reduced pyramidal cells with damaged neurites and synaptic loss. These lesions develop over time to clinically overt dementia[40].
The present data revealed that Al induced oxidative stress in the cortex, hippocampus and striatum. This was evident from the significant increase in lipid peroxidation, which represents the main marker of oxidative stress, and nitric oxide levels in the studied brain regions. In addition, a significant decrease in GSH was recorded in the hippocampus and striatum. Our results agree with the fi ndings of Shateiet al[41] who reported that Al decreased total antioxidant capacity and increased the levels of lipid peroxidation and nitric oxide in the cortex and hippocampus.
Aluminum is a metal characterized by relatively low redox activity;however, it can produce oxidative stress by several mechanisms. It can bind to brain phospholipids which carry negative charges and contain polyunsaturated fatty acids that are readily attacked by free radicals such as O2·-, H2O2, OH·, and OH-[42]. Aluminum can also stimulate lipid peroxidation in the iron-initiated Fenton reaction yielding ROS and. Superoxide (O2·-) forms withan Al-O2·-complex, which enhances the oxidative capacity of O2·-[18].This could explain the elevation in lipid peroxidation in the cortex,hippocampus and striatum induced by Al in the present study.
The present significant increase in NO in the cortex, hippocampus and striatum could be due to the enhancement of nitric oxide synthase induced by Al[43,44]. Nitric oxide can react with ROS such as superoxide forming peroxynitrite which is one of the potent damaging molecules for the nervous system[45]. This may represent one of the mechanisms that mediate Al-induced neurotoxicity.Exaggerating this condition is the present reduction in GSH level in the hippocampus and striatum of Al-intoxicated rats. GSH is a nonenzymatic antioxidant that has a potential role in the brain tissue against free radicals production and neurodegeneration[46]. The depletion of GSH represents an early hallmark in the progressive cell death that results from apoptotic stimuli in several cell types[47,48].The depletion in GSH after Al may be due to the reduced synthesis resulting from the decrease in the activity of glutathione synthetase enzyme which needs ATP as a co-substrate[49]. Therefore, the present decrease in GSH level in the hippocampus and striatum may be due to its exhaustion to get rid of the evolved ROS and failure to replenish its content after Al exposure.
One of the contributing factors in Al-induced neurotoxicity is the high sensitivity of the brain tissue to oxidative stress. This is due to the high content of unsaturated fatty acid in the brain, the high metabolic rate and the relatively low brain antioxidant capacity[50,51].The present findings revealed a significant increase in TNF-α level in the cortex and hippocampus of AlCl3-intoxicated rats. Passoset al.reported that inflammation might contribute to the neurodegenerative effects of AlCl3, through the increased expression of cytokines such as TNF-α in activated microglia around amyloid plaque[52]. This confirms the presence of a direct link between neurotoxicity induced by Aβ and cytokines[53]. Aluminum is a well documented neurotoxin that induces neuroinflammation by several mechanisms. Aluminum triggers oxidative stress, deposition of Aβ, and plaque formation in transgenic mice brain with increased expression of amyloid beta precursor protein. Both Aβ and aluminum act to augment ROS production that may lead to genotoxicity and DNA damage[9].
The observed oxidative stress and neuroinflammation induced by Al in the present study were accompanied by an increase in AchE activity in the cortex, hippocampus and striatum.
Kaizeret al. also observed that Al induced an increase in AchE and lipid peroxidation in several mice brain regions[14]. Aluminum has been reported to cause changes in cholinergic function in the CNS[54]. It has been suggested that aluminum could act as a cholinotoxin by interacting with the cholinergic system[55]. It is hypothesized that the decrease in the integrity of cholinergic neurotransmission is predominantly related to the decline in memory and cognitive functions during aging[56]. Interference in cholinergic signaling has been found to modulate the expression of activityregulated cytoskeletal-associated protein[57], regulated by a neuronal immediate early gene critical for synaptic plasticity[34]. In addition,the exposure to Al even at low doses can result in a dramatic accumulation of Al in the brain leading to a severe impairment in learning and memory abilities, and long-term potentiation in the hippocampus[25].
Therefore, the present increase in AchE in the studied brain regions could result in a decrease in cholinergic activity. This mechanism may underlie the reported deficits in memory and cognition induced by aluminum.
The increase in Na+, K+, ATPase activity in the cortex, hippocampus and striatum of Al-intoxicated rats may be an indicator of the increased brain excitability. The increase in enzyme activity could be a compensatory mechanism to restore the ionic gradient of the cell membrane.
Na+, K+-ATPase is a neuronal membrane-bound enzyme which is crucial for the regulation of membrane potential, cell volume and transmembrane fluxes ofand excitatory neurotransmitters. Na+and K+gradients should be maintained between the intracellular and extracellular compartments for basic cellular homeostasis and other functions especially in specialized cells[58]. The increase in the brain excitability could arise from the stimulant effect of Al on glutamate neurotransmitter which is the main excitatory neurotransmitter in the brain[59]. Therefore, the present increase in Na+, K+, ATPase may be attributed to the state of excitation in an attempt to maintain the ionic gradient across neuronal membranes.
Interestingly, TNF-α, which increases after aluminum exposure,is a key cytokine that triggers glutamate release from microglia, by up-regulating glutaminase and gap junction hemichannels[60,61].The interaction between TNF-α and glutamate is known as immunoexcitotoxicity based on adverse interactions between TNF-α as an inflammatory cytokine and glutamate as an excitotoxin[62].Supporting this explanation is the present observed oxidative stress and the increase in NO which may be occurred following increased glutamate release, increased calcium influx and excitotoxicity[63].It is clear from the present study the AlCl3induced a state of neurotoxicity mediated by oxidative stress, and neuroinflammation in the cortex, hippocampus and striatum of rats. This was associated with reduced cholinergic activity mediated by an enhancement of AchE activity. This effect was more prominent in the hippocampus which is a part of the limbic system needed for formation of various types of memory and learning in mice and other mammalians.In human, any damage to the hippocampus leads to disorders in learning capability, memory, remembering places and other undesired mental effects[64].
Pro-inflammatory cytokines have been reported to impair long-term potentiation, and inhibit neurotrophins which are critical for neuronal survival and function, synaptic plasticity and memory[65]. Moreover,altered density of hippocampal dendritic spines was evident after the exaggeration of the pro-inflammatory cytokine response[66].Alterations in dendritic spines control synaptic plasticity, which, in turn affect learning and memory[67]. Therefore, the present effects induced by AlCl3support the hypothesis that Al may be a suspected factor in the etiology of Alzheimer disease.
The present results showed that the daily pretreatment of ashwagandha extract prevented the oxidative stress resulting from AlCl3in the cortex, hippocampus and striatum. This was indicated from the ability of ashwagandha root extract to prevent the elevation in lipid peroxidation and NO induced by AlCl3and to restore the significant reduction in GSH to control like value.
It has been reported thatW. somniferacontains a high content of phenolic compounds with potent antioxidant activity[68]. Moreover,its extract elevates antioxidant capacity and could be used as a protective agent against cellular damage induced by free radicals[69].The antioxidant effects of ashwagandha root extract could also be due to the high concentrations of withanolides, flavonoids and other components which are potent antioxidants[70]. It has been shown thatW. somniferaroot can increase the synthesis of glutathione in the hippocampus exposed to hypobaric hypoxia[33]. Therefore, the ashwagandha extract could have a potential antioxidant effect in Alintoxicated rats. This effect could be explained by the abundance of phenolic compounds in its structure on one hand and the increased GSH synthesis on the other hand. Moreover, it has been reported that GSH forms S-nitrosoglutathione with NO thus serving as an endogenous reservoir for NO[71]. Thus, the increased GSH synthesis induced by ashwagandha extract can modulate the elevated level of NO induced by Al. This in turn could prevent the toxic effects of NO and prevent the formation of peroxynitrite.
Recently, Shahet al. postulated that the neuroprotective effect of ashwagandha extract may be caused by the reduction of oxidative stress and glutamate insults[29]. In the present study, ashwagandha extract restored the elevated level of TNF-α induced by AlCl3to nonsignificant changes as compared to control value. This in turn will reduce glutamate release and thus the immunoexcitotoxicity induced by AlCl3. Thus ashwagandha-reduced glutamate release could be explained by the decrease in the level of TNF-α and its effect on glutamate release. As a result of this effect, the cascade of events induced by the increased glutamate release including the increased influx of calcium ions and increased free radical and NO production[63] will be prevented. This could explain the reduced level of NO and MDA induced by ashwagandha extract. Moreover,the reduced glutamate release will minimize the membrane depolarization and excitotoxicity and this could explain the present recovery of Na+, K+, ATPase activity induced by ashwagandha extract. Supporting this explanation is the study of Katariaet al[72]who found that low doses of withanone and ashwaganda extract protect against glutamate-induced excitotoxicity.
Several studies reported that ashwagandha increases cholinergic activity. Gautamet al. provided several biochemical and molecular evidence that ashwagandha leaf extract possesses cholinergic properties in brain disorders associated with cholinergic dysfunction[32]. The authors found that the root extract ofW.somnifera(ashwagandha) could augment cholinergic activity in the hippocampus[32]. This augmentation may underlie the protective effects of ashwagandha extract against scopolamine-induced loss of memory function[73]. The root extract of the ashwagandha and withanolide A, one of its active components, have been shown to improve the cognitive deficits and spatial memory impairements in temporal lobe epilepsy and experimental models of stroke[74,75].AchE is the central metabolizing enzyme of acetylcholine. Therefore,the present potential effect of ashwagandha extract against AlCl3-induced increase in AchE activity could play a potential role in restoring the cholinergic activity to its control values. Accordingly,ashwagandha extract could alleviate the reported cholinotoxicity of AlCl3[55].
In the light of the present data, it could be concluded that the alcoholic extract of aswagandha has a protective effect against aluminum neurotoxicity. This effect was mediated by preventing the oxidative stress, neuroinflammation, and excitotoxicity induced by aluminum. In the light of the obtained results, ashwagandha extract could be offered as an adjuvant therapy against Alzheimer’s disease.
Conflict of interest statement
The authors declare that they have no conflict of interest.
[1] Kumar K, Gill KD. Aluminum neurotoxicity: neurobehavioral and oxidative stress.Arch Toxicol2009; 83: 965- 978.
[2] Bondy SC. Prolonged exposure to low levels of aluminum leads to changes associated with brain aging and neurodegeneration.Toxicology2014; 315: 1- 7.
[3] Proudfoot AT. Aluminum and zinc phosphide poisoning.Clinical Toxicol2009; 47: 89-100.
[4] Boullemant A. PM 2.5 emissions from aluminum smelters: coefficients and environmental impact.J Air Waste Manag Assoc2011; 61: 311- 318.
[5] Borgmann U, Couillard Y, Grapentine LC. Relative contribution of food and water to 27 metals and metalloids accumulated by caged Hyalella azteca in two rivers affected by metal mining.Environ Pollut2007; 145:753-765.
[6] Dórea JG. Exposure to mercury and aluminum in early life:developmental vulnerability as a modifying factor in neurologic and immunologic effects.Int J Environ Res Public Health2015;12(2): 1295-1313.
[7] Wang M, Chen JT, Ruan DY, Xu YZ. The influence of developmental period of aluminum exposure on synaptic plasticity in the adult rat dentate gyrusin vivo.Neuroscience2002; 113: 411-419.
[8] Zheng C, Xu Y, Haiyang Z, Haoran W, Wanyue H, Feibo X, et al.Aluminum chloride induces neuroinflammation, loss of neuronal dendritic spine and cognition impairment in developing rat.Chemosphere2016; 151: 289-295.
[9] Said MM, Rabo MM. Neuroprotective effects of eugenol against aluminiuminduced toxicity in the rat brain.Arh Hig Rada Toksikol2017;68(1): 27-37.
[10] Yuan CY, Lee YJ, Hsu GS. Aluminum overload increases oxidative stress in four functional brain areas of neonatal rats.J Biomed Sci2012; 19: 51.
[11] Wahby MM, Mohammed DS, Newairy AA, Abdou HM, Zaky A.Aluminum-induced molecular neurodegeneration: the protective role of genistein and chickpea extract.Food Chem Toxicol2017; 107: 57-67.
[12] Abdel-Salam OME, Hamdy SM, Seadawy SM, Galal AF, Abouelfadl DM, Atrees SS. Effect of piracetam, vincamine, vinpocetine, and donepezil on oxidative stress and neurodegeneration induced by aluminum chloride in rats.Comp Clin Pathol2016; 25: 305-318.
[13] Cordeiro JM, Silva VS, Oliveira CR, Goncalves PP. Aluminium-induced impairment ofmodulatory action on GABA transport in brain cortex nerve terminals.J Inorg Biochem2003; 97: 132-142.
[14] Singh T, Goel RK. Neuroprotective effect ofAllium cepaL. in aluminium chloride induced neurotoxicity.Neurotoxicology2015; 49: 1-7.
[15] Li Q, Liu H, Alattar M, Jiang S, Han J, Ma Y, et al. The preferential accumulation of heavy metals in different tissues following frequent respiratory exposure to PM2.5 in rats.Sci Rep2015; 5: 16936.
[16] Zatta P, Kilyen M, Kiss T.In vivoandin vitroeffects of aluminum on the activity of mouse brain acetylcholinesterase.Brain Res Bull2002; 59: 41-45.
[17] Nayak P, Sharma SB, Chowdary NS. Oxidant handling by hippocampus and Hebb-William maze performance in aluminum-exposed albino Wistar rats.Int J Clin Exp Physiol2014; 1: 106-112.
[18] Exley C. The pro-oxidant activity of aluminum.Free Radic Biol Med2004; 36: 380-387.
[19] Cao Z, Yang X, Zhang H, Wang H, Huang W, Xu F, et al. Aluminum chloride induces neuroinflammation, loss of neuronal dendritic spine and cognition impairment in developing rat.Chemosphere2016; 151: 289-295.
[20] Zaky A, Mohammad B, Moftah M, Kandeel KM, Bassiouny AR. purinic/apyrimidinic endonuclease 1 is a key modulator of aluminum-induced neuroinflammation.BMC Neurosci2013; 14: 26.
[21] Bhattacharjee S, Zhao Y, Hill JM, Percy ME, Lukiw WJ. Aluminum and its potential contribution to Alzheimer’s disease (AD).Front Aging Neurosci2014; 6: 62.
[22] Rajan KE, Preethi J, Singh HK. Molecular and functional characterization of Bacopa monniera. A retrospective review.Evid Based Complement Alternat Med2015; 945217. Doi: http://dx.doi.org/10.1155/2015/945217.
[23] Walton J. Aluminum in hippocampal neurons from human’s with Alzheimer’s disease.Neurotoxicology2006; 27: 385-394.
[24] Zhang H, Yang X, Qin X, Niu Q. Caspase-3 is involved in aluminuminduced impairment of long-term potentiation in rats through the Akt/GSK-3β pathway.Neurotox Res2016; 29: 484-494.
[25] Hendriks HS, Koolen LA, Dingemans MM, Viberg H, Lee I,Leonards PE, et al. Effects of neonatal exposure to the flame retardant tetrabromobisphenol-A, aluminum diethylphosphinate or zinc stannate on long-term potentiation and synaptic protein levels in mice.Arch Toxicol2015; 89: 2345-2354.
[26] Vaishnavi K, Saxena N, Shan N, Singh R, Manjunath K, Uthayakumar M, et al. Differential activities of the two closely related withanolides,withaferin A and withanone: bioinformatics and experimental evidences.PloS One2012; 7: e44419.
[27] Mehrotra V, Mehrotra S, Kirar V, Shyam R, Misra K, Srivastava AK,et al. Antioxidant and antimicrobial activities of aqueous extract ofWithania somniferaagainst methicillin-resistantStaphylococcus aureus.J Microbial Biotech Res2011; 1: 140-145.
[28] Kuboyama T, Tohda C, Komatsu K. Effects of ashwagandha (roots ofWithania somnifera) on neurodegenerative diseases.Biol Pharm Bull2014; 37(6): 892-897.
[29] Shah N, Singh R, Sarangi U, Saxena N, Chaudhary A, Kaur G, et al.Combinations of ashwagandha leaf extracts protect brain-derived cells against oxidative stress and induce differentiation.PLoS One2015; 10(3):e0120554. Doi: 10.1371/journal.pone.0120554.
[30] Singh RH, Narsimhamurthy K, Singh G. Neuronutrient impact of Ayurvedic Rasayana therapy in brain aging.Biogerontology2008; 9: 369-374.
[31] Gautam A, Wadhwa R, Thakur MK. Assessment of cholinergic properties of ashwagandha leaf-extract in the amnesic mouse brain.Ann Neurosci2016; 23: 68-75.
[32] Gautam A, Kaul SC, Thakur MK. Alcoholic extract of ashwagandha leaves protects against amnesia by regulation of arc function.Mol Neurobiol2016; 53(3): 1760-1769.
[33] Baitharu I, Jain V, Deep SN, Shroff S, Sahu JK, Naik PK, et al.Withanolide A prevents neurodegeneration by modulating hippocampal glutathione biosynthesis during hypoxia.PloS One2014; 9: e105311.
[34] Manchanda S, Kaur G.Withania somniferaleaf alleviates cognitive dysfunction by enhancing hippocampal plasticity in high fat diet induced obesity mode.BMC Complement AlternMed2017; 17: 136.
[35] Ruiz-Larrea MB, Leal AM, Liza M, Lacort M, de Groot H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes.Steroids1994; 59: 383-388.
[36] Montgomery HAC, Dymock JF. The determination of nitrite in water.Analyst1961; 86: 414-416.
[37] Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione.J Lab Clin Med1963; 61: 882-888.
[38] Ellman GL, Courtney KD, Andres V, Featherstone R. A new and rapid colorimetric determination of acetylcholinesterase activity.Biochem Pharmacol1961; 7: 88-95.
[39] Tsakiris S, Angelogianni P, Schulpis KH, Behrakis P. Protective effect of l-cysteine and glutathione on rat brain Na+, K+ATPase inhibition induced by free radicals.Z Naturforsch2000; 55: 271-277.
[40] Walton J. Aluminum in hippocampal neurons from human’s with Alzheimer’s disease.Neurotoxicology2006; 27: 385-394.
[41] John J, Nampoothiri M, Kumar N, Mudgal J, Nampurath GK,Chamallamudi MR. Sesamol, a lipid lowering agent, ameliorates aluminium chloride induced behavioral and biochemical alterations in rats.Pharmacogn Mag2015; 11: 327-336.
[42] Verstraeten SV, Nogueira LV, Schreier S, Oteiza PI. Effect of trivalent metal ions on phase separation and membrane lipid packing: role in lipid peroxidation.Arch Biochem Biophys1997; 338: 121-127.
[43] Becaria A, Lahiri DK, Bondy SC, Chen DM, Hamadeh A, Li H, et al.Aluminum and copper in drinking water enhance chronic inflammatory of oxidative events specifically in the brain.J Neururoimmunol2006;176: 16-23.
[44] Colak S, Geyikoglu F, Keles ON, Turkez H, Topal A, Unal B. The neuroprotective role of boric acid on aluminum chloride-induced neurotoxicity.Toxicol Ind Health2011; 27: 700-710.
[45] Poderoso JJ. The formation of peroxynitrite in the applied physiology of mitochondrial nitric oxide.Arch Biochem Biophys2009; 484: 214-220.
[46] Saing T, Lagman M, Castrillon J, Gutierrez E, Guilford FT,Venketaraman V. Analysis of glutathione levels in the brain tissue samples from HIV-1-positive individuals and subject with Alzheimer’s disease and its implication in the pathophysiology of the disease process.BBA Clin2016; 6: 38-44.
[47] Circu ML, Aw TY. Glutathione and apoptosis.Free Radic Res2008; 42:689-706.
[48] Franco R, Schoneveld OJ, Pappa A, Panayiotidis MI. The central role of glutathione in the pathophysiology of human diseases.Arch Physiol Biochem2007; 113: 234-258.
[49] Anand B, Nehru B. Alterations in glutathione system in adult and pup rat brains following chronic aluminum exposure.Ind J Occupat Environ Med2006; 10: 128-132.
[50] Halliwell B. Reactive oxygen species and the central nervous system.J Neurochem1992; 59: 1609-1623.
[51] Bellissimo MI, Amado D Abdalla DS, Ferreira EC, Cavalheiro EA,Naffah-Mazzacoratti MG. Superoxide dismutase, glutathione peroxidase activities and the hydroperoxide concentration are modified in the hippocampus of epileptic rats.Epilepsy Res2001; 46: 121-128.
[52] Passos GF, Figueiredo CP, Prediger RD, Silva KA, Siqueira JM, Duarte FS. Involvement of phosphinositide 3-kinase gamma in the neuroinflammatory response and cognitive impairment induced by beta amyloid 1-40 peptide in mice.Brain Behav Immun2010; 24: 439- 501.
[53] Lee KS, Chung JH, Choi TK, Suh SY, Oh BH, Hong CH. Peripheral cytokines and chemokines in Alzheimer’s disease.Dement Geriatr Cogn Disord2009; 28: 281- 287.
[54] Nampoothiri M, John J, Kumar N, Mudgal J, Nampurath GK,Chamallamudi MR. Modulatory role of simvastatin against aluminium chloride-induced behavioural and biochemical changes in rats.Behav Neurol2015; 2015: 210169.
[55] Gulya K, Rakonczay Z, Kasa P. Cholinotoxic effects of aluminum in rat brain.J Neurochem1990; 54: 1020-1026.
[56] Schliebs R, Arendt T. The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease.J Neural Transm2006;113: 1625-1644.
[57] Soule’ J, Alme M, Myrum C, Schubert M, KanhemaT, Bramham CR.Balancing Arc synthesis, mRNA decay, and proteasomal degradation:maximal protein expression triggered by rapid eye movement sleep-like bursts of muscarinic cholinergic receptor stimulation.J Biol Chem2012;287: 22354-22366.
[58] Albers RW, Siegel GJ. Membrane transport. In: Brady ST, Siegel GJ,Albers RW, Price D, editors. In,Basic neurochemistry: principles of molecular, cellular and medical neurobiology.8th editon. Massachusetts,USA: Elsevier Academic Press; 2012, p. 41-62.
[59] Nayak P, Chatterjee AK. Effects of aluminium exposure on brain glutamate and GABA systems: an experimental study in rats.Food Chem Toxicol2001; 39: 1285-1289.
[60] Tsunoda M, Sharma RP. Modulation of tumor necrosis factor alpha expression in mouse brain after exposure to aluminum in drinking water.Arch Toxicol1999; 73: 419-426.
[61] Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, et al.Tumor necrosis factor alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner.J Biol Chem2006; 281: 21362-21368.
[62] Blaylock RL, Maroon J. Immunoexcitotoxicity as a central mechanism in chronic traumatic encephalopathy-A unifying hypothesis.Surg Neurol Int2011; 2: 107.
[63] Siegel GJ, Albers RW, Brady ST, Price DL.Basic neurochemistry.Molecular, cellular and medical aspects.Burlington, MA, USA: Elsivier Academic Press; 2006.
[64] Anand KS, Dhikav V. Hippocampus in health and disease: an overview.Ann Indian Acad Neurol2012; 15: 239-246.
[65] Minichiello I. TrkB signaling pathwaysin LTP and learning.Nat Rev Neurosc2009; 10: 850- 860.
[66] Le Y, Liu S, Peng M, Tan C, Liao Q, Doan K, et al. Aging differentially affects the loss of neuronal dendritic spine, neuroinflammation and memory impairment at rats after surgery.PloS One2014; 9: e106837.
[67] Segal M. Dendritic spines and long-term plasticity.Nat Rev Neurosc2005; 6: 277- 284.
[68] Harika P, Kannamba B, Ramana GV, Haribabu B. Effect of solvent composition on total phenol and flavonoids content ofWithania somnifera.J Chem Pharm Sci2017; 10: 601-603.
[69] Vidyashankar S, Thiyagarajan OS, Varma SR, Kumar LMS, Badu UV,Patki PS. Ashwagandha (Withania somnifera) supercritical CO2extract derived withanolides mitigates Bisphenol A induced mitochondrial toxicity in HepG2cells.Toxicol Rep2014; 1: 1004-1012.
[70] Parihar MS, Hemnani T. Phenolic antioxidants attenuate hippocampal neuronal cell damage against kainic acid induced excitotoxicity.J Biosci2003; 28: 121-128.
[71] Singh RJ, Hogg N, Joseph J, Kalyanaraman B. Mechanism of nitric oxide release from S-nitrosothiols.J Biol Chem1996; 271: 18596-18603.
[72] Kataria H, Wadhwa R, Kaul SC, Kaur G. Water extract from the leaves ofWithania somniferaprotect RA differentiated C6 and IMR-32 cells against glutamate-induced excitotoxicity.PLoS One2012; 7: e37080.
[73] Konar A, Shah N, Singh R, Saxena N, Kaul SC, Wadhwa R, et al.Protective role of Ashwagandha leaf extract and its component withanone on scopolamine-induced changes in the brain and brain-derived cells.PLoS One2011; 6: e27265.
[74] Soman S, Korah PK, Jayanarayanan S, Mathew J, Paulose CS. Oxidative stress induced NMDA receptor alteration leads to spatial memory deficits in temporal lobe epilepsy: ameliorative effects ofWithania somniferaand withanolide A.Neurochem Res2012; 37: 1915-1927.
[75] Sood A, Kumar A, Dhawan DK, Sandhir R. Propensity ofWithania somniferato attenuate behavioural, biochemical, and histological alterations in experimental model of stroke.Cell Mol Neurobiol2016; 36:1123-1138.
 Asian Pacific Journal of Tropical Biomedicine2018年1期
Asian Pacific Journal of Tropical Biomedicine2018年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Prevalence of coronavirus from diarrheic calves in the Republic of Korea
- Effective Aeromonas specific monoclonal antibody for immunodiagnosis
- Pharmacodynamic profiling of optimal sulbactam regimens against carbapenemresistant Acinetobacter baumannii for critically ill patients
- Efficiency of combining pomegranate juice with low-doses of cisplatin and taxotere on A549 human lung adenocarcinoma cells
- Correlation of phytochemical content with antioxidant potential of various sweet potato (Ipomoea batatas) in West Java, Indonesia
- Larvicidal activity of Xenorhabdus and Photorhabdus bacteria against Aedes aegypti and Aedes albopictus
