Baseline effects of lysophosphatidylcholine and nerve growth factor in a rat model of sciatic nerve regeneration after crush injury
Ryan L. Wood , Keaton S. Karlinsey , Austin D. Thompson Mark N. Rigby Greggory D. Boatright William G. PittBeverly L. Roeder, Scott C. Steffensen , Alonzo D. Cook
1 Department of Chemical Engineering, Brigham Young University, Provo, UT, USA
2 Neuroscience Center, Brigham Young University, Provo, UT, USA
3 Department of Biology, Brigham Young University, Provo, UT, USA
4 Department of Psychology, Brigham Young University, Provo, UT, USA
Introduction
Damage to the peripheral nervous system (PNS) can be a life‐altering event due to the disruption of the normal functions of sensory and motor neurons. Severe damage can lead to permanent loss of nerve function, while minor damage can lead to temporary damage to the axons and Schwann cells (Rotshenker, 2011; Richner et al., 2014). Healing the damage to peripheral nerves occurs by induction of cellular responses resembling cellular activity during development(Shakhabazau et al., 2012). However, the damaged periph‐eral nerves generally regenerate only a fraction of lost motor and sensory function. There remains a need to understand the regeneration process of peripheral nerves after trauma.Lysophosphatidylcholine (LPC) stimulates nerve growth factor receptor (NGFR) expression in Schwann cells and axons (Scherer et al., 1994) by inducing acute demyelination(Pourabdolhossein et al., 2014), which releases the Schwann cells from the axon. As Schwann cells detach from the axon,they begin down‐regulating myelin associated proteins(Jessen and Mirsky, 2016) and up‐regulating developmental markers (Webber et al., 2008) and undergo molecular and gene expression changes necessary for helping with nerve repair (Jessen and Mirsky, 2016). While in this repair state,Schwann cells support axonal regeneration through the production of neurotrophins (e.g., nerve growth factor) and neurotrophic receptors (e.g., NGFRs) (Webber et al., 2008),activation of the innate immune system, formation of bands of Büngner, and activation of autophagy for myelin break‐down (Jessen and Mirsky, 2016). These changes in molecular and gene expression that convert Schwann cells to a repair phenotype are essential for promoting axon remyelination and restoring nerve function (Fan and Gelman, 1992; Cos‐gaya et al., 2002; Jessen and Mirsky, 2016). Hall et al. (1997)found that this repair state increases its NGFR to an optimal concentration between 5 and 8 days after LPC application.
Nerves can produce all the necessary factors and receptors for repair, but they are not always produced fast enough or in high enough quantities (Witzel et al., 2005). Neuro‐trophins guide axonal growth (Yu et al., 2010), however,Zochodne and Cheng (2000) found an insignificant increase in neurotrophins around the proximal nerve stump after damage. Thus, alternative methods, such as cell therapy or neurotrophin injection, are needed to provide the damaged nerve with the necessary factors of regeneration required for complete healing (Toews et al., 1997; Witzel et al., 2005). For example, Wang et al. (2016) increased a transcription factor from the central nervous system in a damaged sciatic nerve and increased the regeneration rate of the nerve. Hoyng et al. (2014) tested the effects of increasing various neurotro‐phins and found that only three neurotrophins increased axonal regeneration: brain‐derived neurotrophic factor(BDNF), nerve growth factor (NGF), and glial cell‐derived neurotrophic factor (GDNF).
Lee et al. (1994) suggested increasing NGF and NGFR levels near the lesion site to help increase the rate of axon regeneration. However, the sciatic nerve is a mixed nerve.Therefore, the treatment applied must be able to affect both motor and sensory nerves. Santos et al. (1999) and Jubran and Widenfalk (2003) have shown that administration of NGF enhances the regeneration of both the motor and sen‐sory components of rat sciatic nerve following direct nerve repair. Kemp et al. (2011) expounded upon these findings by applying a systematicin vivodose. They found that NGF works primarily through the sensory neurons but there are three primary indirect effects on motor neuron regeneration. First, administration of exogenous NGF causes upreg‐ulation of endogenous BDNF, which has been shown to be neurotrophic to motor axonsin vivo. Second, NGF stimulates production of VEGF, allowing the damaged area to be revascularized quickly and accelerate recovery. Finally, they noted that NGF has been shown to affect axonal receptivity to myelination, potentially increasing myelination in the affected area. These findings suggest that NGF will provide the desired effect of enhancing regeneration in both motor and sensory neurons. Kemp et al. (2011) also found that there is an optimal concentration of NGF needed to facilitate regeneration and that surpassing a critical level can have an inhibitory influence. Wood et al. (2016) found that LPC by itself did not provide an adequate increase in NGF and NGFRs to create a faster regeneration.
Therefore, our hypothesis for this study was that a com‐bination of LPC and NGF can produce the needed increase in both NGF and NGFRs to accelerate healing after crushed peripheral nerve injury. In order to elucidate the combined effects that LPC and NGF have on a nerve, we first established the rate of healing of a rat’s sciatic nerve under normal physiological conditions using a single dose and a single injection spaced 7 days apart. This included determining the healing rate for a crushed nerve and a nerve that received NGF without LPC. The healing rates were determined by monitoring the rat’s gait, measuring nerve conduction through electromyography, and measuring the fiber density,fiber size, fiber diameter, axon diameter, and g‐ratio of each nerve. The regeneration of the left sciatic nerves for each group of rats was compared to the undamaged right sciatic nerves at 3 and 6 weeks.
After studying the effects of a single injection of NGF,we hypothesized that multiple NGF injections could be more effective due to the short half‐life of NGF (Tria et al.,1994). However, opening the rat up each time to administer successive intraneural injections would have caused an extensive amount of trauma. To avoid this, we designed an implantable nerve guide device that allowed for transdermal injections of NGF treatments directly into the nerve (see Figure 1). Using the same criteria to measure nerve healing as above, we compared the healing effects of one, two, and three treatments of NGF in combination with a single LPC injection to the effects of one, two, and three treatments of NGF with phosphate buffered saline (PBS) injection controls after 6 weeks.

Figure 1 A diagram of the nerve guide device used for performing multiple direct intraneural injections.
Materials and Methods
Animals
Ninety‐nine female Wistar Albino rats of the speciesRattus norvegicus, weighing 250–300 g, aged 15 ± 2 weeks, were ob‐tained from a breeding colony (BYU Breeding Protocol 15‐0303) and included in this study. They were caged in groups of three until surgery, after which they were separated into individual cages. The total lifespan of the rats was kept at 21 weeks. All procedures were approved by the Institution‐al Animal Care and Use Committee (IACUC) at Brigham Young University (BYU Protocol 14‐0301).
Surgery
In the single injection portion of the study, 51 rats under‐going surgery to expose their left sciatic nerve were divided into four groups. All groups in the single injection study had an initial surgery to perform rat left sciatic nerve damage via crush injury and were given a single injection of LPC, and a second surgery one week later to inject NGF. The crush group (n= 14) had their sciatic nerves crushed without receiving either LPC or NGF. The crush‐NGF group (n=14) had their sciatic nerves crushed and received NGF. The crush‐LPC‐NGF group (n= 14) had their sciatic nerves crushed followed by an intraneural injection of LPC (same day) and then NGF (one week later). For the control group(n= 9) the left sciatic nerve was exposed but no injections were made. Rats in the control group and each experimental rat’s undamaged right sciatic nerve were used to establish normality and reduce variability in the results.
The rats were anesthetizedviaisoflurane (MWI Animal Health, Chicago, IL, USA) inhalation prior to surgery. The left sciatic nerve was exposed through a mid‐crural lateral incision. The nerve was then crushed with dressing forceps for 30 seconds at approximately 10 N of force, approximate‐ly 0.5 cm proximal to its trifurcation at the tibial, sural, and common peroneal branches. The crush‐LPC‐NGF group then received an intraneural injection of 15 μL of the LPC solution, approximately 3 mm proximal to the crush site using a 34‐gauge needle (Hamilton syringe, Sigma‐Aldrich,St. Louis, MO, USA). The LPC used in the study was egg de‐rived LPC (Sigma‐Aldrich). This was delivered to the sciatic nerve in the single injection study at 1 mg/mL in PBS & Fast Green FCF (Sigma‐Aldrich). This was done to compare to our previous study (Wood et al., 2016). When we tested the effects of multiple injections, we used 10 mg/mL (1% wt/vol)LPC. The concentration was changed for the multiple injections study to elicit a greater response than that found by Wood et al. (2016) and for a better comparison to findings from a previous study (Pourabdolhossein et al., 2014).
One week later, the sciatic nerve was re‐exposed and 15 μL of the NGF solution was injected intraneurally 3 mm distal to, but directed towards, the crush site. This was performed identically to the LPC procedure except for the injection location and substance. The NGF used in the study was hu‐man derived beta‐NGF (Sigma‐Aldrich). This was delivered to the nerves at 160 ng/mL in PBS containing Fast Green FCF dye. The Fast Green FCF provided a color to the solution for visual verification during injection. The NGF used to test the effects of multiple NGF treatments in this model was prepared as above to a concentration of 80 ng/mL.The concentrations of the NGF were chosen based on the findings of Kemp et al. (2011). The multiple injection concentration of NGF was lowered in order to not over saturate the nerves while being given multiple doses throughout the recovery process.
Rats in the experiments that tested multiple treatments of NGF received surgery according to the following protocol:An injection of 1% wt/vol LPC was administered immediately after the nerve crush injury was performed. A nerve guide(Figure 1) was placed subcutaneously during the initial operation. Through this guide, NGF (80 ng/mL) was injected directly into the nerve to enhance nerve growth on days 5, 7,and 9 post‐injury to eliminate the need for another surgery to expose the nerve. The nerve guides were 3D‐printed by the Brigham Young University Precision Machine Lab from a FormLabs White (Somerville, MA, USA) proprietary resin.Two holes were drilled near the top of the device, allowing the nerve guide device to be sutured to surrounding muscle fascia. The device was positioned in a manner that allowed the sciatic nerve to remain securely in the channel of the device during the rats’ normal movement. The interior diameter of the channel was designed to be large enough to avoid any possibility of constricting the nerve while still allowing accurate needle placement during injections. After the device was implanted into the rat, transdermal injections directly into the sciatic nerve could be performed by palpating the top of the device and sliding the inserted needle along the face of the guide until it rested in the channel. The exact injection protocol is described in the Methods section.
In the multiple NGF injection experiment, 48 rats were divided into eight groups: crush + PBS control (CS,n= 7),crush + LPC + PBS (CL,n= 5), crush + PBS + 1, 2 or 3 NGF injections (CN, C2N, and C3N,n= 7, 5, and 6 respectively),and crush + LPC + 1, 2, or 3 NGF injections (CLN, CL2N,CL3N,n= 6, 6, and 6). The additional four groups included additional NGF groups, and differentiated the multiple injections study from the single‐injections study. The crush+ LPC + PBS group received an intraneural injection of 15 μL 1% wt/vol LPC after the crush injury was performed, and was given PBS injections as a control at the same time points as the NGF injections for the other groups. The groups treated with both LPC and NGF received LPC injections as described above during the surgical procedure. Each group received 1, 2, or 3 NGF injections after the initial crush injury, and treatments were given at 5, 7, and 9 days post‐injury for the groups that received more than one NGF injection.The crush control group had the same surgery performed without NGF or LPC treatments, but the nerve guide was still attached, and an injection of phosphate‐buffered saline solution (PBS) was given in place of NGF or LPC. The NGF groups received NGF injections via the nerve guide at 5, 7,and 9 days post‐operatively as outlined above, and a PBS injection in place of an LPC injection during the initial surgery. Groups receiving 1 NGF treatment were administered NGF 5 days post‐injury, and PBS as an injection control at 7 and 9 days. Likewise, groups receiving 2 treatments of NGF received injections at 5 and 7 days post‐injury and a PBS injection at 9 days. Groups receiving 3 NGF injections were treated with NGF at 5, 7 and 9 days post‐injury. Data for all groups were compared to baseline values (week 0) measured immediately prior to nerve injury and LPC injection.
Isoflurane inhalant 2–3% in medical grade oxygen was introduced to ratsviaprecision vaporizer (VAS 2001R, Veterinary Anesthesia Systems, Inc., Phoenix, AZ, USA) for 5 minutes in a closed container to induce a plane of anesthesia. Sufficient sedation was confirmed through analgesic ex‐ams by pinching the distal limbs prior to the procedure. The rat was then maintained on inhalant isoflurane anesthesia deliveredviamask with a calibrated precision vaporizer. The surgical site was aseptically prepared from the lumbosacral region, superficially to the gluteus superficialis muscle, and the lateral aspect of the left rear leg. Sterile instruments, drape and aseptic technique were used to perform the procedure.Animals received the nonsteroidal anti‐inflammatory drugs carprofen (BioServ, Flemington, NJ, USA) 4.4 mg/kg preop‐eratively and buprenorphine (Sigma‐Aldrich, St. Louis, MO,USA) 0.1 mg/kg intraperitoneally immediately prior to sur‐gery to provide analgesia. A 1 cm incision was made parallel with and immediately distal to the femur. Two stainless steel strabismus blunt‐ended hooks (and reverse scissoring if necessary) were used to aid in separation of the fascial plane between the rectus femoris and biceps femoris muscles,exposing the sciatic nerve. Blunt‐ended strabismus hooks were then used to separate the sciatic nerve from surrounding fascia to allow proper placement of the nerve guide. The exposed sciatic nerve was crushed with surgical forceps for a period of 20 seconds, followed by a second crush of 10 seconds 2 mm proximal to the trifurcation of the sciatic nerve.Additionally, a strabismus hook was inserted under the nerve distal to the crush in order to expose the nerve for in‐traneural injection, being careful to avoid muscle groups be‐neath the nerve. A 34‐gauge needle (Hamilton syringe) was then used to inject LPC containing Fast Green FCF solution into the sciatic nerve. For rats not receiving LPC, PBS was used as a sham treatment and injected into the nerve. The tracer Fast Green FCF was used to confirm the success of the injection.
Following LPC injections, the sciatic nerve was placed in the channel of a previously sterilized nerve guide device made of FormLabs White resin. The device was positioned to avoid impeding normal gait, while also allowing direct nerve injections through the skin by palpating the subcutaneous nerve guide tab, then placing a 27 ½ ‐gauge needle through the skin and gently gliding it across the surface of the guide into the protective channel surrounding the crush injured sciatic nerve. A 0.75 inch long 30‐gauge needle attached to a Hamilton syringe was then placed through the puncture hole to deliver the PBS or NGF solution. The sciatic nerve,~ 0.5 cm proximal and distal to the crush injury, was placed in the nerve guide’s channel to provide a protective sleeve to allow accurate injection into the nerve without compressing it. The nerve guide was sutured in place to surrounding fascia with 5‐0 or 6‐0 mono filament suture (polypropylene,non‐absorbable) affixed to its tab through two pre‐drilled 1 mm openings (Figure 1). The skin incision was closed using wound clips as necessary.
For subsequent sciatic nerve treatments, after aseptic preparation of the site, the 30‐gauge needle was similarly inserted through the skin, gently moved down the face of the guide and into the nerve at the bottom of the channel for intraneural injections. Delivery of 25 μL of 80 ng/mL NGF was slowly injected into the nerve over a period of 2 minutes. Depending on the experimental group, these injections were performed at 5, 7, and 9 days post‐injury. All other experimental groups received intraneural injections of phosphate‐buffered saline at the same time points according to the same procedure.
Gait analysis
The Basso‐Beattie‐Bresnahan (BBB) scale characterizes rat gait and ranges from 0‐21 (Barros Filho and Molina, 2008).The score tracks recovery and categorizes combinations of rat joint movement, hind limb movements, stepping,forelimb and hind limb coordination, trunk position and stability, paw placement, and tail position. Each rat was as‐sessed one day prior to its surgery using the BBB scale. After surgery, the rats were assessed three times a week using the BBB scale for the duration of the experiment. While the BBB scale may not be the most accurate indication of function for non‐transection injuries, the BBB scale does provide a general indication of overall function and allows for accurate determination of the time at which the leg is weight‐bearing(Wang et al., 2009).
Electromyography
Each rat received transdermal electromyography to measure nerve conductivity. Exact electrode placement was the same as described by Wood et al. (2016). Briefly, two electrodes were placed above the sciatic injury with the stimulating electrode being placed in the sciatic notch. Two electrodes were placed below the sciatic injury with the stimulating electrode being placed near the sciatic nerve branching by the knee. Two electrodes were placed in the ankle with the stimulating electrode being placed in the calf behind the tibia. Lastly, two electrodes were placed in the foot with the recording electrode being placed in the lateral side of the pad of the foot. The non‐stimulating and non‐record‐ing electrodes served as references and were placed 5 mm away from other electrodes subcutaneously. The stimulating electrodes applied a voltage to the nerve and the recording electrode measured the response. Stimulation of the elec‐trodes evoked compound muscle action potentials (CMAPs)that were measured using a National Instruments PXI‐1011 Chassis multi‐function data acquisition instrument (Nation‐al Instruments, Austin, TX, USA) and LabVIEW software(National Instruments, Austin, TX, USA). The rats in each group were assessed one day prior to surgery to establish pre‐surgery amplitudes and velocities. Post‐surgery, the rats received electromyography three days a week for the duration of the rat’s experimental process, either three or six weeks. The first post‐surgery electromyography was per‐formed within 24 hours of the surgery.
The electromyography testing was performed under the same anesthesia regime as for surgery. Isoflurane inhalant 2–3% in medical grade oxygen was introduced to rats via precision vaporizer for 5 minutes in a closed container to induce a plane of anesthesia. Sufficient sedation was confirmed through analgesic exams by pinching the distal limbs prior to placing the needles. The rat was then maintained on inhalant isoflurane anesthesia delivered via mask with a calibrated precision vaporizer for the duration of the electro‐di‐agnostical testing.
Histopathological staining
Phosphate buffered saline (PBS) and Karnovsky’s fixative were used for nerve preservation and fixation, respective‐ly. Spurr’s resin was used for embedding the dehydrated nerves. All solutions were prepared as previously described by Wood et al. (2016).
For histopathologic analysis of the nerve, 1 cm of the nerve was removed 3 mm proximal to the trifurcation. The nerve was then analyzed according to the procedure described by Wood et al. (2016). The excised nerve segments were placed in Karnovsky’s fixative and then in Spurr’s resin. Nerve sections were cut 1 μm thick beginning at the distal end. This provided an ideal area for analyzing the damage and growth of the nerve due to the treatments. Nerve section images were obtained using a Pentax K100 camera (Ricoh, Malvern,PA, USA) attached to a Zeiss Axiovert 135 microscope (Zeiss,Thornwood, NY, USA) and analyzed using ImageJ software(U. S. National Institutes of Health, Bethesda, MD, USA).A custom semi‐automated process (Wood et al., 2016) in ImageJ software, based on the procedure of Urso‐Baiarda and Grobbelaar (2006), was used to calculate morphometric parameters for each nerve. Calculated morphometric parameters were total fascicle areas, myelinated fiber counts,fiber densities, fiber packing, and mean g‐ratio values. A fiber consisted of the axon and myelin together.
Statistical analysis
In the single injection portion of the study, total fascicle areas, myelinated fiber counts, fiber densities, fiber packing,mean g‐ratio values, and conduction amplitudes were com‐pared between all the groups for both 3‐week and 6‐week time points using a one‐way analysis of variance with a Dunnett’s T3post-hoctest.
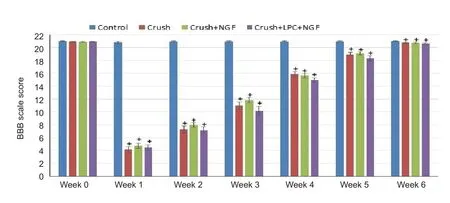
Figure 2 Effects of LPC and NGF on gait of rats after sciatic nerve crush injury.

Figure 3 Representative electromyographic traces after sciatic nerve crush injury in rats treated with LPC and NGF in the singleinjection study.

Figure 4 Effects of lysophosphatidylcholine (LPC) and nerve growth factor (NGF) on amplitude of compound muscle action potential(CMAP) in the Control, Crush, Crush-NGF, and Crush-LPC-NGF groups across a 6-week period in the single injection study.

Figure 5 Effects of lysophosphatidylcholine (LPC) and nerve growth factor (NGF) on amplitude of compound muscle action potential(CMAP) in the Crush + PBS Control (CS), Crush + LPC (CL), Crush+ NGF (CN), Crush + 2NGF (C2N), Crush + 3NGF (C3N), Crush +LPC + NGF (CLN), Crush + LPC + 2NGF (CL2N), and Crush + LPC+ 3NGF (CL3N) groups across all six weeks in the multiple-injection study.
In the experiment studying the effects of multiple treatments of NGF in combination with LPC, conduction amplitudes were compared between all the groups and time points using a two‐way analysis of variance with a Tukey’s multiple comparisons test.
Outliers in data sets were identified using interquartile criteria prior to statistical tests (outliers were above quartile 3 ±1.5 of the interquartile range (IQR) or below quartile 1 ± 1.5 IQR for the data set).P‐values below 0.05 were considered significant. All data were represented as the mean ± SEM.
Results
Healing of injured sciatic nerve
Gait analysis results are shown in Figure 2 beginning with the day before surgery, week 0, through week 6 post‐injury.The control group was significantly different from the other groups until week 6 (P< 0.001). There was no statistical difference between the crush, crush‐NGF, and crush‐LPC‐NGF groups. Each of these groups lost nearly all use of the left leg distal to the knee after surgery. All animals that received a crush injury returned to full gait by week 6.
Electrophysiological function of injured sciatic nerve
Representative CMAP amplitudes are displayed in Figure 3. The complete data set is shown in Figure 4. There was a small difference in the baseline value (week 0) for each of the groups, but none had statistically significant differences.After surgery, a sharp decrease in CMAP amplitude was observed in all of the groups that received a crush. In these groups, CMAP amplitude slowly increased across the 6‐week period until they recovered to 15‐18% of the baseline value.CMAP amplitude at 1, 2, 3, 4, 5 and 6 weeks were significantly different from that at 0 week and the control (P< 0.01),but none of these groups was statistically different from each other at each time point. Neither NGF nor the combination of NGF and LPC was able to restore the action potential by 6 weeks after crush injury.
In the multiple injections experiment, CMAP amplitude was significantly different at weeks 1, 2, 3, 4, 5 and 6 post injury from baseline value (at week 0) in all groups (Figure 5). Baseline values varied between groups but were not statistically different from each other. There was significant difference in CMAP difference between groups at any time point (P= 0.085 or greater for all tests).
Morphology in injured sciatic nerve
Histopathologic analysis was performed to determine the healing of damaged nerves and the Schwann cell’s ability to remyelinate axons. Regenerated nerve fiber profiles were examined at 3 and 6 weeks after damage.
Table 1 lists total fascicular area, total myelinated fiber counts, fiber densities, fiber packing and g‐ratio of the Control, Crush, Crush‐NGF, and Crush‐LPC‐NGF groups at 3 weeks post injury. There was no significant difference in any parameter between groups. In Table 2, the total fascic‐ular area, total myelinated fiber counts, fiber densities, fiber packing and g‐ratio were compared among Control, Crush,Crush‐NGF, and Crush‐LPC‐NGF groups after 6 weeks of healing. There was no significant difference in any parameter between groups at 6 weeks post injury.
Discussion
In the single NGF injection study, injured sciatic nerve re‐generated after administration of a single dose of NGF or a combination of single doses of NGF and LPC. Below, we dis‐cuss the rationale for the original hypothesis for this work,the benefits of the study, alternative explanations based on the observations, and recommendations for future work.
The original hypothesis of this work was that the combination of LPC and NGF would speed up the regeneration of crushed nerves. This hypothesis was based on the knowledge that LPC causes Schwann cells to undergo demyelination and upregulation of NGF receptors (Stoll et al., 1993) and that NGF is a necessary molecule for growth and healing and affects both motor and sensory neurons (Santos et al.,1999; Jubran and Widenfalk, 2003; Kemp et al., 2011). It was anticipated that the LPC would help speed up the degeneration process, and allow the nerve to start the regeneration process sooner. The increase in NGF would then help speedup the regeneration process through increased signaling and saturation of the NGF receptors and by stimulating the production of other endogenous growth factors. The results after single injections of LPC and NGF do not support this hypothesis. However, we believed this hypothesis should not yet be abandoned since the timing, doses and number of injections needed to be tested further.

Table 1 Morphology of injured sciatic nerve in the single injection experiment at 3 weeks post injury
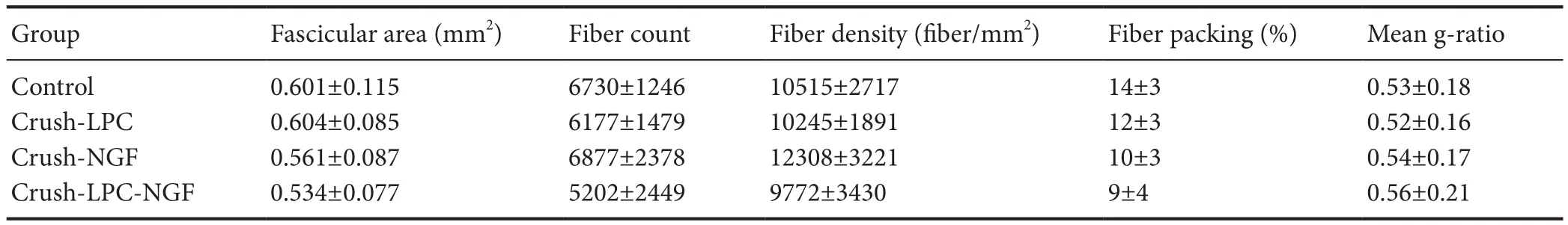
Table 2 Morphology of injured sciatic nerve in the single injection study at 6 weeks post injury
To investigate whether continuous stimulation of growth factors could accelerate nerve regeneration, we performed a second set of experiments using multiple injections of NGF in combination with LPC. Due to the short half‐life of NGF(Tria et al., 1994), we hypothesized that multiple injections of NGF would increase the rate of healing. In order to administer NGF directly into a crushed nerve when the epineurium is still intact, it is necessary to perform an injection. In the first experiment where a single injection of NGF was given, a sec‐ond procedure was performed in each rat to expose the nerve,allowing for a direct injection. When administering multiple injections of NGF to rats, however, a separate surgery for each treatment would be traumatic and could potentially worsen recovery in other ways. This is why the implantable nerve guide device was used to perform multiple direct in‐traneural injections, avoiding the repeated openings of the cut site. However, there were no significant improvements in either regeneration rate or quality between groups according to electrophysiological parameters after multiple injections.
The main benefit of conducting this study was establishing the baseline effects of combining LPC with NGF injections in a rat model of crush injury. Gait and morphological parameters were used to investigate the healing rate. Using age‐matched experimental groups, there was no difference measured in the regeneration between a crushed nerve that received no treatment, a crushed nerve that received NGF and a crushed nerve that received the LPC‐NGF combination. The morphological parameters: mean g‐ratio, total myelinated fiber count, and fiber diameter parameters can reflect Schwann cell damage and healing. Because the morphological parameters were only measured at 3 and 6 weeks, so the data only reveal whether Schwann cells have healed from the damage or not. These parameters show that the Schwann cells in the Crush, Crush‐NGF, and Crush‐NGF‐LPC groups were in the process of repairing at 3 weeks and had fully healed by week 6. The electrophysiological parameter ‐ CMAP amplitude ‐ is the characteristic of the extent of axon damage and healing.The electrophysiological parameters suggested that crushed nerves were still statistically different from the control group at 6 weeks. When we compared the results of rats receiving multiple injections of NGF and an LPC injection to those receiving only NGF, or those receiving only a single injection of NGF, we also found that the results between weeks 0 and 6 were different from each other. However, there were still no differences between groups at any time point.
Because the electrophysiological parameters differ from the gait and morphological parameters, two morphological time points were insufficient to determine a difference in the healing between the groups. These results can be compared to the healing profiles of Crush, LPC and Crush‐LPC groups without receiving NGF, as reported in Wood et al. (2016).The discrepancy of the electrophysiological parameters with the gait and morphological parameters show that additional measurement techniques were needed. Navarro (2016) has provided a review of many analysis techniques for nerve studies and whether they test sensory or motor neurons or both.A sciatic functional index test or a grip strength test could provide additional insights on how well the motor neurons re‐covered at the given time points. The tests chosen were mainly to compare with our previous report (Wood et al., 2016).
Schwann cells recruit macrophages to the injury site. Mac‐rophages then play an important role in the degeneration process by clearing debris from the injury site in preparation for regeneration (Stoll et al., 1989). Once the injury site has been cleared, signaling molecules (e.g. NGF) play an important role in directing the growth cone towards the distal end of the nerve. These signaling molecules work through a concentration gradient, meaning that the growth cone will travel from lower concentration to higher concentration.In order to be effective, the signaling molecule then needs to have its highest concentration near the distal end of the nerve to provide correct direction to the growth cone. Multiple signaling molecules with concentration gradients in different directions lead to “crosstalk,” and result in misdirected growth of the growth cone (Yu et al., 2010). By ap‐plying only NGF at an optimal concentration near the distal end of the nerve, we hoped to eliminate any misdirection and increase the healing rate.
There may be several reasons why LPC was not able to in‐crease the rate of recovery on its own. One of those reasons was that with the addition of LPC, more growth factors may be needed for regeneration due to the increased Schwann cell demyelination. Adding NGF in combination with LPC did not speed up the healing process, even when multiple injections of NGF were administered. Our results show that there was minimal recovery of the motor neurons. The results of Kemp et al. (2011) led us to believe that the application of NGF provided adequate stimulation to speed up the recovery of the motor neurons in conjunction with the increase in recovery of the sensory neurons. But our data showed that while NGF may provide indirect effects upon motor neurons, these effects were not significant enough to increase the rate at which the motor neurons healed with single and multiple injections. From these results, we postulate three reasons why the expected outcome was not achieved. First, the body maybe treats LPC and crush as two separate injuries, and therefore, LPC is not affecting the degeneration or regeneration processes at all. Second, the nerve may need more stimulations of growth factor than injections to accelerate the regeneration process. A third reason could be that the NSAID drug carprofen provides additional myelin degradation (Wang et al., 2009) and therefore provides a similar effect to LPC. Thus there was no difference in the degeneration process. Additionally, the differences between treatment groups would be more pronounced in a more severe injury, such as a transection.
A further study is needed to determine if any of the pro‐posed reasons above are correct. A study could examine macrophage recruitment, signaling molecules and total extent of the damage area with an LPC injection, a nerve crush, and a combination of an LPC injection and a nerve crush. It may also be that the doses of NGF and LPC need to be adjusted in addition to altering when the injections are given. Kemp et al. (2011) investigated the dose response of NGF in a transection injury; it could be worthwhile to determine the dose‐response relationship of NGF and LPC in combination, as well whether the relationship in a transection injury differs from that of a crush.
In conclusion, this study demonstrated that the combination of single or multiple injections of LPC and NGF did not affect the overall healing rate of a crushed sciatic nerve.There was no difference in any of the measured parameters between the Crush group and the Crush‐NGF‐LPC group at any time point. With the findings that the electrophysio‐logical parameters differed from the gait and morphological parameters, it was concluded that using just two morpho‐logical time points was insufficient to determine a difference in the healing of damaged sciatic nerves in rats. Future work is needed to test multiple injections of LPC and NGF at various time points (> 3) and doses.
Acknowledgments:We express our thanks to the following undergraduate students at Brigham Young University who assisted with surgeries, animal care, and electrophysiology measurements: Tyler Brown, Michael Chamberlain, Daniel Elliot, Mitchel Faulkner, Michael Gebhard, Sarah Hansen, Taylor Hansen, Jonathan Jacobs, Lincoln Kartchner, Matthew Landeen, Jordan Miller, Adam Millet, Emil Morco, Trent Taylor, David Walton, Sarah Coffin,Kirk Harter, Michael Lange, Liza Jarman, Heather Mills, Blaise Hill, Kayden Barber, Evan Bogdan, David Lehman, Caleb Dixon, Calvin Panah, Traeden Wilson, and Stephen Wirthlin. Dr. Keiichiro Susuki from Wright State University was helpful in teaching us how to perform the surgeries and analyzing the data.
Author contributions:RLW, KSK, BLR, SCS and ADC conceived the experiments; KSK, ADT, and MNR conducted the experiments; RLW, KSK, GDB and ADC analyzed the results. ADC, BLR, SCS, and WGP edited the manuscript. All authors reviewed and approved the final version of this paper for publication.
Conflicts of interest:The authors declare no conflicts.
Financial support:Funding for animal care and supplies was provided by Brigham Young University, the Don B. Olsen Mentorship to RLW, and a Brigham Young University Office of Research and Creative Activities Student Mentored Research Grant to KSK. The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement:This study was approved by the Institutional Animal Care and Use Committee (IACUC) at Brigham Young University (BYU Protocol 14-0301).
Biostatistics statement:The statistical methods of this study were reviewed by the biostatistician of Brigham Young University in Provo, UT, USA.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Barros Filho TE, Molina AE (2008) Analysis of the sensitivity and reproducibility of the Basso, Beattie, Bresnahan (BBB) scale in Wistar rats.Clinics (Sao Paulo) 63:103‐108.
Cosgaya JM, Chan JR, Shooter EM (2002) The neurotrophin receptor p75(NTR) as a positive modulator of myelination. Science 298:1245‐1248.
Fan X, Gelman BB (1992) Schwann cell nerve growth factor receptor expression during initiation of remyelination. J Neurosci Res 31:58‐67.
Hall SM, Li H, Kent AP (1997) Schwann cells responding to primary demy‐elination in vivo express p75(NTR) and c‐erbB receptors: a light and electron immunohistochemical study. J Neurocytol 26:679‐690.
Hoyng SA, De Winter F, Gnavi S, de Boer R, Boon LI, Korvers LM,Tannemaat MR, Malessy MJA, Verhaagen J (2014) A comparative morpho‐logical, electrophysiological and functional analysis of axon regeneration through peripheral nerve autografts genetically modified to overexpress BDNF, CNTF, GDNF, NGF, NT3 or VEGF. Exp Neurol 261:578‐593.
Jessen KR, Mirsky R (2016) The repair Schwann cell and its function in regen‐erating nerves. J Physiol 594:3521‐3531.
Jubran M, Widenfalk J (2003) Repair of peripheral nerve transections with fibrin sealant containing neurotrophic factors. Exp Neurol 181:204‐212.
Kemp SW, Webb AA, Dhaliwal S, Syed S, Walsh SK, Midha R (2011) Dose and duration of nerve growth factor (NGF) administration determine the extent of behavioral recovery following peripheral nerve injury in the rat.Exp Neurol 229:460‐470.
Lee K, Bachman K, Landis S, Jaenisch R (1994) Dependence on p75 for inner‐vation of some sympathetic targets. Science 263:1447‐1449.
Navarro X (2016) Functional evaluation of peripheral nerve regeneration and target reinnervation in animal models: a critical overview. Eur J Neurosci 43:271‐286.
Pourabdolhossein F, Mozafari S, Morvan‐Dubois G, Mirnaja fi‐Zadeh J, Lo‐pez‐Juarez A, Pierre‐Simons J, Demeneix BA, Javan M (2014) Nogo recep‐tor inhibition enhances functional recovery following lysolecithin‐induced demyelination in mouse optic chiasm. PLoS One 9:e106378.
Richner M, Ulrichsen M, Elmegaard SL, Dieu R, Pallesen LT, Vaegter CB(2014) Peripheral nerve injury modulates neurotrophin signaling in the peripheral and central nervous system. Mol Neurobiol 50:945‐970.
Rotshenker S (2011) Wallerian degeneration: the innate‐immune response to traumatic nerve injury. J Neuroinflammation 8:109.
Santos X, Rodrigo J, Hontanilla B, Bilbao G (1999) Regeneration of the motor component of the rat sciatic nerve with local administration of neurotroph‐ic growth factor in silicone chambers. J Reconstr Microsurg 15:207‐213.
Scherer S, Wang D, Kuhn R, Lemke G, Wrabetz L, Kamholz J (1994) Axons regulate Schwann cell expression of the POU transcription factor SCIP. J Neurosci 14:1930‐1942.
Shakhbazau A, Kawasoe J, Hoyng SA, Kumar R, van Minnen J, Verhaagen J, Midha R (2012) Early regenerative effects of NGF‐transduced Schwann cells in peripheral nerve repair. Mol Cell Neurosci 50:103‐112.
Stoll G, Griffin JW, Li CY, Trapp BD (1989) Wallerian degeneration in the peripheral nervous system: participation of both Schwann cells and macro‐phages in myelin degradation. J Neurocytol 18:671‐683.
Stoll G, Li CY, Trap BD, Griffin JW (1993) Expression of NGF‐receptors during immune‐mediated and lysolecithin‐induced demyelination of the peripheral nervous system. J Neurocytol 22:1022‐1029.
Tria MA, Fusco M, Vantini G, Mariot R (1994) Pharmacokinetics of Nerve Growth Factor (NGF) Following Different Routes of Administration to Adult Rats. Exp Neurol 127:178‐183.
Toews AD, Hostettler J, Barrett C, Morell P (1997) Alterations in gene ex‐pression associated with primary demyelination and remyelination in the peripheral nervous system. Neurochem Res 22:1271‐1280.
Urso‐Baiarda F, Grobbelaar AO (2006) Practical nerve morphometry. J Neu‐rosci Methods 156:333‐341.
Wang X, Budel S, Baughman K, Gould G, Song KH, Strittmatter SM (2009)Ibuprofen enhances recovery from spinal cord injury by limiting tissue loss and stimulating axonal growth. J Neurotrauma 26: 81‐95.
Wang Y, Li W, Sun P, Jin Z, Liu G, Deng L, Guan L (2016) Sciatic nerve regeneration in KLF7‐transfected acellular nerve allografts. Neurol Res 38:242‐254.
Webber CA, Xu Y, Vanneste KJ, Martinez JA, Verge VMK, Zochodne DW(2008) Guiding adult mammalian sensory axons during regeneration. J Neuropathol Exp Neurol 67:212‐222.
Witzel C, Rohde C, Brushart TM (2005) Pathway sampling by regenerating peripheral axons. J Comp Neurol 485:183‐190.
Wood RL, Pitt WG, Steffensen SC, Cook AD (2016) A comparison of lyso‐phosphatidylcholine and crush injury in a rat model of sciatic nerve regen‐eration. Adv Tissue Eng Regen Med Open Access 1:43‐50.
Yu LM, Miller FD, Shoichet MS (2010) The use of immobilized neurotrophins to support neuron survival and guide nerve fiber growth in compartmen‐talized chambers. Biomaterials 31:6987‐6999.
Zochodne DW, Cheng C (2000) Neurotrophins and other growth factors in the regenerative milieu of proximal nerve stump tips. J Anat 196:279‐283.
- 中国神经再生研究(英文版)的其它文章
- Weak phonation due to unknown injury of the corticobulbar tract in a patient with mild traumatic brain injury: a diffusion tensor tractography study
- Exosomes: a novel therapeutic target for Alzheimer’s disease?
- Inhibition of retinal ganglion cell apoptosis:regulation of mitochondrial function by PACAP
- Trillium tschonoskii maxim extract attenuates abnormal Tau phosphorylation
- Association between Alzheimer’s disease pathogenesis and early demyelination and oligodendrocyte dysfunction
- Nogo receptor expression in microglia/macrophages during experimental autoimmune encephalomyelitis progression