Functional changes after spinal lesions: implications for interventions
We have known for many years that the spinal cord can generate some basic locomotor outputs under specific experimental conditions with‐out any input from the brain or the periphery (Stuart and Hultborn,2008). However, when inputs from the brain are lost following spinal cord injury (SCI), the mammalian spinal cord is unable to generate normal, goal‐directed locomotor outputs. In contrast, lower vertebrates spontaneously regenerate axons across lesion sites and recover locomotor function after complete spinal cord lesions (Cohen et al., 1988). The major focus of research into SCI in mammals has been to replicate this lower vertebrate capability by promoting the regeneration of lesioned axons or the sprouting of processes from spared axons, with the aim of reconnecting the spinal cord and thus repairing the damage caused by the injury (Steward et al., 2012). It is not that regeneration cannot occur in mammals, but it is instead actively inhibited. Why this inhibition has evolved in mammals is unknown. It would be useful to consider this question as it may help to explain why the various regeneration strategies that can successfully overcome this inhibition have so far failed to translate into an effective treatment for SCI (Steward et al., 2012). This article reviews work that we have done on the functional changes after recovery from SCI in the lamprey (Parker, 2017), and how these changes may relate to functional recovery in mammalian systems.
The focus on regeneration after SCI seems obvious given that the effects of the injury are caused by the disconnection of the spinal cord from the brain. Regeneration will be necessary for recovery, as some signal needs to be relayed across the lesion site for goal‐directed movement. Regeneration could in principle be replaced by artificial systems that detect signals above the lesion site and relay them electrically to the spinal cord below the lesion. However, this approach faces significant technical challenges (e.g., correctly decoding signals above the lesion site and sending them to appropriate locations in the spinal cord) that would be obviated with intrinsic regeneration. Whilst regeneration would seem necessary for recovery, a distinction has to be made between it being both necessary and sufficient. Something can be sufficient without being necessary if there is redundancy in the system,and it can be necessary without being sufficient if it is one of several essential contributing factors. Regeneration could thus be necessary but not sufficient for recovery if regenerated inputs have to interact with other components. These obvious additional components are spinal cord locomotor networks and sensory systems.
As in other systems, regeneration has been the dominant approach to SCI in the lamprey, a lower vertebrate system for studies of spinal function and recovery after spinal cord lesions (Cohen et al., 1988).However, while regeneration and functional recovery both occur spontaneously in lamprey, regeneration cannot be the only factor underlying recovery. For example, regeneration is incomplete (0–70% of the prelesion value), regenerated axons project short distances below the lesion site, and they can project to ectopic regions compared to the un‐lesioned spinal cord (Cohen et al., 1988). For regeneration to be both necessary and sufficient for recovery in lamprey would logically require that a significant proportion of the normal complement of descending inputs is redundant, and that their specific projections are not import‐ant to normal locomotor function.
Studies of regeneration in lamprey have provided important insights(Selzer, 2003). However, in contrast to the extensive (and in some cases repeated) work on regeneration, very little work has been done on other aspects that could influence recovery. This is despite the fact that the lamprey has been used as a model system for studying the function of spinal cord locomotor networks. We have thus started to examine spinal cord properties below lesion sites. These analyses have identified various changes in cellular (excitability, resting potential) and synaptic properties (increases or decreases of inhibitory and excitatory inputs)below the lesion site 8 weeks after lesioning (i.e., when animals have typically recovered locomotor function). These effects can together influence the excitability of the spinal cord. Sensory feedback is also potentiated below the lesion site (Hoffman and Parker, 2011). Some of these effects correlate with the degree of locomotor recovery, whereas others show no correlation. It seems likely that a range of effects could underlie recovery, no single effect being sufficient or maybe even necessary (Hoffman and Parker, 2011). What these analyses clearly show is that the pre and post‐lesion spinal cord are functionally different.This is not surprising: similar effects occur in the mammalian spinal cord (e.g., Courtine et al., 2009), and even this is unsurprising given that clinical evidence of changes below lesion sites has been known for many years (e.g., spasticity).
Changes below lesion sites may complement regeneration by compensating for the differences in the number and properties of regenerated inputs after lesioning. The descending input,x, will act with spinal cord locomotor networks,y, and sensory feedback,z, to generate a particular output. Each of these influences will carry a certain weight,wx+wy+wz=output. A reduction in one component could thus generate the same output if it was matched by a compensatory change in the weight of the others. This would be a homeostatic‐like mechanism where changes below the lesion site allow a pre‐lesion output to be generated by compensating for the reduction in the descending input.However, assigning single values to these aspects is grossly simplistic.For example, various descending inputs run from different regions of the brain (processing different signals) in different spinal cord tracts to generate region‐specific effects. Recovery of the descending input thus has to consider more than just the percentage of regenerated fibres. A similar point could be made about spinal cord networks, but here the various components and their changes will at least interact to set a certain degree of spinal cord excitability to generate a particular summed output.
We have also shown changes above the lesion site in lamprey (Park‐er, 2017). Similar effects occur in the mammalian and human spinal cord (Grasso et al., 2004; Courtine et al., 2009). These changes may be adaptations that generate stronger local activity above the lesion site that is relayed propriospinally to activate the spinal cord below the lesion. Alternatively, they may reflect a compensatory adjustment to the disturbance of ascending inputs, or to the degeneration of axons above the lesion site.
In addition to changes in spinal cord networks and sensory feed‐back, we have also examined the properties of synaptic inputs from regenerated axons below the lesion site. Inputs from individual regenerated axons have synaptic properties that match those in unlesioned animals. In a homeostatic mechanism, inputs from individual axons might be expected to increase to compensate for the reduction in their overall number. That this does not happen may reflect the heterogeneity and functional specificity of descending inputs: an increase in one input will not necessarily compensate for the reduction of another.However, the maintenance of individual synaptic inputs does involve a compensatory response. Individual regenerated axons make far fewer synaptic contacts below a lesion site than unlesioned axons (Oliphint et al., 2010). This should reduce the synaptic input from individual ax‐ons, but this is prevented by a compensatory increase in postsynaptic response below the lesion site. In contrast to inputs below the lesion,descending inputs above the lesion site are altered to become function‐ally stronger (Parker, 2017). This is due to presynaptic and postsynap‐tic changes, and may again reflect the need to strengthen local signals across the lesion site.
There are thus changes in the spinal cord above and below the lesion site at each of the three general levels of the spinal cord, descending inputs, locomotor networks, and sensory feedback (Figure 1; Parker,2017). Interactions between these components also differ depending on the degree of recovery (Hoffman and Parker, 2011). The simple conclusion of this work is that repairing the lesioned spinal cord is not equivalent to re‐connecting the two cut ends, as you would with a cut electrical cable. In the latter case the two ends are passive and the fault is caused by the interruption of the signal between the two sides, making re‐connection the necessary and sufficient strategy. But in the spinal cord, the areas either side of the lesion site are active, not passive,structures that contribute to normal functions and are thus capable of modification.
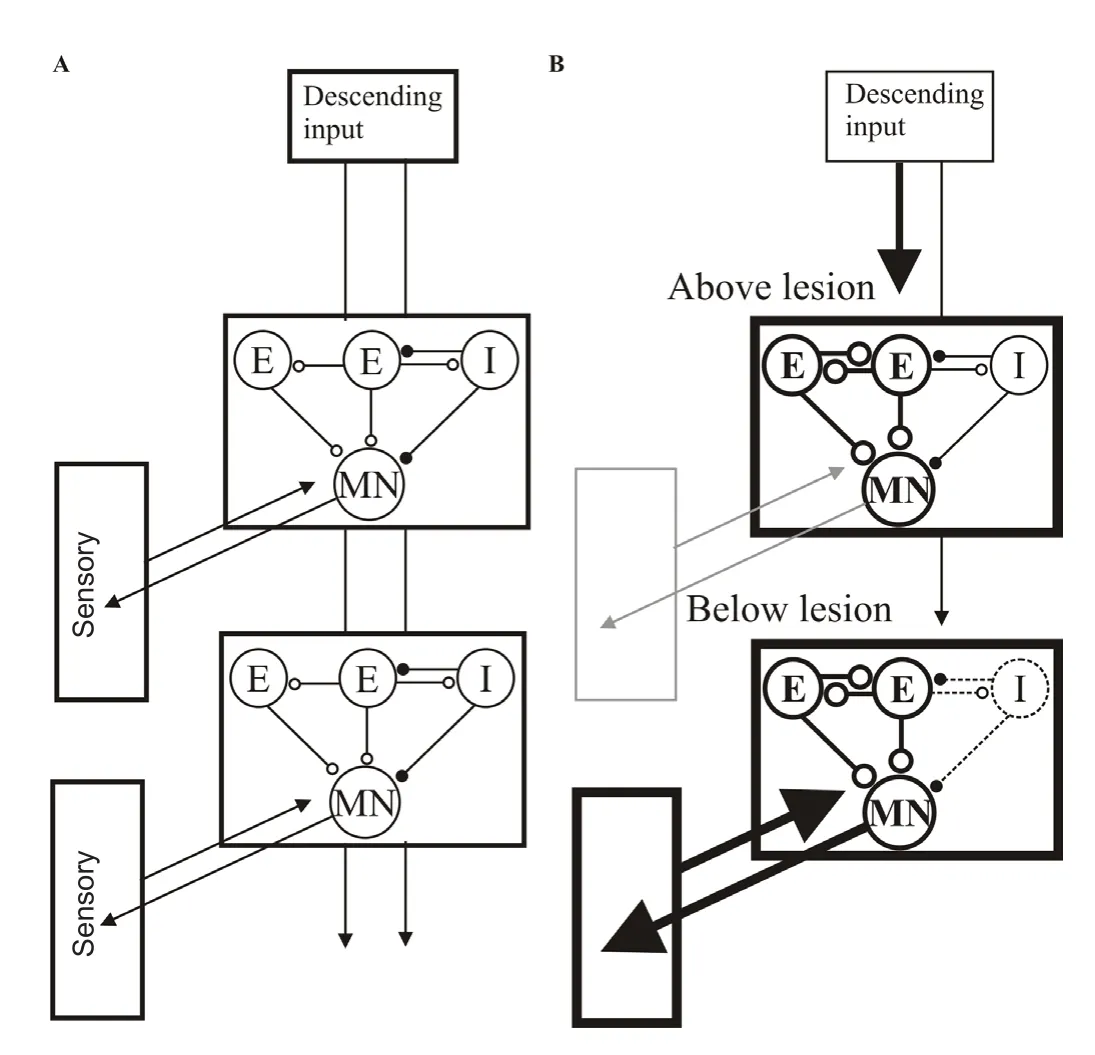
Figure 1 Summary of the changes after recovery from spinal lesions in the lamprey (reviewed in Parker, 2017).
While SCI research focuses on promoting regeneration, other as‐pects have been examined (e.g., Courtine et al., 2009; Johnson et al.,2017). The presence of locomotor networks below the lesion site has inspired attempts to recruit these networks by electrical or pharmacological stimulation. The pharmacological approach has its basis in the concept of fictive locomotion: isolated spinal cord networks can be pharmacologically activated to generate locomotor behaviour (Stuart and Hultborn, 2008). Fictive locomotion is claimed to match normal locomotion, but this claim is often negated by the data used to support it (while the pattern can look the same in the best cases, the frequency is usually an order of magnitude slower; see Parker, 2017). Fictive activity also varies in whether and how it is evoked by the same pharmacological stimulus (Parker, 2017). This lack of control is an obvious issue for any pharmacological activation strategy. Logically, for fictive activity to match normal locomotion requires accepting that normal locomotion does not involve descending inputs from the brain or sensory feedback, both of which are absent under fictive conditions, and that the precise temporal and spatial properties of transmitter systems,which are not replicated in pharmacologically‐evoked fictive locomotion, is unnecessary. These claims would presumably not be accepted,and they are not supported by experimental observations (Li et al.,2009). Fictive activity is the spinal locomotor network component of an integrated locomotor system, and cannot work in isolation.
A more promising approach is neuromodulation of natural activity evoked by targeting transmitter systems that act on G protein‐coupled receptors to modulate the functional state of spinal cord networks.However, while many transmitter systems have been studied there is still little insight into what would constitute an optimal pharmacological approach. There may be several reasons for this, one of which is that the effects of drugs and transmitter systems also change after lesioning. We have shown this for 5‐hydroxytryptamine (5‐HT), gam‐ma‐aminobutyric acid (GABA), and somatostatin in lamprey (Becker and Parker, 2015). Differences in modulatory effects are also seen in mammalian systems (see references in Parker, 2017). This may reflect direct changes in transmitter receptors or signalling pathways, or state‐dependent influences caused by changes in the functional proper‐ties that the modulatory systems act on. Whatever the mechanism, the conclusion is that a rational pharmacological approach to improving function after SCI cannot necessarily be based on drug effects in the unlesioned spinal cord. We instead need to understand how the spinal cord is altered after lesioning, what changes are needed to improve locomotor (or other) functions, and what pharmacological approaches could facilitate these changes.
It has to be emphasised that the consideration of changes either side of the lesion site does not negate the importance of regeneration (i.e.,there is no need for a dichotomy between regeneration and compen‐satory approaches). As outlined above, the functional changes may be a compensatory response to the altered descending input. This would make restoring the pre‐lesion input to the spinal cord, which is an unrealistic goal, unnecessary as the spinal cord is no longer in its pre‐lesion state. In any integrated system, various components interact through feedforward and feedback loops to generate particular outputs.Consideration and understanding of the various changes in motor and sensory properties and their interactions above and below the lesion,changes that show similarities from lamprey to human (Grasso et al.,2004; Courtine et al., 2009; Johnson et al., 2017; Parker, 2017), will al‐low regeneration strategies, electronic prostheses, or pharmacological approaches to be matched to the functional state of the spinal cord.This should lead to greater success in treating SCI than approaches that focus on any single aspect in isolation (Steward et al., 2012).
David Parker*
Department of Physiology, Neuroscience and Development,University of Cambridge, Cambridge, UK
*Correspondence to:David Parker, Ph.D., djp27@cam.ac.uk.
orcid:0000-0002-5345-348X (David Parker)
Accepted:2018-03-12
doi:10.4103/1673-5374.232472
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Ping K. Yip, Queen Mary University of London, UK.
Becker MI, Parker D (2015) Changes in functional properties and 5‐HT modulation above and below a spinal transection in lamprey. Front Neural Circuits 8:148.
Cohen AH, Mackler SA, Selzer ME (1988) Behavioral recovery following spinal transection: functional regeneration in the lamprey CNS. Trends Neurosci 11:227‐231.
Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR (2009)Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci 12:1333‐1342.
Grasso R, Ivanenko YP, Zago M, Molinari M, Scivoletto G, Castellano V, Macellari V, Lacquaniti F (2004) Distributed plasticity of locomotor pattern generators in spinal cord injured patients. Brain 127:1019‐1034.
Hoffman N, Parker D (2011) Interactive and individual effects of sensory potentia‐tion and region‐specific changes in excitability after spinal cord injury. Neurosci‐ence 199:563‐576.
Johnson MD, Frigon A, Hurteau MF, Cain C, Heckman CJ (2017) Reflex wind‐up in early chronic spinal injury: plasticity of motor outputs. J Neurophysiol 117:2065‐2074.
Li WC, Roberts A, Soffe SR (2009) Locomotor rhythm maintenance: electrical coupling among premotor excitatory interneurons in the brainstem and spinal cord of young Xenopus tadpoles. J Physiol 587:1677‐1693.
Oliphint PA, Alieva N, Foldes AE, Tytell ED, Lau BY, Pariseau JS, Cohen AH, Mor‐gan JR (2010) Regenerated synapses in lamprey spinal cord are sparse and small even after functional recovery from injury. J Comp Neurol 518:2854‐2872.
Parker D (2017) The lesioned spinal cord is a “new” spinal cord: evidence from functional changes after spinal injury in lamprey. Front Neural Circuits 11:84.
Selzer ME (2003) Promotion of axonal regeneration in the injured CNS. Lancet Neurol 2:157‐166.
Steward O, Popovich PG, Dietrich WD, Kleitman N (2012) Replication and reproducibility in spinal cord injury research. Exp Neurol 233:597‐605.
Stuart DG, Hultborn H (2008) Thomas Graham Brown (1882‐‐1965), Anders Lund‐berg (1920‐), and the neural control of stepping. Brain Res Rev 59:74‐95.
- 中国神经再生研究(英文版)的其它文章
- Weak phonation due to unknown injury of the corticobulbar tract in a patient with mild traumatic brain injury: a diffusion tensor tractography study
- Exosomes: a novel therapeutic target for Alzheimer’s disease?
- Inhibition of retinal ganglion cell apoptosis:regulation of mitochondrial function by PACAP
- Trillium tschonoskii maxim extract attenuates abnormal Tau phosphorylation
- Association between Alzheimer’s disease pathogenesis and early demyelination and oligodendrocyte dysfunction
- Nogo receptor expression in microglia/macrophages during experimental autoimmune encephalomyelitis progression