Validation of a novel animal model for sciatic nerve repair with an adipose‐derived stem cell loaded fibrin conduit
Maximilian M. Saller, Rosa-Eva Huettl, Julius M. Mayer, , Annette Feuchtinger, Christian Krug, , Thomas Holzbach, , Elias Volkmer, ,
Introduction
Maintenance of proper functionality and homeostasis of any life form requires constant repair of cell damage. In all higher organisms, the peripheral sensory‐motor circuitry is of utmost importance, as it allows for interaction with the environment. Peripheral nerves that innervate the torso and the extremities are capable of regeneration to a certain degree when injured. However, neuronal trauma of critical size imposes severe obstacles for proper regeneration (Lund‐borg et al., 1982; Nectow et al., 2012): Following a complete transection of fibers (axonotmesis), there is an increased risk of neuroma formation, which, when it occurs, often results in functional loss and pain (Bendszus and Stoll, 2005). Ad‐ditional damage to the epi‐ and perineurium (neurotmesis)further contributes to incomplete restoration of connectivity and functional impairment.
Currently, surgeons aim at improving nerve regeneration by either suturing injured nerves (coaptation), or by trans‐planting autologous nerve grafts to bridge a gap between severed nerve endings. For critical‐size nerve defects with a gap larger than 10 to 20 mm, autologous nerve grafts are usually preferable (Lundborg et al., 1982). However, any graft is accompanied by some degree of donor site morbidity(Millesi, 2007). Hence, there is a need for improvement, motivating new strategies to enhance peripheral nerve repair.These include substituting nerve grafts by implanting nerve conduits derived from the vasculature or the implantation of bioresorbable materials, like collagen tubes or de‐celluarized nerve scaffolds, as guiding structures for axonal outgrowth and targeting (Battiston et al., 2000; Weber et al., 2000;Bushnell et al., 2008; He et al., 2015).
Animal models show that the addition of certain cell types can improve the time and final outcome of nerve recovery(Kalbermatten et al., 2008; di Summa et al., 2011). During native regeneration, Schwann cells play a crucial role by re‐leasing cytokines like nerve growth factor (NGF), brain‐de‐rived neurotrophic factor (BDNF), neurotrophin 3 (NTF3),or glial cell derived neurotrophic factor (GDNF), which support axonal growth after injury (Höke et al., 2006). How‐ever, the availability of Schwann cells is limited, and their use has not been clinically approved for cellular therapies.Therefore, several research groups aim to use the power of stem cells to support nerve regeneration (Kalbermatten et al., 2008; di Summa et al., 2010, 2011; McGrath et al., 2012).Currently, researchers are trying to identify the perfect cellular conditions for acceleration and improvement of the healing process. While it is tempting to use gene therapy or to modify cells in the laboratory with modern genetic tools(Hillenbrand et al., 2015), we believe that a strategy closer to the actual clinical application may be more suitable.
We therefore developed a model closer to current clinical practice, which allows for testing the application of different cell typesin vivo. There is evidence that the usage of fibrin glue to seal the sites of coaptation may enhance neuronal re‐generation (Kalbermatten et al., 2009; McGrath et al., 2012;Zhao et al., 2014; Koulaxouzidis et al., 2015). We therefore cast undifferentiated adipose tissue‐derived stem cells in a fibrin gel tube to serve as a nerve conduit around the co‐aptation site. In our model, a 20 mm portion of the sciatic nerve of rats was cut out, flipped around and sutured back in place, thus mimicking an autologous nerve graft with some differences in nerve diameter, axon number and quality. Subsequently, the functional and anatomical outcomes in animals that were treated with stem cell loaded conduits were assessed.
Material and Methods
Ethics statement
Animals were handled and housed according to the federal and institutional guidelines for the care and use of laboratory animals, approved by the government of Upper Bavaria(177‐13).
Animals
Ten (5 per group) 12‐week‐old female rats (Sprague Dawley,Inc., Charles River, MA, USA), weighing approximately 250 g were housed under standard animal laboratory conditions with controlled temperature of 20–22°C and a 12‐hour light/dark cycle, and fed adlibitumin individual ventilated cages with an effective area of 1500 cm2. A maximum of four rats per cage were accommodated.
Autologous adipose-derived mesenchymal stem cell isolation and characterization
For all surgical procedures, individual rats were anesthetized by 2% isoflurane inhalation (cp‐pharma, Burgdorf, Germany) and post‐operatively treated with 0.05 mg/kg buprenor‐phine (Buprenovet, Bayer, Leverkusen, Germany) subcu‐taneously. White fat‐derived mesenchymal stem cells were isolated from inguinal fat pads. The groin region was shaved,disinfected, and the subcutaneous fat was exposed by a 10 to 20 mm long incision. Subsequently, approximately 2.6 g fat was removed, minced with a scalpel, and cells were enzymatically isolated with a commercially available centrifuge(ARC™‐Processing Unit, InGeneron, Houston, TX, USA)following the company’s protocol. The obtained heterogeneous cell population was plated onto T‐225 cell culture flasks (Nunclon, Thermo Fisher, Waltham, MA, USA) and non‐adhered cells were removed by repetitive rinsing with phosphate buffered saline (PBS). The cells were expanded for 3 days in standard culture medium (89% DMEM, 10%heat inactivated fetal calf serum, 1% penicillin/streptomycin,Thermo Fisher Scientific, Inc., Waltham, MA, USA) and directly used for conduit preparation. Stem cell characteris‐tics of obtained cells were determined by the differentiation towards the osteogenic and adipogenic lineage and the sub‐sequent quantification of Alizarin Red (Sigma‐Aldrich, St.Louis, MO, USA) and AdipoRed (Lonza, Basel, Switzerland)staining, using a UV/VIS plate reader according to a previously described protocol (Saller et al., 2012). Furthermore,the expression of CD29, CD90, CD11b/c and CD45 on the isolated cells was quantified by fluorescence‐activated cell sorting (FACS) analyses following an established protocol(Saller et al., 2012). All antibodies and appropriate isotype controls were obtained from BioLegend, San Diego, CA,USA.
Cell carrier preparation
To ensure a spatial localization of the transplanted cells, we decided to use a clinically approved fibrin hydrogel (AR‐TISS®, Baxter, Deerfield, IL, USA), as preliminary results showed a high biocompatibility and metabolic activity over a long culture periodin vitro(data not shown). Cell‐loaded conduits with a length of 25 mm and a 2 mm wall thickness were prepared by mixing 3 × 106cells in standard medium and thrombin solution at a 1:10 ratio. Afterwards the fibrinogen solution was allowed to polymerize in a sterile syringe with a centered metal rod to create a 2 mm lumen.Initial polymerization was carried out for 30 minutes, and after the removal from the syringe, the fibrin conduit was hardened for two hours in complete medium, before gently opening it longitudinally with micro scissors in order to be put around the nerve autograft (Figure 1G). Pre‐operative anesthesia was initiated by intramuscular injection of 0.02 mg/kg fentanyl (Janssen, Germany), 1.0 mg/kg midozilam(Ratiopharm, Ulm, Germany) and 0.2 mg/kg medetomidin(Orion, Espoo, Finnland). Anesthesia was post operative‐ly antagonized with 0.03 mg/kg naloxone (Bristol myers Squibb, New York, NY, USA), 0.1 mg/kg flumazenile (Roche,Basel, Switzerland) and 1.0 mg/kg atipamezole (cp‐pharma,Burgdorf, Germany).
Operation procedure
Under deep anesthesia, rats were immobilized and the dorsal upper hindlimb was shaved from the knee to the spine(Figure 1A). To sufficiently expose the region of interest, a long incision was made and a subcutaneous skin pocket was dissected (Figure 1B, C). By blunt dissection of the femoral muscle, the sciatic nerve was exposed from the spinal cord exit point to the first distal branching point (Figure 1D).To create a critical‐size nerve injury, a 20 mm long portion was cut out (Figure 1E), turned around and microsurgically sutured with three to four perineuronal 10‐0 nylon sutures on both ends (Figure 1F). Long cell‐loaded fibrin conduits of a length of 25 mm were prepared in advance (Figure 1G),wrapped around the nerve defect and finally fixed by suturing the muscles (Figure 1H). Animals that received an autologous nerve graft without cell‐seeded conduits served as controls. All animals were treated daily with 0.05 mg/kg buprenorphine s.c.for three days, and with 1.5 mg/kg fluphenazine (s.c.) and metamizol in the drinking water (ad libitum) within the first postoperative weeks to avoid auto mutilation due to neu‐ropathic pain or ‘foreign body feeling’ of the operated limb(Carr et al., 1992).
Post-operative evaluation of functional recovery
To determine the functional recovery of the sciatic nerve during a 16‐week long post operative phase, we utilized the sciatic function index (SFI) and the static sciatic index (SSI)(de Medinaceli et al., 1982; Bain et al., 1989; Bervar, 2000;Smit et al., 2004). Both indices are validated and reliable methods to evaluate functional peripheral nerve recovery and repair of the rat sciatic nerve after injury. The three variables to compute the SFI are the print length (PL), the toe spread (TS) between the first toe and the fifth toe and the intermediate toe spread (ITS) between the second and the fourth toes. Accordingly, static sciatic index’ parameters are the same, except for the print length, which is not applied in this non dynamic index.
The dynamic footprints for the SFI were documented by bedewing the animals’ hind feet with dark ink and having the animals pass a paper cat walk tunnel of 110 cm in length.A cover at the end of the tunnel presented a shelter for the animals and thus a motivation for them to pass the walking track determinedly to produce accurate footprints. Respectively, the SSI was measured in plantar view photos of the animals’ hind feet that were taken from below while having the rodents sit in a box of acrylic glass with a transparent floor plate. All parameters for the indices were measured manually in ImageJ (NIH, Bethesda, MD, USA) (Schindelin et al., 2012).
Histological evaluation
The possible loss of muscle mass caused by denervation was evaluated by bilaterally weighting gastrocnemius muscles after 16 weeks. Regeneration of sensory and motor axons was assessed in semi thin paraffin sections: sciatic nerves,including at least 5 mm of the proximal and distal injury proportion, were dissected from both legs, fixed overnight in 4% paraformaldehyde in phosphate buffered saline at 4°C and embedded in paraffin. After deparaffinization, sections were stained with a 1% toluidine solution for 2 minutes at room temperature, washed twice in distilled water, and finally mounted. High resolution bright field images were taken with a fully automated microscope (AxioObserver, Zeiss,Jena, Germany), covering the total cross‐sectional distal area of the sciatic nerve defect. These images were subsequently quantified using the commercial image analysis software Definiens Developer XD2 (Definiens AG, Munich, Germany) (Feuchtinger et al., 2015; Helmbrecht et al., 2015). A specific rule set was defined to detect all axons based on col‐or and shape features (Figure 2). Finally, for each image the total number of axons per nerve, the diameter and the pro‐portion of the axon diameter to fiber diameter ratio (g‐ratio)were calculated. Scar tissue formation was calculated by subtracting the total axon area including the myelin sheet from the total cross sectional nerve area.
Statistical analysis
While allin vitroexperiments were carried out in triplicates with cells from three individual animals,in vivoexperiments were performed on five animals. Normal distribution of the data was validated with a Kolmogorow‐Smirnow test, and either a two‐tailedt‐test with or without a Welch’s correction a one‐way ANOVA test with a Bonferronipost-hoctest was used for statistical analysis. AP‐value of 0.05 or lower was considered significant. All values are presented by either the mean and the standard deviation or a box plot with mix to max whiskers.
Results
Adipose-derived cells exhibit stem cell properties
The deployment of bone marrow‐ or adipose tissue‐derived stem cells around peripheral nerve lesions is receiving in‐creasing attention in regenerative medicine (McGrath et al.,2012; Kingham et al., 2014). To validate mesenchymal stem cell characteristics, we carried out osteogenic and adipogenic differentiation assays and screened the cells for the expression of mesenchymal stem cell and hematopoietic markers.While isolated cells that were cultured in osteogenic differentiation medium showed a strong Alizarin Red staining within formed nodules after 21 days (Figure 3A), cells in standard culture medium displayed no mineral deposition(Figure 3A insert). Quantification of Alizarin Red concentration revealed a significant increase in the osteogenic differentiated group (215.1 ± 52.9 mM) when compared to the control group (22.6 ± 4.2 mM, Figure 3A’). Furthermore,differentiation into the adipogenic lineage exposed an in‐tense formation of lipid vacuoles (Figure 3B), visualized by an Oil Red O staining, while cells in control medium showed no lipid deposition (Figure 3B insert). Quantification of lip‐id droplet formation by AdipoRed showed a significant and 14 times higher lipid accumulation (3,872 RFU ‐ Relative Fluorescence Units) in the adipogenic differentiation, when compared to the control group (273 RFU, Figure 3B’). To further evaluate the molecular profile of isolated cells, we performed an FACS analysis on the mesenchymal stem cell markers CD29 and CD90, as well as on the hematopoietic markers CD11b/c and CD45. Remarkably, nearly all cells were positive for CD29 and CD90, while the isolated cells showed no expression of CD11b/c and CD45 (Figure 3C).
Taken together, isolated cells from white adipose tissue could be differentiated into two different lineages and show a mesenchymal surface marker profile. Due to their multi‐potent character, these cells might have a direct or indirect beneficial effect on nerve regeneration after injury.
Application of adipose tissue-derived stem cells leads to improved functional outcome that is accompanied by increased axonal regeneration
To evaluate the potential benefits of cell loaded hydrogel conduits, we utilized a dynamic sciatic function index (SFI)and a static sciatic scatter index (SSI), as toe spreading can be directly correlated to functional regeneration of the sciatic nerve after injury (Bain et al., 1989; Smit et al., 2004).
During the initial four weeks after surgery, no difference in toe positioning was detected between both experimental groups (Figure 4A, left). However, the application of adipose tissue‐derived stem/progenitor cells resulted in a significant improvement after 16 weeks, when compared to animals that received only an autologous nerve graft(Figure 4A, right). Quantification of the SFI (Figure 4B)and SSI (Figure 4C) validated the significant beneficial effect of transplanted cells onto the neuromuscular function already after 8 weeks. This increase in locomotion function was accompanied by a significant reduction of muscle degeneration on the operated side (0.67) when compared to animals with only autologous transplants (0.49; Figure 4D,E). Toluidine blue staining on sections of the distal end of the sciatic nerve defect revealed that the addition of cell seeded conduits increased the axon density, when compared to normal autologous transplants (Figure 5A, A’, B, B’).Quantification of the axon density and scar tissue formation revealed that animals with autologous cells around the site of injury showed a significant increase of axon ingrowth(167.3 axons/ROI; Figure 5D), coinciding with a significant reduction of extracellular matrix deposition (0.53; Figure 5E), when compared to the animals with autologous nerve grafts (134.9 axons/ROI and 0.65%) or to the contralateral side (76.38 axons/ROI and 0.35%). As axonal regeneration and subsequent nerve function also depend on appropriate remyelination (Chomiak and Hu, 2009), we quantified the g‐ratio of all axons (Figure 5F). While animals with autol‐ogous transplants showed a moderate remyelination, when compared to the contralateral side, the additional application of adipose‐derived stem cells revealed considerably larger myelinated axons (Figure 5F, right panel). Quantification of the mean g‐ratio confirmed a significantly improved myelination in combination with cell‐seeded conduits(0.40), when compared to autologous transplants without conduit (0.36) or a native sciatic nerve (0.47; Figure 5G). As the axon diameter can be directly correlated to conduction velocity (Gillespie and Stein, 1983), we quantified the relative frequency of axon diameter as well as axon density (Figure 5H). Autologous transplants showed a significant reduction of the mean axon diameter after 4 months (1.78 μm), and the addition of stem cells to the injury site slightly increased the mean axon diameter (2.02 μm), when compared to the con‐tralateral side (4.18 μm; Figure 5I).
Taken together, the addition of adipose tissue‐derived stem cells to the nerve injury site showed a significant beneficial effect on myelination, axon regeneration, avoidance of muscle atrophy, and locomotive function. However,both treatment options failed to restore the complete function and anatomical nerve parameters when compared to non‐operated animals or the contralateral side (native sciatic nerve) after 16 weeks.
Discussion
The establishment of precise connectivity to peripheral muscular targets and the integration of the corresponding sensory‐motor networks are achieved through highly appropriate temporal and spatial regulation of axonal growth during embryonic development (Huber et al., 2003). Various mechanisms such as the combination of different molecular cues for correct targeting, sorting, and bundling of axons, as well as the communication between heterotypic fiber‐systems,or between axons and their environment, ensure proper fasciculation, accurate path finding, and overall functionality of the network (Huber et al., 2003). Given the accuracy with which these processes have to interact in a stepwise manner during development, re‐generating fibers are challenged with a multitude of processes that inhibit regeneration ca‐pacities after traumatic injuries. Limitation of regeneration or the prevention of axon extension into lesion sites contribute to incomplete restoration of function or, depending on the severity of the injury, may even impede reconnection,resulting in paralysis (Oertle et al., 2003). Despite variable degrees of functional recovery, which is mediated at least in part by re‐organization of neuronal circuitry, re‐establishment of drastically impaired circuits to restore functionality is not yet feasible. Reliable quantitative analyses of re‐estab‐lished connections to their cognate target muscles and the skin, but also assessment of physiological and behavioral consequences at adult age, are therefore a necessity to ex‐tend our understanding of the mechanisms of regenerating neuronal circuitry.
In mouse models, retrograde tracing of re‐established ax‐onal tracts innervating peripheral musculature in the limbs is not only used to evaluate fiber tracts, but also to deter‐mine whether the stereotypic topographic pattern of motor neurons in the lateral or medial aspects of the lateral motor columns (LMC) of the respective dorsal and ventral limb musculature is affected (Kania and Jessell, 2003; Luria et al., 2008). Furthermore, the maintenance or loss of present neuromuscular junctions may contribute to assessing the re‐establishment of connectivity on the muscle site; thus,comprehensive behavior studies, including the assessment of gait, balance and nociception, must be employed to substantiate the investigation (Baumhauer et al., 2014; Helmbrecht et al., 2015). Electrophysiological analyses are required to gauge potential mis‐wiring of single muscle fibers or entire muscle groups after traumatic injury to peripheral nerve tracts. While many molecular mechanisms that are involved in peripheral nerve repair have been identified, the comprehensive complexity of the overall regeneration process is not fully understood yet.
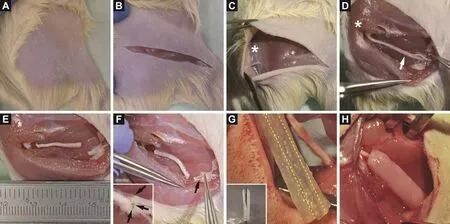
Figure 1 Representative photos of the key steps during the operation procedure.

Figure 2 Automatic image segmentation for quantification of axon parameters.
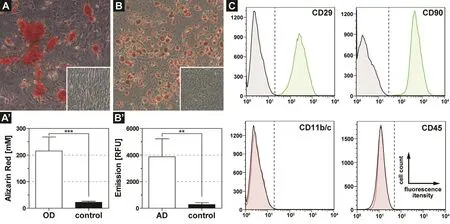
Figure 3 Characteristics of isolated stem cells from white adipose tissue of rats.
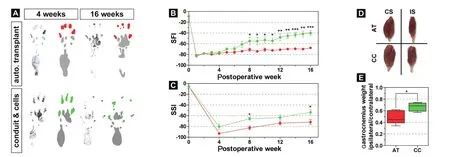
Figure 4 Adipose tissue-derived stem cell loaded fibrin gel conduits improve functional outcome and decrease muscle degradation in rats after sciatic nerve injury.
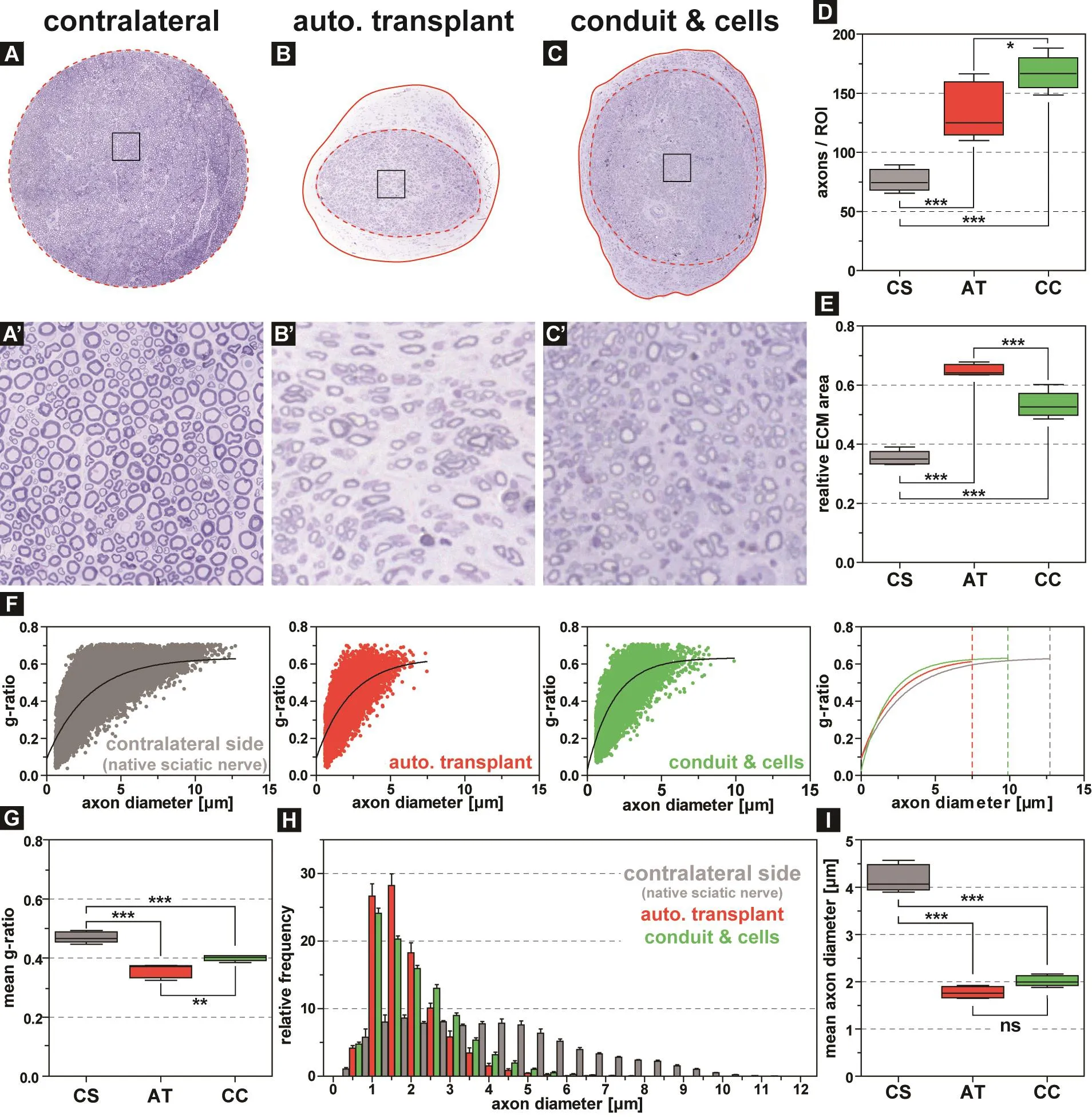
Figure 5 The addition of adipose-derived stem cells leads to an increased myelination and decreased scar tissue formation within the sciatic nerve but no change of the mean axon diameter in comparison to autologous transplants in rats after sciatic nerve injury.
This lack of knowledge is currently a significant drawback for stem cell based approaches in regenerative medicine.While it is generally accepted that transplanted stem cells have a bene ficial effect on the healing process of peripherals nerve injuries inin vivomodels (Hundepool et al., 2014), the underlying molecular mode of action is not clear. Neuro‐trophic factors, released by transplanted cells, are currently the most promising mechanism for causing the increased axonal re‐growth and later myelination (Lopatina et al.,2011; Wang et al., 2017).
Due to the complexity of peripheral nerve surgery in mice,larger animal models have been taken into consideration. To this point, however, we lack reliable, standardized, and validated data in rats. The critical injury size of our sciatic nerve model resembles the situation in humans regarding size, as the majority of peripheral nerve lesions in humans measure around 3–5 cm (He et al., 2014). The axonal regeneration rate in rats is faster, however, reaching approximately 3–4 mm/day (Black and Lasek, 1979), while humans show a 2–4 fold slower regeneration rate (Höke, 2011). In contrast to mouse models, where the smaller size of the sciatic nerve and thus the decreased reproducibility of the operation procedure leads to a highly variable experimental outcome,rat models provide a number of intriguing features, from a surgeon’s point of view: We are able to generate a true crit‐ical size defect model, which means that the defect created in the sciatic nerve would not heal on its own (Lundborg et al., 1982). In consequence, any supplement applied into the gap in order to enhance repair of the defect is the actual cause of any re‐gain of function. Secondly, turning the nerve around and suturing it back in place mimics nerve grafting as performed in current surgical practice: there is a certain size mismatch at the coaptation sites, and there is a mis‐match regarding the distribution and orientation of axons within the nerve. Thirdly, the model as described here is technically close to a potential clinical application. Fibrin gel is already being used in the operating theater on a daily basis throughout the world (Matras, 1985). Likewise, autologous fat cell transplantation is commonly used in clinical practice, e.g., lipo fillings and certain studies proved that adipose‐derived stem cells can be isolated in accordance with GMP guidelines (Aghayan et al., 2015; Larijani et al., 2015). While several studies relied on a differentiation of the stem cells towards a Schwann cell phenotype prior to implantation(di Summa et al., 2010; Scholz et al., 2011; Kingham et al.,2014). Nonetheless, this pre‐differentiation process requires several growth factors, and thus, a translation to human trials is ambitious due to regulatory and ethical issues. Howev‐er, rat models for nerve regeneration are always limited with regard to the transferability to humans (Kaplan et al., 2015).They can only be tools to identify potential therapies, and the results have to be interpreted with care. Especially nerve defects with larger gaps between severed nerve endings are not represented appropriately in rodent models, and fur‐thermore, they bear a species‐specific neurobiological regenerative profile that strongly differs from humans (Kaplan et al., 2015). Additionally, ethical restrictions to processing or pre conditioning these cells before re implantation have to be considered critically, as in most countries, modifications such as centrifuging and accumulating stem cells do require the permission of an ethical committee and/or regulatory agencies.
Taken together, adipose‐derived stem cells lead to a sig‐ni ficant increase of sciatic nerve regeneration in our novel animal model. Furthermore, the model provides a step into the direction of personalized treatment of traumatic nerve injury: different patient‐derived cell sources, different cell types, and different cellular pretreatments, such as changes in oxygen concentrations or growth factors, can be tested in a critical‐size nerve defect model without risking graft rejection or having to depend on donors. Thus, the model at hand presents a powerful tool to search for ways to improve the re‐establishment of peripheral nerve connectivity.
Acknowledgments:We thank Martina Burggraf and Zsuzsanna Farkas(both ExperiMed, LMU) for technical assistance.
Author contributions:Conceived and designed the experiments: MMS,REH, TH, EV. Performed the experiments: MMS, JMM, CK. Analyzed the data: MMS, JMM, AF. Wrote the manuscript: MMS, REH, TH, EV.
Conflicts of interest:The authors declare no competing or financial interests.
Financial support:This study was financially supported by the Faculty of Medicine, LMU (to TH and MMS; FöFole, Project 843 and 955). The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement:Animals were handled and housed according to the federal and institutional guidelines for the care and use of laboratory animals, approved by the government of Upper Bavaria (177–13).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Sameer B. Shah, University of California, San Diego, USA; Huiyin Tu, Zhengzhou University, China.
Additional file:Open peer reviewer reports 1 and 2.
Aghayan HR, Goodarzi P, Arjmand B (2015) GMP‐compliant human adipose tissue‐derived mesenchymal stem cells for cellular therapy.Methods Mol Biol 1283:93‐107.
Bain JR, Mackinnon SE, Hunter DA (1989) Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg 83:129‐138.
Battiston B, Tos P, Cushway TR, Geuna S (2000) Nerve repair by means of vein filled with muscle grafts I. Clinical results. Microsurgery 20:32‐36.
Baumhauer J, Pinzur MS, Donahue R, Beasley W, DiGiovanni C (2014)Site selection and pain outcome after autologous bone graft harvest.Foot ankle Int 35:104‐107.
Bendszus M, Stoll G (2005) Technology insight: visualizing peripheral nerve injury using MRI. Nat Clin Pract Neurol 1:45‐53.
Bervar M (2000) Video analysis of standing‐‐an alternative footprint analysis to assess functional loss following injury to the rat sciatic nerve. J Neurosci Methods 102:109‐116.
Black MM, Lasek RJ (1979) Slowing of the rate of axonal regeneration during growth and maturation. Exp Neurol 63:108‐119.
Bushnell BD, McWilliams AD, Whitener GB, Messer TM (2008) Early clinical experience with collagen nerve tubes in digital nerve repair. J Hand Surg Am 33:1081‐1087.
Carr MM, Best TJ, Mackinnon SE, Evans PJ (1992) Strain differences in autotomy in rats undergoing sciatic nerve transection or repair.Ann Plast Surg 28:538‐544.
Chomiak T, Hu B (2009) What is the optimal value of the g‐ratio for myelinated fibers in the rat CNS? A theoretical approach. PLoS One 4:e7754.
de Medinaceli L, Freed WJ, Wyatt RJ (1982) An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol 77:634‐643.
di Summa PG, Kalbermatten DF, Pralong E, Raffoul W, Kingham PJ,Terenghi G (2011) Long‐term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience 181:278‐291.
di Summa PG, Kingham PJ, Raffoul W, Wiberg M, Terenghi G, Kal‐bermatten DF (2010) Adipose‐derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg 63:1544‐1552.
Feuchtinger A, Stiehler T, Jütting U, Marjanovic G, Luber B, Langer R, Walch A (2015) Image analysis of immunohistochemistry is superior to visual scoring as shown for patient outcome of esophageal adenocarcinoma. Histochem Cell Biol 143:1‐9.
Gillespie MJ, Stein RB (1983) The relationship between axon diameter,myelin thickness and conduction velocity during atrophy of mammalian peripheral nerves. Brain Res 259:41‐56.
He B, Zhu Q, Chai Y, Ding X, Tang J, Gu L, Xiang J, Yang Y, Zhu J, Liu X (2015) Safety and efficacy evaluation of a human acellular nerve graft as a digital nerve scaffold: a prospective, multicentre controlled clinical trial. J Tissue Eng Regen Med 9:286‐295.
He B, Zhu Z, Zhu Q, Zhou X, Zheng C, Li P, Zhu S, Liu X, Zhu J (2014)Factors predicting sensory and motor recovery after the repair of upper limb peripheral nerve injuries. Neural Regen Res 9:661‐672.
Helmbrecht MS, Soellner H, Truckenbrodt AML, Sundermeier J,Cohrs C, Hans W, de Angelis MH, Feuchtinger A, Aichler M, Fouad K, Huber AB (2015) Loss of Npn1 from motor neurons causes post‐natal deficits independent from Sema3A signaling. Dev Biol 399:2‐14.
Hillenbrand M, Holzbach T, Matiasek K, Schlegel J, Giunta RE (2015)Vascular endothelial growth factor gene therapy improves nerve re‐generation in a model of obstetric brachial plexus palsy. Neurol Res 37:197‐203.
Höke A (2011) A (heat) shock to the system promotes peripheral nerve regeneration. J Clin Invest 121:4231‐4234.
Höke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW,Brushart TM (2006) Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci 26:9646‐9655.
Huber AB, Kolodkin AL, Ginty DD, Cloutier JF (2003) Signaling at the growth cone: ligand‐receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci 26:509‐563.
Hundepool CA, Nijhuis THJ, Mohseny B, Selles RW, Hovius SER(2014) The effect of stem cells in bridging peripheral nerve defects: a meta‐analysis. J Neurosurg 121:195‐209.
Kalbermatten DF, Kingham PJ, Mahay D, Mantovani C, Pettersson J,Raffoul W, Balcin H, Pierer G, Terenghi G (2008) Fibrin matrix for suspension of regenerative cells in an artificial nerve conduit. J Plast Reconstr Aesthet Surg 61:669‐675.
Kalbermatten DF, Pettersson J, Kingham PJ, Pierer G, Wiberg M,Terenghi G (2009) New fibrin conduit for peripheral nerve repair. J Reconstr Microsurg 25:27‐33.
Kania A, Jessell TM (2003) Topographic motor projections in the limb imposed by LIM homeodomain protein regulation of ephrin‐A:E‐phA interactions. Neuron 38:581‐596.
Kaplan HM, Mishra P, Kohn J (2015) The overwhelming use of rat models in nerve regeneration research may compromise designs of nerve guidance conduits for humans. J Mater Sci Mater Med 26:226.
Kingham PJ, Kolar MK, Novikova LN, Novikov LN, Wiberg M (2014)Stimulating the neurotrophic and angiogenic properties of human adipose‐derived stem cells enhances nerve repair. Stem Cells Dev 23:741‐754.
Koulaxouzidis G, Reim G, Witzel C (2015) Fibrin glue repair leads to enhanced axonal elongation during early peripheral nerve regeneration in an in vivo mouse model. Neural Regen Res 10:1166‐1171.
Larijani B, Aghayan H, Goodarzi P, Mohamadi‐Jahani F, Norouzi‐Jav‐idan A, Dehpour AR, Fallahzadeh K, Azam Sayahpour F, Bidaki K,Arjmand B (2015) Clinical grade human adipose tissue‐derived mes‐enchymal stem cell banking. Acta Med Iran 53:540‐546.
Lopatina T, Kalinina N, Karagyaur M, Stambolsky D, Rubina K, Re‐vischin A, Pavlova G, Parfyonova Y, Tkachuk V (2011) Adipose‐de‐rived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS One 6:e17899.
Lundborg G, Dahlin LB, Danielsen N, Gelberman RH, Longo FM,Powell HC, Varon S (1982) Nerve regeneration in silicone chambers:In fluence of gap length and of distal stump components. Exp Neurol 76:361‐375.
Luria V, Krawchuk D, Jessell TM, Laufer E, Kania A (2008) Specification of motor axon trajectory by ephrin‐B:EphB signaling: symmetrical control of axonal patterning in the developing limb. Neuron 60:1039‐1053.
Matras H (1985) Fibrin seal: the state of the art. J Oral Maxillofac Surg 43:605‐611.
McGrath AM, Brohlin M, Kingham PJ, Novikov LN, Wiberg M, No‐vikova LN (2012) Fibrin conduit supplemented with human mes‐enchymal stem cells and immunosuppressive treatment enhances regeneration after peripheral nerve injury. Neurosci Lett 516:171‐176.
Millesi H (2007) Bridging defects: autologous nerve grafts. Acta Neuro‐chir Suppl 100:37‐38.
Nectow AR, Marra KG, Kaplan DL (2012) Biomaterials for the development of peripheral nerve guidance conduits. Tissue Eng Part B Rev 18:40‐50.
Oertle T, van der Haar ME, Bandtlow CE, Robeva A, Burfeind P, Buss A,Huber AB, Simonen M, Schnell L, Brösamle C, Kaupmann K, Vallon R, Schwab ME (2003) Nogo‐A inhibits neurite outgrowth and cell spreading with three discrete regions. J Neurosci 23:5393‐5406.
Saller MM, Prall WC, Docheva D, Schönitzer V, Popov T, Anz D, Clau‐sen‐Schaumann H, Mutschler W, Volkmer E, Schieker M, Polzer H(2012) Increased stemness and migration of human mesenchymal stem cells in hypoxia is associated with altered integrin expression.Biochem Biophys Res Commun 423:379‐385.
Schindelin J, Arganda‐Carreras I, Frise E, Kaynig V, Longair M, Pi‐etzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J‐Y,White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012)Fiji: an open‐source platform for biological‐image analysis. Nat Methods 9:676‐682.
Scholz T, Sumarto A, Krichevsky A, Evans GRD (2011) Neuronal differentiation of human adipose tissue‐derived stem cells for peripheral nerve regeneration in vivo. Arch Surg 146:666‐674.
Smit X, van Neck JW, Ebeli MJ, Hovius SER (2004) Static footprint analysis: a time‐saving functional evaluation of nerve repair in rats.Scand J Plast Reconstr Surg hand Surg 38:321‐325.
Wang C, Lu CF, Peng J, Hu CD, Wang Y (2017) Roles of neural stem cells in the repair of peripheral nerve injury. Neural Regen Res 12:2106‐2112.
Weber RA, Breidenbach WC, Brown RE, Jabaley ME, Mass DP (2000)A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg 106:1036‐1045.
Zhao Z, Wang Y, Peng J, Ren Z, Zhang L, Guo Q, Xu W, Lu S (2014)Improvement in nerve regeneration through a decellularized nerve graft by supplementation with bone marrow stromal cells in fibrin.Cell Transplant 23:97‐110.
- 中国神经再生研究(英文版)的其它文章
- Weak phonation due to unknown injury of the corticobulbar tract in a patient with mild traumatic brain injury: a diffusion tensor tractography study
- Exosomes: a novel therapeutic target for Alzheimer’s disease?
- Inhibition of retinal ganglion cell apoptosis:regulation of mitochondrial function by PACAP
- Trillium tschonoskii maxim extract attenuates abnormal Tau phosphorylation
- Association between Alzheimer’s disease pathogenesis and early demyelination and oligodendrocyte dysfunction
- Nogo receptor expression in microglia/macrophages during experimental autoimmune encephalomyelitis progression